Abstract
Maintaining hematopoietic stem cell (HSC) quiescence is a critical property for the life-long generation of blood cells. Approximately 75% of cells in a highly enriched long-term repopulating HSC (LT-HSC) pool (Lin−Sca1+c-KithiCD150+CD48−) are quiescent, with only a small percentage of the LT-HSCs in cycle. Transcription factor GATA-3 is known to be vital for the development of T cells at multiple stages in the thymus and for Th2 differentiation in the peripheral organs. Although it is well documented that GATA-3 is expressed in HSCs, a role for GATA-3 in any prethymic progenitor cell has not been established. In the present study, we show that Gata3-null mutant mice generate fewer LT-HSCs and that fewer Gata3-null LT-HSCs are in cycle. Furthermore, Gata3 mutant hematopoietic progenitor cells fail to be recruited into an increased cycling state after 5-fluorouracil–induced myelosuppression. Therefore, GATA-3 is required for the maintenance of a normal number of LT-HSCs and for their entry into the cell cycle.
Introduction
Multiple hematopoietic cell lineages are generated from hematopoietic stem cells (HSCs) that are found in a very small fraction of the Lin−Sca1+c-Kithi (LSK) population of BM cells. Long-term repopulating HSCs (LT-HSCs) are even more rare, and can be purified almost to homogeneity in the LSK CD150+CD48−CD34−Flt3− immunophenotypic population of murine adult BM cells.1-3 Less than 5% of LT-HSCs are actively cycling in the S + G2/M phases to produce more HSCs for maintaining life-long hematopoiesis and for the generation of terminally differentiated hematopoietic cells. Conversely, approximately 75% of LT-HSCs are quiescent (in the G0 phase), thus maintaining both stemness and proliferative capacity.4,5 Studies using various mutant model mice support the contention that the maintenance of HSC quiescence is essential for their long-term repopulating function.6 The balance between quiescence and cell-cycle entry is controlled by signals from the HSC niche through a variety of signaling pathways, cyclin-dependent kinases, and transcription factors. The molecular mechanisms that control HSC cell-cycle entry are not fully understood, although recent studies have identified several nuclear proteins that are involved in restricting or promoting cell-cycle entry; these include: GFI1,7 MEF/ELF4,8 FOXO3A,9 GFI1B,10 EGR1,11 JUNB,12 and perhaps both c- and N-Myc.13
The GATA transcription factors all bind to a WGATAR DNA sequence motif found in the promoters and enhancers of thousands of target genes to control their transcription. Recent genome-wide ChIP-seq experiments confirmed that WGATAA is the preferred sequence bound by GATA proteins in vivo.14-17 The vertebrate GATA family is composed of 6 members, somewhat artificially divided into the hematopoietic (GATA-1, GATA-2, and GATA-3) and endodermal (GATA-4, GATA-5, and GATA-6) subfamilies. GATA-2 and GATA-3 are both expressed in HSCs.18-21 Whereas Gata2-null mutant embryonic stem (ES) cells fail to generate HSCs or progenitors in reconstituted chimeric mice,22 Gata3-null ES cells are able to generate myeloid and B-lymphoid cells, but not T cells.23 Therefore, GATA-2 is required for the generation of HSCs, but GATA-3 is not. More specifically, GATA-2 is required for HSC proliferation and viability during definitive hematopoiesis, and even haploinsufficiency of GATA-2 results in a reduced number of functional HSCs.22,24-26 GATA-3 is vital for the development of T cells at multiple stages in T-cell development and for Th2 differentiation in peripheral organs, but is dispensable for the generation of myeloid and B cells.27
We recently discovered that GATA-3 is required for the generation of early T-lineage progenitor (ETP) cells, the most immature cells in the thymus, which have been shown to have complete developmental potential for T-lineage development, and that GATA-3 plays a critical role immediately around the time of thymic entry of T-cell progenitors.28 To further explore a possible role for GATA-3 in pre-thymic T-cell progenitors, we examined GATA-3 mRNA expression profiles in various hematopoietic progenitor populations in the BM, and found that GATA-3 is highly expressed in the primitive LT-HSC population (LSK CD34−CD150+CD48−). This prompted us to hypothesize that GATA-3 might control one or more LT-HSC–specific functions.
Buza-Vidas et al reported recently that GATA-3 is dispensable for HSC regulation under conditions of steady-state maintenance and transplantation stress by analyzing Gata3flox/flox:TgVav-Cre cells in 1-week-old mice.29 In contrast, in the present study, we report that GATA-3 is in fact required for the maintenance of normal LT-HSC numbers and for promoting HSC cell-cycle entry in adult mice.
Methods
Wild-type and mutant mice
C57Bl/6 (B6, CD45.2) mice and C57Bl/6-Ly5.1 (B6-SJL, CD45.1) mice were purchased from The Jackson Laboratory. Gata3z-,30 Gata3flox-,31 and TgMx1Cre32 –transgenic mice were generated as described previously. Gata3z/z B6 embryos were rescued by norepinephrine administration.33,34 The Mx1Cre transgene was bred into the Gata3flox (CD1/B6 mixed) background; deletion of the floxed alleles was induced with polyinosinic-polycytidylic acid [poly(I:C)] (P1530; Sigma-Aldrich) treatment (3 IP injections of 300 μg every 2 days) into 6- to 9-week-old mice; cells were collected 3 weeks after the first injection. Poly(I:C) injection did not affect LT-HSC number or HSC cell-cycle status in wild-type or TgMx1Cre mice 3 weeks after the first injection (Essers et al35 and data not shown). All mice used in this study were housed in the Unit for Laboratory Animal Medicine at the University of Michigan, and all experiments were approved by the University Committee on Use and Care of Animals.
Cell preparation and flow cytometry
Whole BM cells were flushed from 2 femurs and 2 tibias with PBS (Invitrogen) supplemented with 2% FBS (Invitrogen). Cells were filtered through a cell strainer to obtain a single-cell suspension. Cell suspensions were first treated with FcBlock (BD Pharmingen) and then incubated with various Ab combinations. Mature hematopoietic lineage cells (Lin+) were labeled with a cocktail of purified mAbs that recognize the B220 (RA3-6B2), TER119 (TER119), Gr1 (RB6-8C5), and CD2 (RM2-5) antigens, and excluded by magnetic bead depletion with sheep anti–rat IgG-conjugated Dynabeads (Invitrogen) following the manufacturer's instructions. Depending on the specific analysis, the following Abs were also used: CD3 (145-2C11), CD4 (RM4-5), CD8 (53-6.7), CD19 (1D3), CD34 (RAM34), CD45.1 (A20), CD45.2 (104), CD48 (HM48.1), CD150 (mshad150), c-Kit (2B8), Mac-1 (M1/70), Sca1 (D7), and Thy1.2 (53-2.1). Cells were resuspended in propidium iodide or 4′,6-diamidion-2-phenylindole (DAPI) solution to discriminate live from dead cells. All Abs were purchased from BD Pharmingen, BioLegend, or eBioscience. Cells were analyzed on a FACSCanto II flow cytometer and sorted on a FACSAria cell sorter (both BD Pharmingen). Files were analyzed using FlowJo Versions 8.8.6 to 9.4.4 software (TreeStar).
Adoptive transfer
B6-SJL × B6 F1 (CD45.1/CD45.2 heterozygotes) recipient mice were irradiated using an orthovoltage X-ray source delivering approximately 113.5 rads/min. After receiving 2 doses of 550 rads from 2-4 hours apart, recipient mice were transplanted within the following 12 hours. For primary transplantation, CD45.2+ embryonic day 14.5 (e14.5) fetal liver cells from Gata3+/+ or Gata3z/z mice were mixed at a 1:1 ratio with competitor CD45.1+ BM cells (5 × 105 cells each) and transplanted into CD45.1/CD45.2 recipients as described previously.28 Chimerism in peripheral blood was analyzed at 4, 8, 12, and 16 weeks after transplantation. Animals were considered positive for engraftment if > 1% of test cells were in the Gr1+Mac1+ myeloid cell population. For secondary transplantation, irradiated recipients were transplanted with a diluted number (9600, 3200, 800, 200, and 50) of LSK CD45.2+CD45.1− donor cells recovered from primary recipients (donors varied in age from 17-21 weeks after transplantation). Test cells were injected intravenously via the retro-orbital sinus, along with 2 × 105 fresh competitor cells (CD45.1+). Animals were considered positive for engraftment if > 1% of test cells were in the Gr1+Mac1+ myeloid cell and CD19+ B-cell populations. The HSC frequency was calculated using L-Calc Version 1.1.1 software (StemCell Technologies).
Apoptosis analysis
After cell-surface staining, apoptosis was analyzed using annexin V and DAPI staining in annexin V binding buffer, as described by the manufacturer (BD Pharmingen).
Cell-cycle and cell-proliferation assays
Cells were first stained with cell-surface markers, followed by fixation and permeabilization. Cell-cycle analysis was performed by 5-bromodeoxyuridine (BrdU) incorporation and flow cytometry using the BrdU Flow Kit according to the manufacturer's protocol (BD Pharmingen). Mice were injected IP with 1.5 mg of BrdU and maintained with 1 mg/mL of BrdU (Sigma-Aldrich) in their drinking water for 19 hours before analysis. Cell proliferation was assessed by Ki67 immunostaining (BD Pharmingen) and DAPI staining. Mice in the myelosuppressive treatment experiment received a single IP injection of 5-fluorouracil (5-FU; Sigma-Aldrich) at 150 μg/g body weight, 15 days after the first poly(I:C) treatment. Cells were analyzed 6 days after 5-FU administration.
qRT-PCR
For quantitative RT-PCR (qRT-PCR), RNA was purified with RNeasy Micro Kit (QIAGEN) and used to synthesize cDNA with the SuperScript III First-Strand Synthesis Kit (Invitrogen). Real-time PCR was performed with TaqMan probe or SYBR Green dye on an ABI Prism 7000 system (Applied Biosystems). Primers used were as follows: HPRT,36 GATA-3,37 and GATA-2: forward 5′-GCAGAGAAGCAAGGCTCGC-3′ and reverse 5′-CAGTTGACACACTCCCGGC-3′; MEF,38 FOXO3A,39 c-Myc and N-Myc,13 EGR1,11 JUNB,12 and GFI1 (Mm00515855_m1; Applied Biosystems) and GFI1B (Mm00492319_m1; Applied Biosystems). All results of qRT-PCR were quantified relative to the mRNA expression of the endogenous reference gene Hprt for SYBRGreen assay or ribosomal RNA for TaqMan assay (Applied Biosystems).
Statistical analysis
Statistical significance was determined with the Student t test. Data were considered statistically significant when P < .05.
Results
Expression of the Gata3 gene during the early stages of definitive hematopoiesis
GATA-3 is not only expressed in HSCs, but also in multipotent progenitor cells (MPPs), common lymphoid progenitors (CLPs), and pre-pro-B cells in the BM.18,19,21,40-42 To address whether GATA-3 is involved in any function of pre-thymic T-cell progenitors, its mRNA expression was determined by qRT-PCR in purified HSCs or progenitor populations in the BM and at various stages of T-cell development in the thymus and spleen.
Cells collected from adult C57Bl/6 mouse BM were sorted into LT-HSCs (LSK CD150+CD48−CD34−) or LSK Flt3−, MPPs (LSK Flt3lo), lymphoid-primed multi-potential progenitor/LMPPs; LSK Flt3hi), and CLPs (lineage−Sca1loc-KitloIL-7Rα+Flt3+). The LSK CD150+CD48−CD34− population of murine BM cells is highly enriched for HSCs,1,3 and represents only approximately 0.003% of total BM cells. In addition, T cells at various stages of development were sorted from the thymus and spleen: ETP (Lin−c-KithiCD25−), DN2 (Lin−c-KithiCD25+), DN3 (Lin−c-Kitlo/−CD25+), DN4 (Lin−c-Kitlo/−CD25−), DP (CD4+CD8+), thymic CD8 SP T cells (CD4−CD8+), thymic CD4 SP T cells (CD4+CD8−), splenic CD8 T cells (CD3+CD8+CD4−), and splenic CD4 T cells (CD3+CD4+CD8−). Splenic B cells (CD19+B220+) were included for comparison. Sorted cells were analyzed by qRT-PCR for the expression of GATA-3.
Whereas the level of GATA-3 expression was high in CD4 T cells, it was low in CD8 T cells and barely detectable in B cells, in agreement with previous observations.43,44 As reported previously, GATA-3 mRNA was detected in all of the following immature progenitor populations: HSCs (LSK Flt3−), MPPs, LMPPs, and CLPs in the BM18,19,21,40-42 and ETP and DN2 in the thymus.45,46 Somewhat surprisingly, GATA-3 mRNA was even more abundant in the LT-HSC fraction than in any other BM progenitor fraction (Figure 1A). We found that LT-HSCs express GATA-3 at levels that are similar to ETP, in which GATA-3 is known to exert an essential function. These data therefore prompted us to explore a possible role for GATA-3 in prethymic progenitor cells, specifically in LT-HSCs.
GATA-3 is abundantly expressed in LT-HSCs and plays a role in maintenance of the HSC pool. (A) qRT-PCR analysis of GATA-3 mRNA relative abundance in purified BM, thymus, and spleen populations recovered from adult (6- to 9-week-old) C57Bl/6 mice. LSK CD150+CD48−CD34− (LT-HSCs CD34−), LSK Flt3− (HSCs Flt3−), LSK Flt3lo (MPPs), LSK Flt3hi (LMPPs), Lin−Sca1loc-KitloIL-7Rα+Flt3+ (CLPs), Lin−c-KithiCD25− (ETPs), Lin−c-KithiCD25+ (DN2), Lin−c-Kitlo/−CD25+ (DN3), Lin−c-Kitlo/−CD25− (DN4), CD4+CD8+ (DP), CD4−CD8+ (thymic CD8 SP T cells), CD4+CD8− (thymic CD4 SP T cells), CD3+CD8+CD4− (splenic CD8 T cells), CD3+CD4+CD8− (splenic CD4 T cells), and CD19+ B220+ (splenic B cells). (−) indicates the no-template control. Data represent the summary of 3 independent experiments. (B) Loss of GATA-3 expression in LSK cells was confirmed through comparison of adult Gata3flox/flox:TgMx1Cre [f/f(+)] to control Gata3flox/flox [f/f(−)] or Gata3flox/+:TgMx1Cre [f/+(+)] 3 weeks after the first poly(I:C) injection. The level of GATA-3 mRNA of the f/f(−) mice was set at 100%. Data represent the summary of 5 mice of each genotype determined in 3 independent experiments with SEM. *P < .001. (C) Representative gating strategy of HSC populations in flow cytometric analysis. Lin− BM cells were isolated from f/f(−) and f/f(+) mice after TgMx1Cre induction by poly(I:C), and analyzed for surface expression of c-Kit and Sca1 to mark the LSK population, then further analyzed for CD150 and CD48 expression. Numbers adjacent to the boxed areas indicate the mean percentage of cells in the gate. (D) The absolute numbers of LSK CD150+CD48−CD34− cells per mouse (2 femurs plus 2 tibias) in f/f(−), f/+(+), and f/f(+) mice 3 weeks after poly(I:C) treatment. Data represent the summary of 12 mice of each genotype from 5 independent experiments with SEM. *P < .001; #P < .008. NS indicates not significant. (E) LSK CD150+CD48−CD34− cells were sorted from f/f(−), f/+(+), and f/f(+) mice 3 weeks after poly(I:C) treatment. Complete deletion of the conditional allele was verified in f/f(+) LT-HSCs. Data represent the summary of 3 mice of each genotype from 2 independent experiments with SEM. *P < .009; #P < .03. NS indicates not significant; ND, not detectable.
GATA-3 is abundantly expressed in LT-HSCs and plays a role in maintenance of the HSC pool. (A) qRT-PCR analysis of GATA-3 mRNA relative abundance in purified BM, thymus, and spleen populations recovered from adult (6- to 9-week-old) C57Bl/6 mice. LSK CD150+CD48−CD34− (LT-HSCs CD34−), LSK Flt3− (HSCs Flt3−), LSK Flt3lo (MPPs), LSK Flt3hi (LMPPs), Lin−Sca1loc-KitloIL-7Rα+Flt3+ (CLPs), Lin−c-KithiCD25− (ETPs), Lin−c-KithiCD25+ (DN2), Lin−c-Kitlo/−CD25+ (DN3), Lin−c-Kitlo/−CD25− (DN4), CD4+CD8+ (DP), CD4−CD8+ (thymic CD8 SP T cells), CD4+CD8− (thymic CD4 SP T cells), CD3+CD8+CD4− (splenic CD8 T cells), CD3+CD4+CD8− (splenic CD4 T cells), and CD19+ B220+ (splenic B cells). (−) indicates the no-template control. Data represent the summary of 3 independent experiments. (B) Loss of GATA-3 expression in LSK cells was confirmed through comparison of adult Gata3flox/flox:TgMx1Cre [f/f(+)] to control Gata3flox/flox [f/f(−)] or Gata3flox/+:TgMx1Cre [f/+(+)] 3 weeks after the first poly(I:C) injection. The level of GATA-3 mRNA of the f/f(−) mice was set at 100%. Data represent the summary of 5 mice of each genotype determined in 3 independent experiments with SEM. *P < .001. (C) Representative gating strategy of HSC populations in flow cytometric analysis. Lin− BM cells were isolated from f/f(−) and f/f(+) mice after TgMx1Cre induction by poly(I:C), and analyzed for surface expression of c-Kit and Sca1 to mark the LSK population, then further analyzed for CD150 and CD48 expression. Numbers adjacent to the boxed areas indicate the mean percentage of cells in the gate. (D) The absolute numbers of LSK CD150+CD48−CD34− cells per mouse (2 femurs plus 2 tibias) in f/f(−), f/+(+), and f/f(+) mice 3 weeks after poly(I:C) treatment. Data represent the summary of 12 mice of each genotype from 5 independent experiments with SEM. *P < .001; #P < .008. NS indicates not significant. (E) LSK CD150+CD48−CD34− cells were sorted from f/f(−), f/+(+), and f/f(+) mice 3 weeks after poly(I:C) treatment. Complete deletion of the conditional allele was verified in f/f(+) LT-HSCs. Data represent the summary of 3 mice of each genotype from 2 independent experiments with SEM. *P < .009; #P < .03. NS indicates not significant; ND, not detectable.
Deletion of Gata3 affects HSC maintenance
To define a role for GATA-3 in LT-HSCs, we first determined their numbers in various Gata3 mutant mice. Because the early lethality of Gata3-null mutant embryos47 prohibits investigation of HSC function in adult animals, Gata3flox/flox:TgMx1Cre conditional knockout (cKO) mice31,32 were generated, and Cre expression was activated using poly(I:C) to conditionally inactivate the Gata3 gene. Littermates bearing either a Gata3flox/flox (ie, without a transgene, pseudo–wild-type) or Gata3flox/+:TgMx1Cre genotype were used as controls in these experiments; all mice received poly(I:C) administration. As shown in Figure 1B, 3 IP injections of 300 μg of poly(I:C) into 6- to 9-week-old mice were sufficient to completely inactivate the floxed Gata3 allele, and therefore GATA-3 mRNA was reduced by 98.9% within the BM LSK population. Furthermore, a significant reduction (51%) was observed in the absolute number of LT-HSCs in the cKO mice 3 weeks after the first poly(I:C) treatment (Figure 1C-D) compared with pseudo–wild-type control littermates. To confirm that Gata3 was efficiently ablated even in the LT-HSCs, the few HSCs that could be recovered after poly(I:C) administration were examined by qRT-PCR. GATA-3 mRNA was undetectable in the LT-HSCs recovered from cKO mice (Figure 1E), demonstrating that the survival of the remaining LT-HSCs was not a consequence of incomplete inactivation of Gata3.
To further address the role of GATA-3 in maintaining LT-HSC number, lethally irradiated mice were competitively reconstituted with Gata3-null mutant hematopoietic cells28 and recipient BM cells were analyzed by flow cytometry 16-20 weeks after adoptive transfer. For these experiments, we used a Gata3-null allele (Gata3z) that was maintained on an inbred C57Bl/6 background28,30 instead of the Gata3flox allele that was maintained on a mixed background (50%-75% outbred).31 The advantage of using the Gata3z/z-null cells is that we were able to test the consequence of 100% Gata3 deficiency, whereas Gata3flox/flox:TgMx1Cre LSK cells still express 1%-2% residual GATA-3 mRNA (compared with pseudo–wild-type controls) after poly(I:C) treatment (Figure 1B). Because catecholamine-rescued Gata3z/z-null mutant mice are not able to survive beyond approximately e18.5,33,34 we used e14.5 fetal livers as the HSC donor cells to obtain Gata3-null mutant adult HSCs, as we have done previously.28 In agreement with the results observed in cKO animals, a reduction (69%) in absolute number was observed in the most primitive CD45.2+CD45.1−Gata3-null mutant donor–derived LSK CD150+CD48−CD34− cells (Figure 2A and supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Furthermore, these recipient mice exhibited a 55% reduction in CD45.2+CD45.1− LSK cell numbers 16-20 weeks after adoptive transfer (Figure 2A and supplemental Figure 1), indicating a diminished contribution of Gata3-null mutant cells to HSCs and progenitor cell expansion than control wild-type cells in a competitive situation. Of note, when LSK numbers recovered from the Gata3z/z mutant hematopoietic cells are compared at earlier and later times after transplantation, more profound reduction was observed later (55% reduction at 16-20 weeks) than earlier (40% reduction at 10 weeks, a reduction that nevertheless was not statistically meaningful28 ). These data suggest that Gata3-null fetal liver HSCs initially engraft normally, but gradually lose their ability to maintain a wild-type number of cells in the HSCs pool in the normal adult BM environment (niche). These results thereby demonstrate that GATA-3 plays an important role in maintaining the stem cell number within the adult LT-HSCs pool.
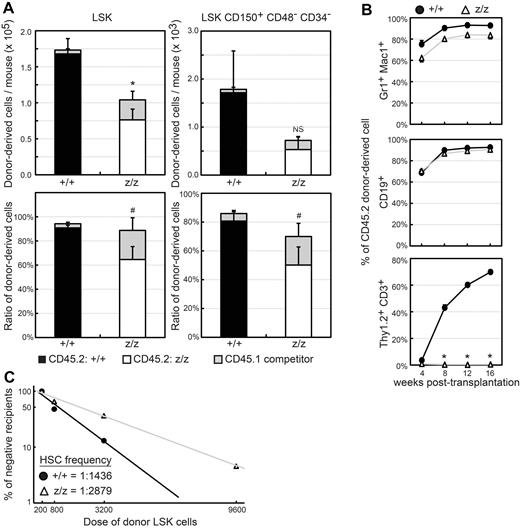
A role for GATA-3 in the maintenance of hematopoiesis. (A) Chimerism was analyzed in primary recipient mice 16-20 weeks after injection of a 1:1 ratio of fetal liver cells from Gata3z/z (CD45.2) or littermate control Gata3+/+ (CD45.2) and adult BM cells (CD45.1). Donor-derived absolute cell numbers (top) per mouse (2 femurs plus 2 tibias) and cell ratios (bottom) in BM LSK (left panels) and LSK CD150+CD48−CD34− (right panels) populations were determined by flow cytometry. Recipient mice were considered to be engrafted when > 1% CD45.2+CD45.1− myeloid cells were of the donor immunophenotype. Data represent the summary of 4 independent experiments and 6 recipient mice in each group with SEM. *P < .003; #P < .03. NS indicates not significant. (B) Mature lineage cells in peripheral blood were analyzed by flow cytometry every 4 weeks for 16 weeks after transplantation for lineage-specific markers and CD45. The contribution of fetal liver donor-derived cells (CD45.2+CD45.1−) to myeloid (Gr1+ Mac1+), B-lymphoid (CD19+), and T-lymphoid (Thy1.2+CD3+) lineages from Gata3+/+ (+/+, ●) or Gata3z/z (z/z, ▵) cells are shown. Data represent the summary of 5 independent experiments and an average of 12 recipient mice in each group with SEM. *P < .001. (C) The frequency of long-term repopulating cells was determined in vivo by administering different doses of donor LSK cells. The indicated number (horizontal axis) of Gata3+/+ (+/+, ●) or Gata3z/z (z/z, ▵) LSK CD45.2+CD45.1− cells from primary recipients were transplanted into lethally irradiated secondary recipients along with 2 × 105 CD45.1 adult BM cells. Recipient mice that were engrafted by more than 1% with CD45.2+CD45.1− myeloid and B cells in peripheral blood for 16 weeks after transplantation were scored as positive and were used to calculate the HSC frequency in the primary recipients (also see supplemental Table 1).
A role for GATA-3 in the maintenance of hematopoiesis. (A) Chimerism was analyzed in primary recipient mice 16-20 weeks after injection of a 1:1 ratio of fetal liver cells from Gata3z/z (CD45.2) or littermate control Gata3+/+ (CD45.2) and adult BM cells (CD45.1). Donor-derived absolute cell numbers (top) per mouse (2 femurs plus 2 tibias) and cell ratios (bottom) in BM LSK (left panels) and LSK CD150+CD48−CD34− (right panels) populations were determined by flow cytometry. Recipient mice were considered to be engrafted when > 1% CD45.2+CD45.1− myeloid cells were of the donor immunophenotype. Data represent the summary of 4 independent experiments and 6 recipient mice in each group with SEM. *P < .003; #P < .03. NS indicates not significant. (B) Mature lineage cells in peripheral blood were analyzed by flow cytometry every 4 weeks for 16 weeks after transplantation for lineage-specific markers and CD45. The contribution of fetal liver donor-derived cells (CD45.2+CD45.1−) to myeloid (Gr1+ Mac1+), B-lymphoid (CD19+), and T-lymphoid (Thy1.2+CD3+) lineages from Gata3+/+ (+/+, ●) or Gata3z/z (z/z, ▵) cells are shown. Data represent the summary of 5 independent experiments and an average of 12 recipient mice in each group with SEM. *P < .001. (C) The frequency of long-term repopulating cells was determined in vivo by administering different doses of donor LSK cells. The indicated number (horizontal axis) of Gata3+/+ (+/+, ●) or Gata3z/z (z/z, ▵) LSK CD45.2+CD45.1− cells from primary recipients were transplanted into lethally irradiated secondary recipients along with 2 × 105 CD45.1 adult BM cells. Recipient mice that were engrafted by more than 1% with CD45.2+CD45.1− myeloid and B cells in peripheral blood for 16 weeks after transplantation were scored as positive and were used to calculate the HSC frequency in the primary recipients (also see supplemental Table 1).
Long-term reconstitution potential of Gata3 mutant HSCs
Although we showed that Gata3-null mutant fetal liver cells were able to produce both B and myeloid cells in the peripheral blood up to 10 weeks after donor cell injection in earlier adoptive-transfer experiments,28 long-term repopulating potential is more commonly evaluated by analyzing reconstitution for more than 16 weeks.48 To test the long-term repopulating potential of fetal Gata3-null mutant HSCs, recipient mice reconstituted with wild-type and Gata3z/z-null mutant fetal liver cells were monitored for donor cell reconstitution in the myeloid (Gr1+ Mac1+), B lymphoid (CD19+) and T lymphoid (Thy1.2+CD3+) populations in peripheral blood every 4 weeks for 16 weeks. As shown in Figure 2B, for 16 weeks Gata3-null fetal liver donor hematopoietic cells were able to contribute to the peripheral myeloid and B cell populations at levels that were similar to control wild-type cells. These data show that Gata3-null mutant fetal HSCs retain long-term repopulating potential. This conclusion is consistent with the observation by Buza-Vidas et al that GATA-3 is dispensable for the self-renewal function of HSCs when isolated from 1-2 week-old mice.29
To further investigate the long-term repopulating potential of the Gata3-null adult HSCs and to accurately measure the reduction in number of functional HSCs in the Gata3 mutants, we injected different numbers of either wild-type or Gata3z/z C57Bl/6 CD45.2+CD45.1− LSK cells that were isolated from the primary adoptive transfer recipients into secondary recipients. As summarized in Figure 2C and supplemental Table 1, the data exhibited a diminished LT-HSC frequency in Gata3z/z-null cells compared with control wild-type cells. These results demonstrate that whereas GATA-3 is dispensable for the long-term repopulating potential of HSCs, it is essential for maintaining a wild-type number of functional LT-HSCs.
Loss of Gata3 results in impaired HSC cell-cycle entry and proliferation
To delve more deeply into the nature of the cellular defect in Gata3-deficient HSCs, cell survival of HSCs of poly(I:C)–treated, compound mutant–floxed, transgenic (TgMx1Cre) animals was examined by flow cytometry using an early apoptosis marker (annexin V) and a marker for dead cells (DAPI). Because no significant difference in annexin V binding was observed in LSK CD150+CD34− cells between the cKO and control littermate mice (Figure 3A-B), we concluded that the reduced LT-HSC number in Gata3 mutant animals was not likely to be because of reduced cell viability.
GATA-3 participates in HSC cell-cycle control. (A) Profile of annexin V and DAPI staining in BM LSK CD150+CD34− cells 3 weeks after poly(I:C) treatment. Numbers adjacent to the boxed areas indicate the mean percentage of cells in the gate. (B) Ratio of annexin V+DAPI− and annexin V+DAPI+ cells in LSK CD150+CD34− cells from adult Gata3flox/flox [f/f(−)], Gata3flox/+:TgMx1Cre [f/+(+)], and Gata3flox/flox:TgMx1Cre [f/f(+)] mice 3 weeks after poly(I:C) treatment. Data represent the summary of at least 5 mice of each genotype from 3 independent experiments with SEM. NS indicates not significant. (C) Profile examining the number of cells in cycle assessed after 19 hours of BrdU labeling with DNA content (DAPI) in BM LSK CD150+CD48− (top panel) and LSK CD150+CD48−CD34− (bottom panel) populations 3 weeks after poly(I:C) treatment. Numbers in the boxed areas indicate the mean percentage of cells in each gate. (D) Ratio of BrdU+ cells (S phase) in BM LSK (left group), LSK CD150+CD48− (middle group), and LSK CD150+CD48−CD34− (right group) populations 3 weeks after poly(I:C) treatment. Data represent the summary of 5 mice of each genotype from 5 independent experiments with SEM. *P < .05; #P < .002. NS indicates not significant. (E) Cell-cycle analysis using intracellular Ki67 and DNA content (DAPI) staining in BM LSK CD150+CD34− cells 3 weeks after poly(I:C) treatment. Numbers adjacent to the boxed areas indicate the mean percentage of cells in gate. (F) Ratio of cells in the G0, G1, and S + G2/M phases in BM LSK CD150+CD34− cells from adult f/f(−) (black bars), f/+(+) (gray bars), and f/f(+) (white bars) 3 weeks after poly(I:C) treatment. Data represent the summary of at least 5 mice of each genotype from 3 independent experiments with SEM. *P < .008; #P < .02; +P < .03. NS indicates not significant. (G) Profile of DNA content (DAPI) staining in BM LSK cells without (top panels) or with (bottom panels) 5-FU treatment for 6 days; cells were analyzed 3 weeks after poly(I:C) treatment. Numbers above the indicator bars quantify the mean percentage of cells in that gate. (H) Ratio of cells in the S + G2/M phase in the LSK population (top) and the absolute LSK cell numbers (bottom) per mouse (2 femurs plus 2 tibias) either without (−) or with (+) 5-FU treatment. Data represent the summary of at least 6 mice of each genotype and treatment groups from 3 independent experiments with SEM. *P < .001; #P = .05; +P < .03. NS indicates not significant.
GATA-3 participates in HSC cell-cycle control. (A) Profile of annexin V and DAPI staining in BM LSK CD150+CD34− cells 3 weeks after poly(I:C) treatment. Numbers adjacent to the boxed areas indicate the mean percentage of cells in the gate. (B) Ratio of annexin V+DAPI− and annexin V+DAPI+ cells in LSK CD150+CD34− cells from adult Gata3flox/flox [f/f(−)], Gata3flox/+:TgMx1Cre [f/+(+)], and Gata3flox/flox:TgMx1Cre [f/f(+)] mice 3 weeks after poly(I:C) treatment. Data represent the summary of at least 5 mice of each genotype from 3 independent experiments with SEM. NS indicates not significant. (C) Profile examining the number of cells in cycle assessed after 19 hours of BrdU labeling with DNA content (DAPI) in BM LSK CD150+CD48− (top panel) and LSK CD150+CD48−CD34− (bottom panel) populations 3 weeks after poly(I:C) treatment. Numbers in the boxed areas indicate the mean percentage of cells in each gate. (D) Ratio of BrdU+ cells (S phase) in BM LSK (left group), LSK CD150+CD48− (middle group), and LSK CD150+CD48−CD34− (right group) populations 3 weeks after poly(I:C) treatment. Data represent the summary of 5 mice of each genotype from 5 independent experiments with SEM. *P < .05; #P < .002. NS indicates not significant. (E) Cell-cycle analysis using intracellular Ki67 and DNA content (DAPI) staining in BM LSK CD150+CD34− cells 3 weeks after poly(I:C) treatment. Numbers adjacent to the boxed areas indicate the mean percentage of cells in gate. (F) Ratio of cells in the G0, G1, and S + G2/M phases in BM LSK CD150+CD34− cells from adult f/f(−) (black bars), f/+(+) (gray bars), and f/f(+) (white bars) 3 weeks after poly(I:C) treatment. Data represent the summary of at least 5 mice of each genotype from 3 independent experiments with SEM. *P < .008; #P < .02; +P < .03. NS indicates not significant. (G) Profile of DNA content (DAPI) staining in BM LSK cells without (top panels) or with (bottom panels) 5-FU treatment for 6 days; cells were analyzed 3 weeks after poly(I:C) treatment. Numbers above the indicator bars quantify the mean percentage of cells in that gate. (H) Ratio of cells in the S + G2/M phase in the LSK population (top) and the absolute LSK cell numbers (bottom) per mouse (2 femurs plus 2 tibias) either without (−) or with (+) 5-FU treatment. Data represent the summary of at least 6 mice of each genotype and treatment groups from 3 independent experiments with SEM. *P < .001; #P = .05; +P < .03. NS indicates not significant.
At any given time, less than 5% of BM LT-HSCs are actively cycling and found in the S + G2/M phases of the cell cycle, whereas most (75%) LT-HSCs are quiescent in the G0 phase.4,5 Studies examining different murine mutants support the hypothesis that unchecked proliferation leads to the exhaustion of adult HSCs.6 We showed previously that the percentage of S-phase cells in Lin−CD27+C-KithiCD25− lymphoid progenitors was slightly higher in Gata3z/z mutants (62%) than in Gata3+/+ animals (50%).28 Similarly, the percentage of S-phase cells in e18.5 ETP and DN2 was slightly higher in Gata3g/g (hypomorphic mutant) ETP- and DN2-stage thymocytes (51% and 56%, respectively) than in Gata3+/+ ETP and DN2 cells (39% and 47%, respectively; our unpublished observations). Intriguingly, the loss of Gata3 has also been reported to lead to elevated cell-cycle progression in mammary gland luminal cells.49 Based on the more frequent cycling status of Gata3 mutants during early T-cell progenitor stages and in the mammary gland, we hypothesized that Gata3 mutant HSCs might also exhibit more active cycling than control HSCs, and therefore that one consequence of this more active cycling could be early exhaustion of LT-HSCs.
To experimentally test this “early exhaustion” hypothesis, the cell-cycle status of wild-type and mutant HSCs was investigated by in vivo labeling of cycling cells with BrdU.4,50 Unlike what was observed in early T-cell progenitors, there was no significant effect of Gata3 deletion on the percentage of cycling LSK cells, whereas the percentage of LSK CD150+CD48− or LSK CD150+CD48−CD34− cells that exited G0 and entered the cell cycle statistically decreased in Gata3 mutant cells compared with controls (Figure 3C-D). To validate this observation, we also investigated the expression of Ki67 (negative in G0) and DNA content (DAPI) by flow cytometry. As shown in Figure 3E and F, the percentage of Gata3 mutant LSK CD150+CD34− cells that were quiescent in G0 increased compared with controls. Therefore, in contrast to our initial hypothesis, these data suggest that the reduced LT-HSC numbers in Gata3 mutants were not a consequence of an increase in the ratio of cells exiting G0 to enter the cell cycle, thereby leading to HSC exhaustion. Instead, the data support the possibility that GATA-3 may somehow be involved in recruiting HSCs out of the quiescent state.
To underscore the involvement of GATA-3 in HSC cell-cycle regulation, we analyzed the cycling status and BM LSK numbers of Gata3 cKO mutants after recovery from myelosuppression by 5-FU.51 Because 5-FU leads to massive induction of cell-cycle activity in HSCs, we hypothesized that this might allow a different evaluation of the impact of GATA-3 on cell-cycle progression.52 Fifteen days after poly(I:C) treatment, mice were challenged with a single injection of 5-FU, and then assessed for cell-cycle status, as shown by DNA content, 6 days after 5-FU treatment (at their proliferative peak) during BM ablation and recovery. In agreement with previous studies, wild-type animals exhibited enhanced progression into the cell cycle to recover from the loss of LSK cells after 5-FU treatment (Figure 3G-H). In contrast, the Gata3 mutants exhibited no enhancement of cycling, which resulted in significantly delayed LSK expansion after 5-FU treatment. These data demonstrate that GATA-3 promotes cell-cycle entry and proliferation of HSCs directly.
Enhanced B-lymphocyte development in Gata3-null mutant HSCs
As shown in Figure 2B, Gata3-null mutant hematopoietic progenitor cells retain the capability to generate myeloid and B cells. Intriguingly, the ratio of B cells to myeloid cells that are derived from Gata3z/z adult LSK cells in secondary recipients was higher than the ratio derived from control Gata3+/+ adult LSK cells at weeks 4-16 (Figure 4A). In addition, this increased production of B cells was only observed 16 weeks after primary transplantation of fetal liver HSC donor cells (Figure 4A). The cell number in mature lineages of the BM was analyzed 16-20 weeks after transplantation, and an increased number of B-lineage cells was again observed only in secondary recipients (Figure 4B). These data suggest that GATA-3, either directly or indirectly, represses B-cell reconstitution in these adoptive-transfer experiments.
GATA-3 inhibits the generation of adult B-lymphoid cells. (A) Peripheral blood was analyzed from primary recipient (1°-, top) and secondary recipient (2°-, bottom) mice by flow cytometry every 4 weeks for 16 weeks after transplantation. Ratios of donor-derived (CD45.2+CD45.1−) B-lymphoid (CD19+) to myeloid (Gr1+Mac1+) cells in peripheral blood from Gata3+/+ (+/+, ●) or Gata3z/z (z/z, ▵) cells are shown. Data represent the summary of 5 independent experiments with an average of 11 recipient mice for 1°- and 2 independent experiments with an average of 40 recipient mice for 2°- in each group with SEM. *P < .03. (B) The absolute numbers of donor-derived cells (CD45.2+CD45.1−) in myeloid (Gr1+Mac1+, left panels), B-lymphoid (CD19+B220+, middle panels), and T-lymphoid (Thy1.2+CD3+, right panels) populations per mouse (2 femurs plus 2 tibias) from +/+ or z/z donor cells in BM. Chimerism was analyzed from 1°- (top panels) and 2°- (bottom panels) recipient mice 16-20 weeks after transplantation. Data represent the summary of 3 independent experiments with an average of 12 recipient mice for 1°- and 2 independent experiments with an average of 20 recipient mice for 2°- in each group with SEM. *P < .03. NS indicates not significant.
GATA-3 inhibits the generation of adult B-lymphoid cells. (A) Peripheral blood was analyzed from primary recipient (1°-, top) and secondary recipient (2°-, bottom) mice by flow cytometry every 4 weeks for 16 weeks after transplantation. Ratios of donor-derived (CD45.2+CD45.1−) B-lymphoid (CD19+) to myeloid (Gr1+Mac1+) cells in peripheral blood from Gata3+/+ (+/+, ●) or Gata3z/z (z/z, ▵) cells are shown. Data represent the summary of 5 independent experiments with an average of 11 recipient mice for 1°- and 2 independent experiments with an average of 40 recipient mice for 2°- in each group with SEM. *P < .03. (B) The absolute numbers of donor-derived cells (CD45.2+CD45.1−) in myeloid (Gr1+Mac1+, left panels), B-lymphoid (CD19+B220+, middle panels), and T-lymphoid (Thy1.2+CD3+, right panels) populations per mouse (2 femurs plus 2 tibias) from +/+ or z/z donor cells in BM. Chimerism was analyzed from 1°- (top panels) and 2°- (bottom panels) recipient mice 16-20 weeks after transplantation. Data represent the summary of 3 independent experiments with an average of 12 recipient mice for 1°- and 2 independent experiments with an average of 20 recipient mice for 2°- in each group with SEM. *P < .03. NS indicates not significant.
GATA-3 contributes to a transcriptional network controlling HSC cell-cycle entry
GATA-2 is well known to be required for the production of HSCs and for the expansion of immature hematopoietic cells.22,24-26 Because GATA family proteins can apparently all bind to a GATA core DNA element and have been shown to be at least partially compensatory,53 we hypothesized that GATA-2 may partially compensate for the loss of Gata3. To test this hypothesis, we examined GATA-2 mRNA levels in Gata3 mutant LSK cells and LT-HSCs. As shown in Figure 5A, GATA-2 expression was not significantly affected in either LSK cells or LT-HSCs recovered from Gata3 mutant mice. Although these data indicate that compensatory induction of Gata2 transcription is not observed in the absence of Gata3, it does not exclude the possibility that wild-type GATA-2 abundance or activity is sufficient to compensate for any role normally executed by GATA-3 in LT-HSCs.
GATA-3 participates in a transcription network governing the HSC cell cycle. (A) qRT-PCR analysis of GATA-2 mRNA relative expression in BM LSK (top panel) and LSK CD150+CD48−CD34− (bottom panel) populations from adult Gata3flox/flox [f/f(−)], Gata3flox/+:TgMx1Cre [f/+(+)], and Gata3flox/flox:TgMx1Cre [f/f(+)] 3 weeks after poly(I:C) treatment. Data represent the summary of at least 3 mice of each genotype from 3 independent experiments with SEM. NS indicates not significant. (B) Relative expression of various transcription factors (horizontal axis) in BM LSK (top panel) and LSK CD150+CD48−CD34− (bottom panel) cells from adult f/f(−) (black bars), f/+(+) (gray bars), and f/f(+) (white bars) 3 weeks after poly(I:C) treatment. The mRNA expression level was normalized to that of f/f(−) in each set. Data represent the summary of 5 mice from 3 independent experiments of each genotype with SEM. *P < .05. NS indicates not significant.
GATA-3 participates in a transcription network governing the HSC cell cycle. (A) qRT-PCR analysis of GATA-2 mRNA relative expression in BM LSK (top panel) and LSK CD150+CD48−CD34− (bottom panel) populations from adult Gata3flox/flox [f/f(−)], Gata3flox/+:TgMx1Cre [f/+(+)], and Gata3flox/flox:TgMx1Cre [f/f(+)] 3 weeks after poly(I:C) treatment. Data represent the summary of at least 3 mice of each genotype from 3 independent experiments with SEM. NS indicates not significant. (B) Relative expression of various transcription factors (horizontal axis) in BM LSK (top panel) and LSK CD150+CD48−CD34− (bottom panel) cells from adult f/f(−) (black bars), f/+(+) (gray bars), and f/f(+) (white bars) 3 weeks after poly(I:C) treatment. The mRNA expression level was normalized to that of f/f(−) in each set. Data represent the summary of 5 mice from 3 independent experiments of each genotype with SEM. *P < .05. NS indicates not significant.
The data presented herein demonstrate a function for GATA-3 in promoting cell-cycle entry of quiescent HSCs. We therefore next evaluated the effect of Gata3 loss on the expression of transcription factors that have been shown to be important for HSC quiescence and/or cell-cycle regulation in LSK and LT-HSC populations (Figure 5B). In the LSK population, GFI1B, FOXO3A, N-Myc, and JUNB were each reduced in abundance after the deletion of Gata3, whereas GFI1, GFI1B, and EGR1 were the most significantly diminished in the LT-HSC population. GFI1B in particular has been shown previously to cross-regulate Gata3 in T-lymphoma cells.54 Therefore, these expression data indicate that GATA-3 participates, along with other well-characterized cell-cycle nuclear regulatory proteins, in a complex transcriptional network that controls the balance between quiescence and cell-cycle entry in HSCs.
Discussion
Although Gata3 has been known for some time to be expressed in HSCs, a possible functional role for GATA-3 in these cells has not been investigated. In the present study, we show that Gata3 is abundantly transcribed in LT-HSCs and is required for maintaining a normal number of LT-HSCs. We drew this conclusion after analyzing mice in which the Gata3 gene was conditionally ablated and after analysis of adoptively transferred animals reconstituted with different numbers of Gata3-null mutant HSCs. Three weeks after somatic Gata3 deletion in HSCs, LT-HSC numbers were reduced to 49% of controls. In adoptively transferred recipient animals, Gata3-null mutant HSCs generated fewer LT-HSCs and LSK cells by 16-20 weeks after transplantation: 31% and 45% compared with wild-type cells, respectively. Note that the number of Gata3-null mutant LSK cells was 60% of wild-type LSK cells even by 10 weeks after transplantation.28 These data suggest that Gata3-null mutant fetal HSCs are able to reconstitute the adult HSC compartment initially, but are unable to maintain the normal HSC abundance in the adult BM environment, and also demonstrate that GATA-3 contributes to maintaining the number of LT-HSCs in a cell-autonomous manner. The data do not exclude the possibility that there may be additional non–cell-autonomous contributions of GATA-3 to the number of LT-HSCs through the regulation of niche size or number.
Buza-Vidas et al reported recently that GATA-3 is dispensable for HSC regulation at steady-state maintenance and in self-renewal.29 They reached this conclusion through the analysis of Gata3flox/flox:TgVav-Cre cells in 1-week-old mice born on a C57Bl/6 genetic background, conclusions apparently in contrast to the results described herein. In the present study, we conditionally ablated the Gata3 gene in 9- to 12-week-old mice in a mixed CD1/C57Bl/6 genetic background by inducing Cre expression through TgMx1Cre. Because both studies describe nearly complete deletion of the Gata3 gene, the difference could be explained by differences in mouse strains or by differing functions of GATA-3 at the adult versus newborn stages. In adoptive-transfer experiments, Buza-Vidas et al used BM cells isolated from Gata3flox/flox:TgVav-Cre mice at 1-2 weeks of age as donors, whereas we used Gata3z/z (complete-null30 ) cells as donors, because our conditional mutant was not yet purely C57Bl/6 inbred. Because the embryonic lethality of Gata3-null mutants precludes recovery of HSCs from adult BM, we used null mutant e14.5 fetal liver cells as the primary HSC donor population. Neither genotype showed significant differences in long-term repopulating potential of Gata3 mutant HSCs after serial transplantation. Because Buza-Vidas et al did not show the number of HSCs and progenitors recovered in their adoptive-transfer experiments,29 we are unable to directly compare results. However, the data reported here suggest that Gata3-null mutant HSCs only gradually lose the ability to maintain the normal cell numbers of HSCs in the total pool, and that this property is exacerbated at later time points after transplantation. These same observations could also be used to support the hypothesis that GATA-3 controls LT-HSC number only at the adult stage, but possibly not at 1-2 weeks of age.
Approximately 75% of LT-HSCs are quiescent, and only a small number are slowly cycling.4,5 Proliferation is clearly detrimental to HSC long-term function: diminished LT-HSC number coincides with increased cycling in p21, Gfi1, Pten, FoxO, Cdc42, or Tie2 mutant cells, whereas an increase in HSCs or progenitor number coincides with reduced cycling in Mef- or JunB-deficient (expansion of myeloid progenitors) HSCs.6,12 Exceptions to this rule were observed in other mutant mice in which both the p18Ink4 knock-outs55 and Gfi1b cKOs10 (accompanied by increased mobilization) displayed an increased number of HSCs, whereas Egr1 knock-outs displayed only modestly increased numbers of BM HSCs but striking increases in peripheral HSCs,11 even when accompanied by increased proliferation. Gata3 mutant LT-HSC cells exhibited a reduced ratio of BrdU-positive cycling cells after 19 hours of labeling. Ki67 staining coupled with DNA content analysis also showed that more Gata3 mutant LSK CD150+CD34− cells were quiescent and that fewer cells were in the S + G2/M phases. Furthermore, Gata3 mutant LSK cells fail to augment cell cycling to recover from myelosuppression after 5-FU treatment. Therefore, the Gata3 mutant mouse appears to be the first genetic model that exhibits impaired cell-cycle entry of HSCs while at the same time displaying a reduction in LT-HSC number.
The effect of GATA-3 on the HSC cell cycle seen in the present study was unanticipated, because previous reports showed that Gata3-null mutant lymphoid progenitors exhibited an increase in cells in cycle in OP9-DL1 cocultures,28 in the Gata3 hypomorphic ETP and DN2 intrathymic stage cells (our unpublished observations), and in luminal cells of the mammary gland.49 Therefore our data reveal a possibly bimodal effect of GATA-3 on cell-cycle regulation at different developmental stages and in different tissues.
Because Gata3-null mutant ES cells are able to generate myeloid and B-lymphoid cells in chimeric animals,23 in in vitro OP9 cocultures,56 and in adoptively transferred animals reconstituted with Gata3-null HSCs,28 GATA-3 is clearly dispensable for the generation of HSCs and descendant hematopoietic lineages, except in the T- and natural killer cell lineages. The data presented herein show that LT-HSC number is diminished, but also that the remaining 31% is sufficient to support hematopoiesis (again, with the exception of T-lineage cells) in Gata3-null mutant animals. The co-expression of GATA-2 and GATA-3 in LT-HSCs suggests a possible compensatory regulation of ablated GATA-3 function by GATA-2. Although no difference in the level of GATA-2 expression was observed in LT-HSCs when Gata3 was conditionally inactivated, GATA-2 mRNA was approximately 25-fold more abundant than GATA-3 in these cells (supplemental Figure 2). This raises the possibility that abundant GATA-2 at least partially masks any uncomplemented role for GATA-3 in LT-HSCs.
In secondary adoptive-transfer experiments, Gata3-null hematopoietic progenitor cells showed faster and greater B-lymphoid cell repopulating ability than did wild-type cells at weeks 4-16. In addition, the production of B cells in the BM from Gata3-null progenitor cells is modestly augmented in secondary adoptively transferred animals 16-20 weeks after transplantation. This increased B-cell generation in the absence of GATA-3 is only observed at week 16 in primary adoptive-transfer experiments in which fetal liver cells were used as HSC donors. In OP9 coculture, e11.5 Gata3-null mutant fetal liver hematopoietic cells developed into B cells indistinguishably from wild-type fetal liver cells.56 These data suggest that GATA-3 represses B-cell development during the adult stage, but not at the fetal stage, although further studies will be required to confirm or refute this hypothesis. In contrast to the adult-specific repressive effect of GATA-3 on B-cell development, GATA-3 is required for ETP development at both the fetal and adult stages.28
HSCs are generally defined by their ability to contribute 1% or more of both circulating myeloid and lymphoid cells at 16 weeks (or longer) after transplantation.48 Although by definition every LT-HSC is able to generate all myeloid and lymphoid lineage cells for more than 16 weeks, their differentiation potential often appears to be heterogeneous. By analyzing dynamic changes in WBC production from a large number of transplanted single cells, Dykstra et al proposed that LT-HSCs could be divided into 3 functionally defined groups: myeloid-biased (α cell), balanced (β cell), and lymphoid-biased (γ and δ cell) HSCs.57 Initially, we suspected that there might be a shift in the balance toward lymphoid fate in the Gata3 mutants based on the WBC output of Gata3 mutant donor cells from week 4-16 after transplantation. However, lymphoid-biased HSCs exhibit active cycling,58 whereas Gata3 mutant LT-HSCs exhibit obviously diminished cycling. Therefore, it is likely that augmented production of B-lymphoid cells over myeloid-lineage cells is a consequence of loss of B-lineage repression, rather than a shift in the balance of Gata3 mutant HSCs, although further studies will be required to confirm or refute this hypothesis.
We were initially concerned that the tendency of Gata3-null HSCs to produce more B cells relative to myeloid cells could affect the interpretation of the transplantation results. To determine whether the transplantation interpretations were affected, we also calculated progenitor frequency using 2 different criteria: by considering engraftment as defined by > 1% CD45.2+ cells in myeloid cells or in B-lymphoid lineages alone. Progenitor frequency calculated using these 2 different criteria did not change the outcome of the results, suggesting that the tendency of Gata3-null HSCs to produce more B cells did not affect interpretation of the transplantation results presented herein.
The factor(s) that lies downstream of GATA-3 that precisely maintains the number of LT-HSCs and that determines the fraction of those LT-HSCs that can enter into the cell cycle is currently unknown. The change in the abundance of multiple transcription factors that have been implicated in the regulation of the cell cycle in Gata3 mutant hematopoietic cells suggests that GATA-3 contributes to a transcriptional network that, at some level, regulates quiescence and entry of HSCs into cycle. Diminished expression of GFI1B and EGR1 in heterozygous (Gata3flox/+:TgMx1Cre) LT-HSCs demonstrates that even 30%-40% of Gata3 abundance compared with pseudo–wild-type [Gata3flox/flox:Tg(−)] is not sufficient to maintain wild-type mRNA levels of these presumptive GATA-3 target gene mRNAs. Rather, our data suggest that GATA-3 controls the mRNA level of multiple transcription factors involved in the regulation of LT-HSC function and the output of this regulatory network, but that no single gene may be responsible for the overall downstream effects of GATA-3 function. Although ChIP experiments with anti–GATA-3 Abs have not been conducted in HSCs, ChIP-seq experiments analyzing immature and mature T cells exhibit binding of GATA-3 near the Gfi1, Mef, and Foxo3a genes.17 It seems likely that some of these same factors might also be regulated directly by GATA-3 in HSCs. Clearly, much more extensive investigation will be required to provide a clear picture of the transcriptional network controlling this critical developmental decision.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank their laboratory colleagues for advice and detailed discussions; their laboratory technicians, Mary Clifford and Carmen Yu; and the flow core facility and the Unit for Laboratory Animal Medicine at the University of Michigan for excellent instrument and animal maintenance.
This work was initially supported by a research grant from the National Institutes of Health (GM28896).
National Institutes of Health
Authorship
Contribution: C.-J.K. designed and performed the experiments, analyzed the data, and assisted in writing the manuscript; T.H. and J.D.E. designed the experiments, analyzed the data, and assisted in writing the manuscript; and I.M. critiqued and analyzed the data and assisted in writing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: James Douglas Engel, 3071 BSRB, 109 Zina Pitcher Pl, University of Michigan Medical School, Ann Arbor, MI 48109; e-mail: engel@umich.edu.

![Figure 1. GATA-3 is abundantly expressed in LT-HSCs and plays a role in maintenance of the HSC pool. (A) qRT-PCR analysis of GATA-3 mRNA relative abundance in purified BM, thymus, and spleen populations recovered from adult (6- to 9-week-old) C57Bl/6 mice. LSK CD150+CD48−CD34− (LT-HSCs CD34−), LSK Flt3− (HSCs Flt3−), LSK Flt3lo (MPPs), LSK Flt3hi (LMPPs), Lin−Sca1loc-KitloIL-7Rα+Flt3+ (CLPs), Lin−c-KithiCD25− (ETPs), Lin−c-KithiCD25+ (DN2), Lin−c-Kitlo/−CD25+ (DN3), Lin−c-Kitlo/−CD25− (DN4), CD4+CD8+ (DP), CD4−CD8+ (thymic CD8 SP T cells), CD4+CD8− (thymic CD4 SP T cells), CD3+CD8+CD4− (splenic CD8 T cells), CD3+CD4+CD8− (splenic CD4 T cells), and CD19+ B220+ (splenic B cells). (−) indicates the no-template control. Data represent the summary of 3 independent experiments. (B) Loss of GATA-3 expression in LSK cells was confirmed through comparison of adult Gata3flox/flox:TgMx1Cre [f/f(+)] to control Gata3flox/flox [f/f(−)] or Gata3flox/+:TgMx1Cre [f/+(+)] 3 weeks after the first poly(I:C) injection. The level of GATA-3 mRNA of the f/f(−) mice was set at 100%. Data represent the summary of 5 mice of each genotype determined in 3 independent experiments with SEM. *P < .001. (C) Representative gating strategy of HSC populations in flow cytometric analysis. Lin− BM cells were isolated from f/f(−) and f/f(+) mice after TgMx1Cre induction by poly(I:C), and analyzed for surface expression of c-Kit and Sca1 to mark the LSK population, then further analyzed for CD150 and CD48 expression. Numbers adjacent to the boxed areas indicate the mean percentage of cells in the gate. (D) The absolute numbers of LSK CD150+CD48−CD34− cells per mouse (2 femurs plus 2 tibias) in f/f(−), f/+(+), and f/f(+) mice 3 weeks after poly(I:C) treatment. Data represent the summary of 12 mice of each genotype from 5 independent experiments with SEM. *P < .001; #P < .008. NS indicates not significant. (E) LSK CD150+CD48−CD34− cells were sorted from f/f(−), f/+(+), and f/f(+) mice 3 weeks after poly(I:C) treatment. Complete deletion of the conditional allele was verified in f/f(+) LT-HSCs. Data represent the summary of 3 mice of each genotype from 2 independent experiments with SEM. *P < .009; #P < .03. NS indicates not significant; ND, not detectable.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/10/10.1182_blood-2011-07-366070/4/m_zh89991287420001.jpeg?Expires=1767723335&Signature=n7Iik4GB7tPbXCQNfpCymjV-jH1iioou1ohgndeByQvMFYO9S9oh1Pu4-ksm82muvJf-rVcJPsTBzenDf8Pwyrv3yu-mvMkG6TFbAe9-uzJzU1rCHPdt3OVOtc8wK2uViND7IDC0xgoiPMJA30T5aJ9s9D8iO5hOLZTQRB3efc8OhN6VbyieXlGhQetaeO7rdfVgdirkE~FqK-Xm8UKknIZBqI--oVflJ-lGAnvgg4jJufXArfdzK5Pu3rAoKsJI6EEel307bWLUcI62xo-LQjvsBf2WL7K4MKDZ~kRshNe5hHQc9WXtvTpXtRC9sKlfazWoky-K49VhimaMaHFtYw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. GATA-3 participates in HSC cell-cycle control. (A) Profile of annexin V and DAPI staining in BM LSK CD150+CD34− cells 3 weeks after poly(I:C) treatment. Numbers adjacent to the boxed areas indicate the mean percentage of cells in the gate. (B) Ratio of annexin V+DAPI− and annexin V+DAPI+ cells in LSK CD150+CD34− cells from adult Gata3flox/flox [f/f(−)], Gata3flox/+:TgMx1Cre [f/+(+)], and Gata3flox/flox:TgMx1Cre [f/f(+)] mice 3 weeks after poly(I:C) treatment. Data represent the summary of at least 5 mice of each genotype from 3 independent experiments with SEM. NS indicates not significant. (C) Profile examining the number of cells in cycle assessed after 19 hours of BrdU labeling with DNA content (DAPI) in BM LSK CD150+CD48− (top panel) and LSK CD150+CD48−CD34− (bottom panel) populations 3 weeks after poly(I:C) treatment. Numbers in the boxed areas indicate the mean percentage of cells in each gate. (D) Ratio of BrdU+ cells (S phase) in BM LSK (left group), LSK CD150+CD48− (middle group), and LSK CD150+CD48−CD34− (right group) populations 3 weeks after poly(I:C) treatment. Data represent the summary of 5 mice of each genotype from 5 independent experiments with SEM. *P < .05; #P < .002. NS indicates not significant. (E) Cell-cycle analysis using intracellular Ki67 and DNA content (DAPI) staining in BM LSK CD150+CD34− cells 3 weeks after poly(I:C) treatment. Numbers adjacent to the boxed areas indicate the mean percentage of cells in gate. (F) Ratio of cells in the G0, G1, and S + G2/M phases in BM LSK CD150+CD34− cells from adult f/f(−) (black bars), f/+(+) (gray bars), and f/f(+) (white bars) 3 weeks after poly(I:C) treatment. Data represent the summary of at least 5 mice of each genotype from 3 independent experiments with SEM. *P < .008; #P < .02; +P < .03. NS indicates not significant. (G) Profile of DNA content (DAPI) staining in BM LSK cells without (top panels) or with (bottom panels) 5-FU treatment for 6 days; cells were analyzed 3 weeks after poly(I:C) treatment. Numbers above the indicator bars quantify the mean percentage of cells in that gate. (H) Ratio of cells in the S + G2/M phase in the LSK population (top) and the absolute LSK cell numbers (bottom) per mouse (2 femurs plus 2 tibias) either without (−) or with (+) 5-FU treatment. Data represent the summary of at least 6 mice of each genotype and treatment groups from 3 independent experiments with SEM. *P < .001; #P = .05; +P < .03. NS indicates not significant.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/10/10.1182_blood-2011-07-366070/4/m_zh89991287420003.jpeg?Expires=1767723335&Signature=sYI7kiWoeERnA-GLz3Rx92rgSV9DWlKvlqiw9rdG9BnhesyPqU71belwmDU5AbFtAIZE1l4K6AY3u8aDp1X7Rfkgs8Fbk8HaKQSh2t5zh2ji8Tg0SmEpsv47Q244TSYAa6YmCO1n1Dm9~984M4YTYzXxmdl~wvpZ0-Gepfz26UTB2qfuB8w5MOHtKmKTdSpcwuxtmANwjdX2uPh~t~S344~8ZpoWQjVlKspEp0lB6bvll3fdkCcIx8ciSx6iRyvvLrXK4ziUgBi-RRBfq-vbw2l9VzxcYFKaBSa3dl9---q81M5mn~HNvP7uO1cvgf-KRTOlolX3P2kYl19~~YpRXw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 5. GATA-3 participates in a transcription network governing the HSC cell cycle. (A) qRT-PCR analysis of GATA-2 mRNA relative expression in BM LSK (top panel) and LSK CD150+CD48−CD34− (bottom panel) populations from adult Gata3flox/flox [f/f(−)], Gata3flox/+:TgMx1Cre [f/+(+)], and Gata3flox/flox:TgMx1Cre [f/f(+)] 3 weeks after poly(I:C) treatment. Data represent the summary of at least 3 mice of each genotype from 3 independent experiments with SEM. NS indicates not significant. (B) Relative expression of various transcription factors (horizontal axis) in BM LSK (top panel) and LSK CD150+CD48−CD34− (bottom panel) cells from adult f/f(−) (black bars), f/+(+) (gray bars), and f/f(+) (white bars) 3 weeks after poly(I:C) treatment. The mRNA expression level was normalized to that of f/f(−) in each set. Data represent the summary of 5 mice from 3 independent experiments of each genotype with SEM. *P < .05. NS indicates not significant.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/10/10.1182_blood-2011-07-366070/4/m_zh89991287420005.jpeg?Expires=1767723335&Signature=GAFiPL-8x6lcUcNXFjDEQjWj1rUZQeyt~Ez8J0tCb4RVvxSU4KZ0tEJdGpypoDneKxOLb-nXfqEPjYr5O25BH7xoz2fQD9GxAtSUiI6Qhp~nzUdLrBjqEbdXkmQ6pSUgj7i9OPU15LKpegwbyAumUsX6NQwuDoSJxwsMY0lCYicT4PxSrJtfl4RebjhS-1g6IXed2vFmB92172lvkbS-FgRdbkDVwA0iND~RG1SWAgCh1NtCgz3~ihUh8Zw6IbNNf~t3CEXRvDHcLtlrfRKIFZgFfBv0ADCdKz-eekfipxRDR7ckNIwltclrahWLTDQ~3O0B9gXZBdXHNB6tb78LPQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal