Abstract
We investigated whether body mass index (BMI) correlates with distinct outcomes in newly diagnosed acute promyelocytic leukemia (APL). The study population included 144 patients with newly diagnosed and genetically confirmed APL consecutively treated at a single institution. All patients received All-trans retinoic acid and idarubicin according to the GIMEMA protocols AIDA-0493 and AIDA-2000. Outcome estimates according to the BMI were carried out together with multivariable analysis for the risk of relapse and differentiation syndrome. Fifty-four (37.5%) were under/normal weight (BMI < 25), whereas 90 (62.5%) patients were overweight/obese (BMI ≥ 25). An increased BMI was associated with older age (P < .0001) and male sex (P = .02). BMI was the most powerful predictor of differentiation syndrome in multivariable analysis (odds ratio = 7.24; 95% CI, 1.50-34; P = .014). After a median follow-up of 6 years, the estimated cumulative incidence of relapse at 5 years was 31.6% (95% CI, 22.7%-43.8%) in overweight/obese and 11.2% (95% CI, 5.3%-23.8%) in underweight/normal weight patients (P = .029). Multivariable analysis showed that BMI was an independent predictor of relapse (hazard ratio = 2.45, 95% CI, 1.00-5.99, in overweight/obese vs under/normal weight patients, P = .049). An increased BMI at diagnosis is associated with a higher risk of developing differentiation syndrome and disease relapse in APL patients treated with AIDA protocols.
Introduction
Obesity has received considerable attention in the medical community because of its negative impact on human health. More recently, excess of body weight has been associated with an increased risk of a wide range of malignancies.1-3 In a meta-analysis of cohort studies, Larsson and Wolk reported epidemiologic evidence suggesting that an excess of body weight is a risk factor for several hematologic cancers, including leukemia, non-Hodgkin lymphoma, and multiple myeloma.4 In particular, compared with nonoverweight persons, the relative risk of leukemia for overweight and obese persons was reported to be 1.14 and 1.39, respectively, whereas a 5-kg/m2 increase in body mass index (BMI) was associated with a 13% increased risk of leukemia.4 As to the impact of BMI on treatment response and outcome, a number of studies have shown that obesity is associated with a poorer overall survival and disease-free survival in patients with breast cancer.5,6 By contrast, the correlation between BMI and treatment outcome has not yet been investigated in patients with leukemia.
Acute promyelocytic leukemia (APL), once known as the most rapidly fatal leukemia subtype, is nowadays considered the most frequently curable acute leukemia of adults.7 Indeed, modern combined approaches with all-trans retinoic acid and anthracycline chemotherapy yield complete remission rates greater than 90% and long-term cure rates of 70% to 75%.8-11 Disease relapse and death during induction because of hemorrhage, infection, or differentiation syndrome account for treatment failures.8-12 Estey et al reported in 1997 that an increasing BMI was strongly associated with a diagnosis of APL among patients affected by acute myeloid leukemia (AML).13 In a cohort of 1245 patients with newly diagnosed AML, which included 120 APLs, they found a mean BMI of 27.6 ± 7.7 in APL compared with 25 ± 5.28 in non-APL AML patients. The impact of BMI in terms of response to therapy and outcome was not investigated in either the report by Estey et al13 or in other APL studies.
In the present study, we analyzed the clinicobiologic features and outcome according to the BMI in a large series of APL patients who received uniform therapy according to the AIDA (All-trans retinoic acid plus idarubicin) protocols adopted by the Italian cooperative group GIMEMA.10,14 We report a significant association of an increased BMI in APL with both the differentiation syndrome and disease relapse.
Methods
Patient selection and treatment
All patients in the study were diagnosed and consecutively treated between January 1993 and December 2010 at the Sapienza University of Rome using AIDA-0493 or AIDA-2000 protocols.10,14 Diagnosis was confirmed at the genetic level by RT-PCR identification of the PML/RARA hybrid, as previously described.15 Immunophenotype was performed by flow cytometry using a wide panel of monoclonal antibodies, including CD13, CD33, HLA-DR, CD34, CD2, CD7, CD15, CD9, CD117, CD56, and MPO (BD Biosciences). Relapse risk at the time of diagnosis was established according to the criteria defined by Sanz et al.16 Seventy-five patients diagnosed between January 1993 and May 2000 received uniform treatment based on the AIDA-0493 regimen,10 whereas 69 patients diagnosed after May 2000 were treated according to the risk-adapted regimen AIDA-2000 with distinct postinduction approaches based on the initial risk stratification as reported.14 Patients in the Sanz low- and intermediate-risk category enrolled in the AIDA-2000 received only anthracycline/anthracenadione agents and all-trans retinoic acid (ATRA), whereas patients in the high-risk group also received cytarabine and etoposide in addition to ATRA, idarubicin, and mitoxantrone.14
Sequentially collected bone marrow aspirates were analyzed in all patients after consolidation therapy for molecular monitoring of minimal residual disease as reported.15 Molecular relapse was defined as a positive PML/RARA test detected in 2 successive marrow samples collected at any time after consolidation therapy using RT-PCR assay with a sensitivity of 10−4 and in the absence of morphologically detectable blasts in both marrow or peripheral blood.
Differentiation syndrome
According to the criteria of Frankel et al, the diagnosis of definitely present retinoic acid syndrome (now renamed, differentiation syndrome) was clinically established by the presence of at least 3 of the following signs: weight gain, respiratory distress, unexplained fever, interstitial pulmonary infiltrates, and pleural or pericardial effusions.17
BMI calculation
BMI was defined as the person's body weight divided by the square of his or her height18 (universally used formula producing a unit of measure of kg/m2). According to the World Health Organization,19 patients were stratified into 4 categories: underweight (BMI < 18.5 kg/m2), normal weight (BMI 18.5-25 kg/m2), overweight (BMI 25-30 kg/m2), and obese (BMI ≥ 30 kg/m2). For children and adolescents (< 20 years old), body weight categories were defined according to age- and sex-specific BMI percentiles, as follows: underweight, less than the 5th percentile; normal weight, from the 5th percentile to less than the 85th percentile; overweight, from the 85th percentile to less than 95th percentile; and obese, equal or greater than the 95th percentile (Center for Disease Control and Prevention; http://apps.nccd.cdc.gov/dnpabmi/). The study population included 12 patients younger than 20 years.
Outcome estimate and statistical analysis
The distributions of clinical features at diagnosis (ie, age, sex, French-American-British morphologic form, type of transcript, white blood cell count, platelet count, hemoglobin level) were compared between the BMI categories using the χ2 test.
The primary outcome was the cumulative incidence of relapse (CIR), defined as the time from the end of induction therapy to the date of hematologic relapse or molecular relapse, whichever occurred first, and considering death in remission as a competitive event. Cumulative incidence curves were estimated by the Kalbfleisch and Prentice method, considering competing causes.20 The Gray test was used to assess CIR differences between groups identified by the patients' characteristics.21 The prognostic impact of patient characteristics on the hazard of relapse was assessed by multivariable Cox proportional hazards regression models and expressed as hazard ratios with 95% CIs. To investigate the shape of the relationship between BMI and the hazard of relapse, restricted cubic spline models were used.22 The impact of patient characteristics on the risk of differentiation syndrome was assessed by multivariable logistic regression models and expressed as odds ratios with 95% CI. The analyses were carried out with the SAS statistical package (SAS Institute) and the R software (http://cran.r-project.org/) with the cmprsk package developed by Gray (http://biowww.dfci.harvard.edu/∼gray) and the Design package developed by Harrell (http://biostat.mc.vanderbilt.edu/twiki/bin/view/Main/Design). All reported P values are 2-sided.
Results
Patient BMI and clinical features at presentation
The main demographic and clinical characteristics of patients by BMI at diagnosis are shown in Table 1. Fifty-four (37.5%) were underweight/normal, whereas 90 (62.5%) patients were overweight/obese. A total of 66% of overweight/obese patients were older than 40 years (P = .02). BMI increased with age in APL patients: the median age was 19 years in underweight patients, 36 years in normal weight, 50 in overweight, and 53 years in obese APL patients. Moreover, we found an association between BMI and sex (33% and 53% of males in underweight/normal and overweight/obese groups, respectively, P = .02). No statistically significant associations were detected when BMI groups were analyzed in terms of French-American-British morphology, PML/RARA transcript type, white blood cell (WBC), hemoglobin, and platelet counts.
Patient characteristics at diagnosis by BMI
| . | Underweight/normal . | Overweight/obese . | P* . | ||
|---|---|---|---|---|---|
| Median (range) . | N (%) . | Median (range) . | N (%) . | ||
| All patients | — | 54 (100) | — | 90 (100) | |
| Age at diagnosis, y | 33 (1-76) | 50 (1-77) | |||
| ≤ 40 | — | 38 (70) | — | 31 (34) | < .0001 |
| > 40 | — | 16 (30) | — | 59 (66) | |
| Sex | |||||
| Male | — | 18 (33) | — | 48 (53) | .02 |
| Female | — | 36 (67) | — | 42 (47) | |
| French-American-British subtype | |||||
| Typical | — | 45 (83) | — | 75 (83) | 1.00 |
| Variant | — | 9 (17) | — | 15 (17) | |
| PML/RARα | |||||
| BCR1-2 | — | 32 (59) | — | 51 (57) | .76 |
| BCR3 | — | 22 (41) | — | 39 (43) | |
| WBC | 2.9 (0.4-77) | 2.4 (0.4-145) | |||
| ≤ 10 × 109/L | — | 41 (76) | — | 67 (74) | .84 |
| > 10 × 109/L | — | 13 (24) | — | 23 (26) | |
| Platelets | 29 (7-210) | 28.5 (8-196) | |||
| ≤ 40 × 109/L | — | 35 (65) | — | 63 (70) | .52 |
| > 40 × 109/L | — | 19 (35) | — | 27 (30) | |
| Hemoglobin | 9.2 (4-13.6) | 9.4 (5-15) | |||
| ≤ 10 g/dL | — | 36 (67) | — | 57 (63) | .69 |
| > 10 g/dL | — | 18 (33) | — | 33 (37) | |
| Sanz risk score | |||||
| Low | — | 14 (26) | — | 20 (22) | .78 |
| Intermediate | — | 27 (50) | — | 44 (49) | |
| High | — | 13 (24) | — | 26 (29) | |
| . | Underweight/normal . | Overweight/obese . | P* . | ||
|---|---|---|---|---|---|
| Median (range) . | N (%) . | Median (range) . | N (%) . | ||
| All patients | — | 54 (100) | — | 90 (100) | |
| Age at diagnosis, y | 33 (1-76) | 50 (1-77) | |||
| ≤ 40 | — | 38 (70) | — | 31 (34) | < .0001 |
| > 40 | — | 16 (30) | — | 59 (66) | |
| Sex | |||||
| Male | — | 18 (33) | — | 48 (53) | .02 |
| Female | — | 36 (67) | — | 42 (47) | |
| French-American-British subtype | |||||
| Typical | — | 45 (83) | — | 75 (83) | 1.00 |
| Variant | — | 9 (17) | — | 15 (17) | |
| PML/RARα | |||||
| BCR1-2 | — | 32 (59) | — | 51 (57) | .76 |
| BCR3 | — | 22 (41) | — | 39 (43) | |
| WBC | 2.9 (0.4-77) | 2.4 (0.4-145) | |||
| ≤ 10 × 109/L | — | 41 (76) | — | 67 (74) | .84 |
| > 10 × 109/L | — | 13 (24) | — | 23 (26) | |
| Platelets | 29 (7-210) | 28.5 (8-196) | |||
| ≤ 40 × 109/L | — | 35 (65) | — | 63 (70) | .52 |
| > 40 × 109/L | — | 19 (35) | — | 27 (30) | |
| Hemoglobin | 9.2 (4-13.6) | 9.4 (5-15) | |||
| ≤ 10 g/dL | — | 36 (67) | — | 57 (63) | .69 |
| > 10 g/dL | — | 18 (33) | — | 33 (37) | |
| Sanz risk score | |||||
| Low | — | 14 (26) | — | 20 (22) | .78 |
| Intermediate | — | 27 (50) | — | 44 (49) | |
| High | — | 13 (24) | — | 26 (29) | |
— indicates not applicable.
χ2 test comparing distributions among BMI groups.
Cumulative incidence of relapse
Three patients died during induction therapy. The median follow-up of the remaining 141 patients considered for the CIR estimate was 6 years (range, 0.1-13.8 years). Table 2 shows the 5-year CIR values according to BMI, age, sex, French-American-British subtype, blood counts, and PML/RARA transcript.
CIR at 5 years after the end of induction therapy, by patient characteristics
| . | 5-year CIR (95% CI) . | P* . |
|---|---|---|
| All patients | 23.4 (17.1-31.8) | |
| Body fat category | ||
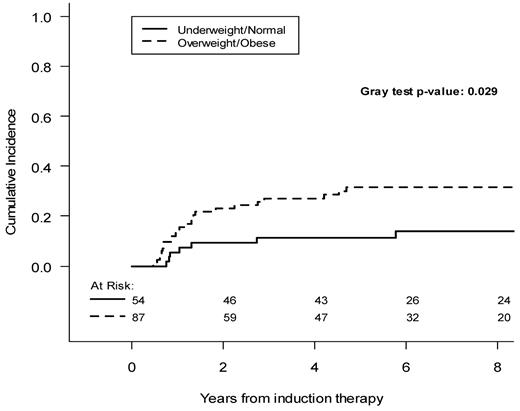
| Underweight/normal | 11.2 (5.3-23.8) | .029 |
| Overweight/obese | 31.6 (22.7-43.8) | |
| Age at diagnosis | ||
| ≤ 40 years | 18.7 (11.2-31.3) | .34 |
| > 40 years | 27.8 (18.9-40.8) | |
| Sex | ||
| Male | 32.2 (22.2-46.7) | .079 |
| Female | 16.4 (9.7-27.6) | |
| French-American-British subtype | ||
| Typical | 22 (15.4-31.4) | .16 |
| Variant | 30.4 (16.4-56.5) | |
| PML/RARα | ||
| BCR1-2 | 16.1 (9.6-27.2) | .027 |
| BCR3 | 32.6 (22.5-47.2) | |
| WBC | ||
| ≤ 10 × 109/L | 24.4 (17.2-34.6) | .99 |
| > 10 × 109/L | 20.6 (10.6-39.8) | |
| Platelets | ||
| ≤ 40 | 21.7 (14.7-32) | .70 |
| > 40 | 27.4 (16.4-45.5) | |
| Hemoglobin | ||
| ≤ 10 g/dL | 26 (18.1-37.3) | .21 |
| > 10 g/dL | 18.7 (10.4-33.8) | |
| Sanz risk score | ||
| Low | 24.7 (13.5-45.2) | .94 |
| Intermediate | 25.2 (16.5-38.7) | |
| High | 18.9 (9.7-36.9) | |
| Differentiation syndrome | ||
| No | 22.8 (16.3-32.1) | .98 |
| Yes | 26.1 (12.2-55.7) |
| . | 5-year CIR (95% CI) . | P* . |
|---|---|---|
| All patients | 23.4 (17.1-31.8) | |
| Body fat category | ||
| Underweight/normal | 11.2 (5.3-23.8) | .029 |
| Overweight/obese | 31.6 (22.7-43.8) | |
| Age at diagnosis | ||
| ≤ 40 years | 18.7 (11.2-31.3) | .34 |
| > 40 years | 27.8 (18.9-40.8) | |
| Sex | ||
| Male | 32.2 (22.2-46.7) | .079 |
| Female | 16.4 (9.7-27.6) | |
| French-American-British subtype | ||
| Typical | 22 (15.4-31.4) | .16 |
| Variant | 30.4 (16.4-56.5) | |
| PML/RARα | ||
| BCR1-2 | 16.1 (9.6-27.2) | .027 |
| BCR3 | 32.6 (22.5-47.2) | |
| WBC | ||
| ≤ 10 × 109/L | 24.4 (17.2-34.6) | .99 |
| > 10 × 109/L | 20.6 (10.6-39.8) | |
| Platelets | ||
| ≤ 40 | 21.7 (14.7-32) | .70 |
| > 40 | 27.4 (16.4-45.5) | |
| Hemoglobin | ||
| ≤ 10 g/dL | 26 (18.1-37.3) | .21 |
| > 10 g/dL | 18.7 (10.4-33.8) | |
| Sanz risk score | ||
| Low | 24.7 (13.5-45.2) | .94 |
| Intermediate | 25.2 (16.5-38.7) | |
| High | 18.9 (9.7-36.9) | |
| Differentiation syndrome | ||
| No | 22.8 (16.3-32.1) | .98 |
| Yes | 26.1 (12.2-55.7) |
Gray test, comparing cumulative incidences among categories.
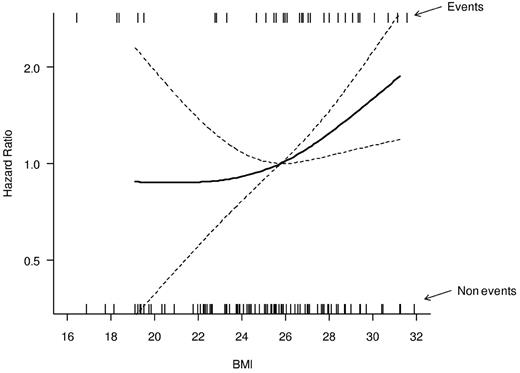
As shown in Figure 1, in which BMI is considered as a continuous variable, the hazard of relapse increases progressively as the BMI increases, with an increase in the curve slope for BMIs more than 25 kg/m2. Therefore, to highlight CIR differences with increased statistical power, we pooled patients into 2 categories: overweight and obese (BMI > 25 kg/m2) and normal-underweight (BMI < 25 kg/m2). Overweight/obese patients had a 5-year CIR of 31.6% (95% CI, 22.7%-43.8%), compared with 11.2% (95% CI, 5.3%-23.8%) for underweight/normal weight patients (P = .029, Figure 2).
Relationship between BMI, on a continuous scale, and the hazard of relapse. The relationship is modeled by a restricted cubic spline model (bold line). Model-based 95% CIs are also reported (dashed lines). The median BMI value (25.8) was considered as the reference in the estimation of the hazard ratio. Estimates were adjusted for sex and age in a Cox proportional hazard model. The rug plots placed at the top and bottom of the graph show the BMI values in patients that relapsed (top) and in those who did not relapse (bottom).
Relationship between BMI, on a continuous scale, and the hazard of relapse. The relationship is modeled by a restricted cubic spline model (bold line). Model-based 95% CIs are also reported (dashed lines). The median BMI value (25.8) was considered as the reference in the estimation of the hazard ratio. Estimates were adjusted for sex and age in a Cox proportional hazard model. The rug plots placed at the top and bottom of the graph show the BMI values in patients that relapsed (top) and in those who did not relapse (bottom).
The analysis of CIR rates according to the patients' baseline characteristics showed no significant impact of WBC count, platelet count, hemoglobin level, and occurrence of differentiation syndrome, whereas a significant outcome difference was detected when considering the PML/RARA transcript type; the 5-year CIR was indeed 32.6% (95% CI, 22.5%-47.2%) and 16.1% (95% CI, 9.6%-27.2%) for patients with the BCR3 and BCR1 or BCR2 types, respectively (P = .027, Table 2). Cox multivariable regression analysis was performed for hazard of relapse for the 141 patients achieving CR after induction (Table 3). An increased BMI had an independent prognostic role in terms of probability of relapse (hazard ratio = 2.45; 95% CI, 1-5.99; P = .049). Similarly, the type of PML/RARA transcript retained an independent prognostic value with the BCR3 type conferring a hazard ratio of 2.36 (95% CI, 1.11-5.05; P = .026) compared with the BCR1 or BCR2 types. At the last follow-up evaluation, 17 patients (19.5%) had died in the overweight/obese category, compared with 4 patients (7.4%) in the underweight/normal weight group. The 5-year overall survival was 81.1% (95% CI, 70.5%-88.2%) in the overweight/obese category and 91.9% in the underweight/normal weight group (95% CI, 79.8%-96.9%; P = .029).
Multivariable Cox regression analysis for hazard of relapse after the end of induction therapy
| Variable . | Levels . | Hazard ratio (95% CI) . | P . |
|---|---|---|---|
| BMI | Overweight/obese vs normal/underweight | 2.45 (1.00-5.99) | .049 |
| Age at diagnosis | > 40 y vs ≤ 40 y | 1.18 (0.55-2.51) | .67 |
| Sex | Male vs female | 1.98 (0.93-4.23) | .077 |
| PML/RARα | BCR3 vs BCR1-2 | 2.36 (1.11-5.05) | .026 |
| WBC | ≤ 10 × 109/L vs > 10 × 109/L | 2.24 (0.78-6.42) | .13 |
| Hemoglobin | ≤ 10 g/dL vs > 10 g/dL | 2.41 (1.04-5.58) | .040 |
| Platelets | > 40 × 109/L vs ≤ 40 × 109/L | 1.18 (0.55-2.54) | .67 |
| French-American-British subtype | Variant vs typical | 2.65 (0.93-7.58) | .069 |
| ATRA syndrome | Yes vs no | 0.72 (0.27-1.95) | .52 |
| Variable . | Levels . | Hazard ratio (95% CI) . | P . |
|---|---|---|---|
| BMI | Overweight/obese vs normal/underweight | 2.45 (1.00-5.99) | .049 |
| Age at diagnosis | > 40 y vs ≤ 40 y | 1.18 (0.55-2.51) | .67 |
| Sex | Male vs female | 1.98 (0.93-4.23) | .077 |
| PML/RARα | BCR3 vs BCR1-2 | 2.36 (1.11-5.05) | .026 |
| WBC | ≤ 10 × 109/L vs > 10 × 109/L | 2.24 (0.78-6.42) | .13 |
| Hemoglobin | ≤ 10 g/dL vs > 10 g/dL | 2.41 (1.04-5.58) | .040 |
| Platelets | > 40 × 109/L vs ≤ 40 × 109/L | 1.18 (0.55-2.54) | .67 |
| French-American-British subtype | Variant vs typical | 2.65 (0.93-7.58) | .069 |
| ATRA syndrome | Yes vs no | 0.72 (0.27-1.95) | .52 |
Association between BMI and risk of differentiation syndrome development
Two patients (4%) experienced a differentiation syndrome in the underweight/normal weight categories compared with 19 patients (21%) in the overweight/obese group. We tested in a multivariable logistic regression model the clinicopathologic features at baseline potentially related to an increased risk of differentiation syndrome (Table 4). BMI only was identified as an independent risk factor for development of differentiation syndrome (odds ratio = 7.4; 95% CI, 1.0-34.5, P = .014).
Association between patients' characteristics at diagnosis and risk of differentiation syndrome
| Variable . | Levels . | N . | ATRA syndrome, N (%) . | OR (95% CI)* . | P* . |
|---|---|---|---|---|---|
| Body fat category | Normal/underweight | 54 | 2 (4) | Reference | |
| Overweight/obese | 90 | 19 (21) | 7.24 (1.50-34.95) | .014 | |
| Age at diagnosis | ≤ 40 y | 69 | 8 (12) | Reference | |
| > 40 y | 75 | 13 (17) | 1.09 (0.39-3.06) | .88 | |
| Sex | Female | 78 | 11 (14) | Reference | |
| Male | 66 | 10 (15) | 0.90 (0.32-2.47) | .83 | |
| PML/RARα | BCR1-2 | 83 | 10 (12) | Reference | |
| BCR3 | 61 | 11 (18) | 1.46 (0.50-4.25) | .49 | |
| WBC | > 10 × 109/L | 36 | 7 (19) | Reference | |
| ≤ 10 × 109/L | 108 | 14 (13) | 0.59 (0.16-2.13) | .42 | |
| Hemoglobin | > 10 g/dL | 51 | 8 (16) | Reference | |
| ≤ 10 g/dL | 93 | 13 (14) | 0.81 (0.29-2.27) | .69 | |
| Platelets | ≤ 40 × 109/L | 98 | 16 (16) | Reference | |
| > 40 × 109/L | 46 | 5 (11) | 0.71 (0.22-2.24) | .55 | |
| French-American-British subtype | Typical | 120 | 18 (15) | Reference | |
| Variant | 24 | 3 (13) | 0.47 (0.10-2.24) | .34 |
| Variable . | Levels . | N . | ATRA syndrome, N (%) . | OR (95% CI)* . | P* . |
|---|---|---|---|---|---|
| Body fat category | Normal/underweight | 54 | 2 (4) | Reference | |
| Overweight/obese | 90 | 19 (21) | 7.24 (1.50-34.95) | .014 | |
| Age at diagnosis | ≤ 40 y | 69 | 8 (12) | Reference | |
| > 40 y | 75 | 13 (17) | 1.09 (0.39-3.06) | .88 | |
| Sex | Female | 78 | 11 (14) | Reference | |
| Male | 66 | 10 (15) | 0.90 (0.32-2.47) | .83 | |
| PML/RARα | BCR1-2 | 83 | 10 (12) | Reference | |
| BCR3 | 61 | 11 (18) | 1.46 (0.50-4.25) | .49 | |
| WBC | > 10 × 109/L | 36 | 7 (19) | Reference | |
| ≤ 10 × 109/L | 108 | 14 (13) | 0.59 (0.16-2.13) | .42 | |
| Hemoglobin | > 10 g/dL | 51 | 8 (16) | Reference | |
| ≤ 10 g/dL | 93 | 13 (14) | 0.81 (0.29-2.27) | .69 | |
| Platelets | ≤ 40 × 109/L | 98 | 16 (16) | Reference | |
| > 40 × 109/L | 46 | 5 (11) | 0.71 (0.22-2.24) | .55 | |
| French-American-British subtype | Typical | 120 | 18 (15) | Reference | |
| Variant | 24 | 3 (13) | 0.47 (0.10-2.24) | .34 |
Estimated using a multivariable logistic regression model.
Discussion
We report here that an increased BMI correlates with a worse prognosis in APL patients receiving ATRA and chemotherapy. To our knowledge, this is the first study that describes a prognostic impact of BMI in leukemia. Estey et al originally reported on the correlation between an increased BMI and an APL diagnosis among AML patients;13 this observation was subsequently confirmed by Thomas et al in a smaller series23 and is further corroborated by the present study.
There is at present no comprehensive explanation for the inferior outcome of APL patients with an increased BMI, a finding that is however in line with reports in solid tumors and in particular in patients with breast cancer.5 A number of factors, such as altered chemotherapy and/or ATRA drug metabolism and/or bioavailability, might be involved in these patients, but these mechanisms require specific laboratory investigations. On the other hand, emerging evidence suggests that systemic metabolic alterations associated with obesity might significantly impact on the biology of neoplasms, for instance, by modulating the strength of shared signaling pathways or through other indirect mechanisms (eg, endocrine dysfunction, immunologic impairment). Obese subjects have increased circulating levels of metabolically active mediators, such as leptin, insulin, or IGF1. Interestingly, the more active long isoform of the leptin receptor, which has proliferative and antiapoptotic effects when activated, is selectively up-regulated in leukemic cells compared with normal promyelocytes in APL patients.24,25 This may provide APL cells with a survival advantage in the leptin-rich milieu of obese subjects. Other potential mechanisms that might account for this association include a loss of cancer protective effect because of reduced physical activity in the obese, and the release of cancer-promoting factors (hormones and adipokines) from adipose tissue.26,27
One additional explanation for inferior outcome in overweight/obese cancer patients might be related to suboptimal doses of drugs; indeed, a frequently adopted policy for patients who are overweight is that of adjusting chemotherapy doses to the ideal weight. However, we think this not to be the case in our current series, as with the only exception of 2 cases no dose modifications according to the ideal weight were made.
Patients overweight and obese had in the present study a significantly increased risk of differentiation syndrome. To obtain more reliable data in this respect, we excluded from the analysis the “indeterminate” differentiation syndrome category defined by Frankel et al17 and selected only patients with or without a “definitely present” syndrome. Our results are in keeping with the observations originally reported by Jeddi et al who analyzed a series of 36 patients, 11 of whom developed differentiation syndrome.28 Six of the 9 patients (66.6%) with a BMI more than 30 kg/m2 versus 5 of 27 patients (18.5%) with a BMI less than 30 kg/m2 developed a differentiation syndrome in that study.28 However, multivariate analysis in the study of Jeddi et al showed that both an increased BMI and a WBC more than 20 × 109/L were independent factors predictive of differentiation syndrome,28 whereas our analysis identified only an increased BMI as a strong predictor of the syndrome. Increased WBC counts as well as elevated creatinine levels were predictive factors for differentiation syndrome in a larger study of the Spanish PETHEMA group.29 Compared with the aforementioned study,29 the lack of association between WBC and differentiation syndrome in our series might depend on the inclusion of patients with definitely present differentiation syndrome only. In agreement with Montesinos et al,29 our study showed that the occurrence of differentiation syndrome had no impact on outcome for patients receiving risk-adapted therapy. We think that the association of differentiation syndrome with an increased BMI might have practical clinical implications. For example, overweight patients might be administered more intensive prophylaxis with steroids, or at least they could be more closely monitored clinically by attending physicians during induction therapy, in particular with respect to respiratory function, creatinine levels, and diuresis.
Together with the PML/RARA short isoform (BCR3), an increased BMI clearly predicted for an increased risk of relapse in our series. Other outcomes, such as disease-free survival and overall survival, were not calculated in this investigation because of the confounding impact on these estimates of death in remission and of the heterogeneous salvage treatments adopted over the long study period.
With regard to the impact of the PML/RARA transcript, several studies, including the GIMEMA,15 PETHEMA,16 German,30 and MRC31 multicenter trials, had previously reported a trend toward an inferior outcome for APL patients harboring the BCR3 isoform receiving simultaneous ATRA and chemotherapy. On a more mature follow-up, we found that patients with the short BCR3 PML/RARA transcript had a significantly higher relapse rate, as reported also in the earlier experiences with ATRA32,33 and in a more recent trial with ATRA plus chemotherapy from Canada.34 After the adoption by our group of a risk-adapted approach for patients with WBC more than 10 × 109/L, which resulted in a significant outcome improvement especially for this category,14 it is not surprising that the WBC count at diagnosis has lost its prognostic significance. Indeed, after the adoption of the AIDA-2000, we observed only 2 relapses of 23 patients with high-risk disease (8.7%), whereas there were 6 relapses in the 16 high-risk patients (37.6%) receiving the AIDA-0493 (37.6%). Interestingly, we also observed a lower than expected frequency of the BCR3 PML/RARA isoform in hyperleukocytic patients (44% vs ∼ 60% being reported in the literature).32-34
In conclusion, we highlight that BMI is a strong outcome predictor in APL across all ages and suggest that treatment modifications might be needed for both differentiation syndrome prevention and antileukemic therapy in APL patients who are overweight and obese at presentation. We hope that our findings may stimulate novel laboratory and clinical research in this area.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by Associazione Italiana per la Ricerca sul Cancro and Associazione Italiana contro le Leucemie.
Authorship
Contribution: F.L.-C., P.G.P., M.B., and L.M. conceived and designed the study; M.B., A.M.T., G.L., G.A., M.C.P., G.C., R.L., and R.F. provided study materials or patients; M.B., V.B., D.D., L.M. collected, assembled, analyzed, and interpreted data; and M.B., L.M., F.L.-C., P.G.P., V.B., D.D., and R.F. wrote the manuscript and gave final approval of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Massimo Breccia, Department of Cellular Biotechnologies and Hematology, Sapienza University, Via Benevento 6, 00161 Rome, Italy; e-mail: breccia@bce.uniroma1.it.
References
Author notes
M.B. and L.M. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal