Abstract
Hematopoietic stem cells (HSCs) are rare residents of the bone marrow responsible for the lifelong production of blood cells. Regulation of the balance between HSC self-renewal and differentiation is central to hematopoiesis, allowing precisely regulated generation of mature blood cells at steady state and expanded production at times of rapid need, as well as maintaining ongoing stem cell capacity. Erg, a member of the Ets family of transcription factors, is deregulated in cancers; and although Erg is known to be required for regulation of adult HSCs, its precise role has not been defined. We show here that, although heterozygosity for functional Erg is sufficient for adequate steady-state HSC maintenance, Erg+/Mld2 mutant mice exhibit impaired HSC self-renewal after bone marrow transplantation or during recovery from myelotoxic stress. Moreover, although mice functionally compromised for either Erg or Mpl, the receptor for thrombopoietin, a key regulator of HSC quiescence, maintained sufficient HSC activity to sustain hematopoiesis, Mpl−/−Erg+/Mld2 compound mutant mice displayed exacerbated stem cell deficiencies and bone marrow failure. Thus, Erg is a critical regulator of adult HSCs, essential for maintaining self-renewal at times of high HSC cycling.
Introduction
Adult hematopoietic stem cells (HSCs) are a rare population of bone marrow cells responsible for the production of multiple functionally specialized lineages of blood cells. In addition to maintaining exquisitely regulated numbers of cells during the normal daily replacement of billions of aged or expended blood cells, HSCs are also critical for regeneration and maintenance of hematopoiesis in response to major hematologic stresses, such as after myelotoxic therapy or stem cell transplantation. Life-long maintenance of the blood cell system requires HSC self-renewal, a specialized cell division in which one or both daughter cells retain full long-term HSC pluripotency. Regulation of the appropriate balance between HSC self-renewal and differentiation is the essence of hematopoiesis, allowing production of sufficient effector blood cells while protecting sufficient pluripotential reserve capacity for future hematopoietic supply. Analysis of the effects of genetic mutation or gene dysregulation have defined diverse molecular regulators of physiologic and stress-induced HSC self-renewal, including transcription factors, epigenetic regulators, cell cycle modulators, and cell extrinsic regulators and their intracellular signal transducers.1,2
The E-twenty-six (ETS)–related gene (ERG, OMIM ID 165080) is a member of the ETS family of transcription factors.3 A key role for ERG in cancer has emerged with the observation that up to 75% of prostate cancers are characterized by ERG deregulation. This commonly occurs via a chromosomal rearrangement that fuses the 5′ untranslated region of the TMPRSS2 (androgen-regulated transmembrane protease, serine 2, OMIM ID 602060) gene with the 3′ region of ERG, resulting in expression of a TMPRSS2:ERG fusion protein.4 Chromosomal translocations that fuse ERG with the TLS/FUS RNA binding protein (OMIM ID 137070) are also associated with rare myeloid and lymphoid leukemias,5-7 overexpression of ERG is associated with poor prognosis in acute myeloid leukemia with normal karyotype,8 and ERG DNA deletions have been linked with a cluster of high-risk acute lymphoblastic leukemia patients with superior relapse-free survival.9 In the mouse, ectopic expression of Erg causes T-acute lymphoblastic leukemia accompanied by acquisition of Notch1 (OMIM ID 190198) mutations.10 ERG is also implicated in the development of transient myeloproliferative disorder and acute megakaryocytic leukemia (DS-AMKL) associated with Down syndrome. ERG is located on chromosome 21,11 is expressed in primary DS-AMKL samples and DS-AMKL-derived cell lines12 and can induce megakaryocytic differentiation of human cell lines and primary murine hematopoietic progenitor cells.12-14 In a mouse model of myeloproliferation associated with Down syndrome, genetic manipulation to specifically revert Erg to functional disomy corrected the hematologic and pathologic features of disease, suggesting that ERG is a key component of trisomy-21 in human Down syndrome that predisposes to AMKL.15
We recently isolated an allele of the murine Erg gene with a missense mutation in the ETS domain-encoding region, termed Mld2, that disrupts the ability of Erg to transactivate gene expression. Mice homozygous for the ErgMld2 mutation die at midgestation with a failure of definitive hematopoiesis.16,17 While remaining healthy, adult Erg+/Mld2 mice produce fewer lineage-negative Sca1+cKit+ (LSK) bone marrow cells, a population enriched for HSCs, and Erg+/Mld2 marrow displays a profound functional inability to compete with normal marrow for hematopoietic reconstitution after transplantation, consistent with a HSC defect.16 Conversely, trisomy of Erg in a mouse model of Down syndrome is responsible for an increase in the number of LSK cells in the bone marrow and the development of myeloproliferative disease.15 Thus, Erg gene dosage is critical for maintenance of normal HSC regulation. In HSCs, Erg appears likely to work, at least in part, in concert with the related ETS family member Fli-1 (OMIM ID 193067), as recently suggested in vitro by genome-wide chromatin immunoprecipitation studies showing Erg and Fli-1 colocalization, along with several other transcription factors, in the regulatory domains of multiple loci,18 and definitively demonstrated in vivo in studies demonstrating synergistic exacerbation of HSC defects in mice with compound heterozygous mutation in Fli-1 and Erg.19
Although previous studies suggest that Erg is essential for normal adult HSC function, its precise role has not been defined. Here, we show that, although heterozygosity for functional Erg permits adequate steady-state HSC maintenance, wild-type levels of Erg are essential to maintain HSC self-renewal during stress hematopoiesis associated with high levels of stem cell cycling.
Methods
Mice
Erg+/Mld2 mice were generated and maintained as previously described.16 Mice expressing the Aequorea victoria green fluorescent protein (GFP) protein (GFP+) under the human ubiquitin C promoter were obtained from The Jackson Laboratory.20 Experiments were performed using procedures approved by the Walter and Eliza Hall Institute of Medical Research Animal Ethics Committee.
Hematology
Blood was collected into tubes containing ethylenediaminetetraacetic acid (Sarstedt) and analyzed with an Advia 120 analyzer (Bayer). Single-cell suspensions from bone marrow were collected in balanced salt solution (BSS-CS: 0.15M NaCl, 4mM KCl, 2mM CaCl2, 1mM MgSO4, 1mM KH2PO4, 0.8mM K2HPO4 and 15mM HEPES supplemented with 2% vol/vol BCS). Lympho-myeloid analysis on blood was performed after cells were suspended in buffered 156mM NH4Cl for erythrocyte lysis and resuspended in BSS-CS with BCS. For clonogenic assays, up to 400 cells sorted by flow cytometry were cultured in 1 mL of 0.3% agar in Iscove modified Dulbecco medium containing 20% newborn calf serum and cytokines. After incubation for 7 days in a fully humidified atmosphere of 10% CO2 in air, fixed, dried onto glass slides, stained for acetylcholinesterase, Luxol fast blue, and hematoxylin, and then the number and type of colonies were determined. For analysis of hematopoietic recovery, Erg+/+ or Erg+/Mld2 mice were exposed to a single dose of 350 cGy of γ-irradiation and the bone marrow compartment assessed at 3, 7, and 14 days after irradiation.
Bone marrow transplantation
Recipient CD45.1 C57BL/6 mice at 7-10 weeks of age were exposed to 11 Gy of γ-irradiation (2 doses of 550 cGy) followed by intravenous injection of unfractionated bone marrow cells suspended in BSS-CS. For competitive transplantation assays, a total of 2 × 106 nucleated cells were injected into irradiated CD45.1 recipients in a 1:1 or 10:1 ratio of test CD45.2 GFP+ donor to CD45.2 competitor bone marrow. A total of 1 × 106 total CD45.2 GFP+ nucleated cells were injected into CD45.2-irradiated recipients for noncompetitive transplantation assays, unless otherwise stated. The proportion of donor-derived cells in the blood or hematopoietic tissues was determined by flow cytometric analysis at 4 weeks, 20 weeks, and/or 6 months after transplantation from the percentage of hematopoietic cells expressing GFP and/or CD45.2 and specific lineage markers. For the competitive repopulating unit assay, irradiated CD45.1 recipient mice were transplanted with 2 × 105 unfractionated CD45.1+ competitor bone marrow cells mixed with various doses of unfractionated CD45.2+Erg+/+Mpl+/+ or Erg+/Mld2Mpl+/+ test bone marrow. The blood of recipients was analyzed by flow cytometry 16 weeks after the transplantation, and mice with > 1% of Gr-1+/Mac-1+ myeloid cells, B220+ B cells, and CD4+/CD8+ T lymphocytes derived from the CD45.2 test bone marrow were deemed to have received a detectable number of long-term HSCs in the test marrow and scored as positive. The frequency of competitive repopulating unit was estimated from the number of positive mice at each test dose using the ELDA program.21 For colony-forming unit–spleen assays, 400 cells sorted by flow cytometry were injected into each irradiated recipient. After 8 days, spleens were collected into Carnoy fixative (60% ethanol, 30% chloroform, 10% glacial acetic acid) and macroscopic colonies counted.
Flow cytometry
Bone marrow from 7-week-old to 12-month-old mice was harvested into BSS-CS. Lineage staining was performed with fluorochrome-conjugated or biotinylated monoclonal antibodies against Ter119, Gr1, Mac1, B220, CD4, and CD8 for analysis of blood and bone marrow compartments. Antibodies against Mac1 were omitted for lineage specification when assessing LSK cell reconstitution after sublethal irradiation. LSK subset delineation was performed with antibodies against c-Kit, Sca-1, Flt3R, CD3422 and CD150 and CD4823 with secondary staining using streptavidin- peridinin chlorophyll protein-Cy5.5 or streptavidin phycoerythrin-Texas Red. Cells were analyzed on the LSR II (BD Biosciences) or LSR I (BD Biosciences) or sorted by flow cytometry on a FACSAria (BD Biosciences).
Cell cycle analysis
Mice were administered intraperitoneal bromodeoxyuridine (BrdU) 0.5 mg/kg and maintained on oral BrDU (0.5 mg/L) in the drinking water for up to 3 days, or were maintained only on oral BrDU (0.5 mg/L) for 12 days to 4 weeks. The proportion of BrdU uptake in sorted LSK fractions was determined after fixation, permeabilization, DNAase treatment, and staining with anti-BrdU antibody and 7-amino-actinomycin D (BD Biosciences PharMingen) according to the manufacturer's instructions, with analysis by flow cytometry on an LSR II (BD Biosciences Pharmingen).
Statistical and bioinformatic analysis
Student unpaired 2-tailed t tests were used, with Holm modification for multiple testing where stated.24
Microarray analysis
Previously published microarray data available at Array Expression (www.ebi.ac.uk/arrayexpress) under access number E-TABM-371 and E-TABM-105016,25 were analyzed in R (Version 2.10.0) using the limma (Version 2.8) package of the Bioconducor software project (www.bioconductor.org).26 Each mutant was analyzed with its independent set of control wild-type mice. Raw intensities were normalized with the normexp background correction followed by quantile normalization across the whole dataset.27 Probes were filtered if not detected above background (expression level < 7) in any sample. Differential expression was assessed using linear modeling and empirical Bayes moderated t statistics.28 Benjamini-Hochberg multiple testing correction was applied, and probes with false discovery rate < 0.2 in either mutant were judged differentially expressed. This yielded 142 differentially expressed probes (139 unique genes) in the Mpl−/− LSK cells and 100 differentially expressed probes (96 unique genes) in the Erg+/Mld2 LSK cells. The correlation between the Mpl−/− and Erg+/Mld2 expression profiles was determined by applying Spearman rank correlation to the log2-fold changes, for all probes differentially expressed in either mutant. Heat maps and Venn diagrams were generated using the limma (Version 2.8) and gplots (Version 2.8.0) Bioconductor packages in R (Version 2.10.0).
Results
Erg+/Mld2 HSCs are numerically and functionally sustained at steady state
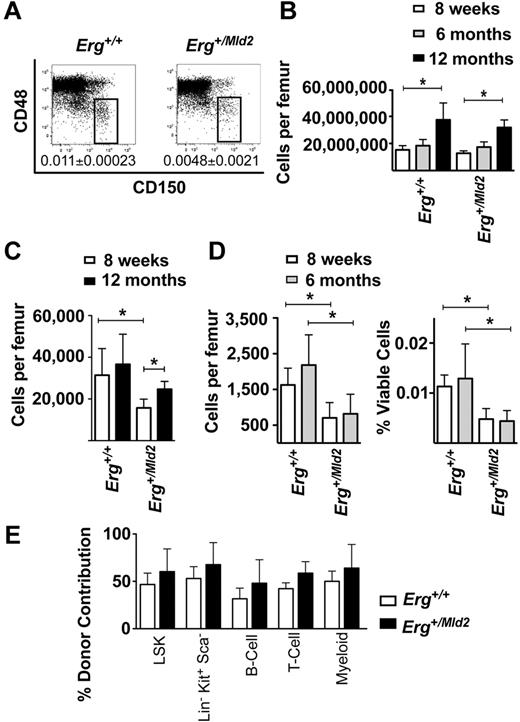
Previous studies have established that Erg+/Mld2 bone marrow, or the HSC-enriched LSK subset, are defective in lympho-myeloid reconstitution during competitive transplantation.16 To examine HSC function in steady-state hematopoiesis, we examined the number and function of HSCs in young and aged cohorts of Erg+/Mld2 mice. HSC-enriched LSK29 and CD150+CD48−23 bone marrow fractions were enumerated (Figure 1A-D). Although there were fewer LSK16 and CD150+CD48− LSK cells in Erg+/Mld2 marrow compared with littermate controls (Figure 1A,C-D), the number of cells in each of these fractions did not decline with age (Figure 1C-D).
Steady-state HSC activity in Erg+/Mld2 mice. (A) Representative flow cytometric analyses of the LSK bone marrow fraction with CD150 and CD48 SLAM markers. The box indicates the CD150+CD48−, HSC-enriched population as a percentage of viable bone marrow cells at 8 weeks of age. Data are mean ± SD of 6 mice per genotype. (B) Bone marrow cellularity represented as number of viable nucleated cells per femur, with (C) numbers of total LSK cells per femur at 8 weeks and 12 months of age and (D) CD150+CD48− LSK cells at 8 weeks and 6 months of age expressed as the number of cells per femur (left panel) and percentage of viable nucleated bone marrow cells (right panel). Data are mean ± SD of 6 mice per genotype for each time point shown. *P < .02. (E) Relative contribution to LSK cells, hematopoietic progenitor (Lin−Sca−cKit+), myeloid, T, and B cells in recipients of mixtures of 12-month-old and 10-week-old genotype-matched bone marrow in a 1:1 competitive transplant. Data are mean ± SD for each reconstituted fraction for 3 independent competitive transplant experiments. P > .05 for comparison of data from Erg+/Mld2 and wild-type donors for each population examined.
Steady-state HSC activity in Erg+/Mld2 mice. (A) Representative flow cytometric analyses of the LSK bone marrow fraction with CD150 and CD48 SLAM markers. The box indicates the CD150+CD48−, HSC-enriched population as a percentage of viable bone marrow cells at 8 weeks of age. Data are mean ± SD of 6 mice per genotype. (B) Bone marrow cellularity represented as number of viable nucleated cells per femur, with (C) numbers of total LSK cells per femur at 8 weeks and 12 months of age and (D) CD150+CD48− LSK cells at 8 weeks and 6 months of age expressed as the number of cells per femur (left panel) and percentage of viable nucleated bone marrow cells (right panel). Data are mean ± SD of 6 mice per genotype for each time point shown. *P < .02. (E) Relative contribution to LSK cells, hematopoietic progenitor (Lin−Sca−cKit+), myeloid, T, and B cells in recipients of mixtures of 12-month-old and 10-week-old genotype-matched bone marrow in a 1:1 competitive transplant. Data are mean ± SD for each reconstituted fraction for 3 independent competitive transplant experiments. P > .05 for comparison of data from Erg+/Mld2 and wild-type donors for each population examined.
Erg+/Mld2 HSCs from aged mice were then functionally tested in transplant assays in competition with young Erg+/Mld2 HSCs. Unfractionated bone marrow cells from 12-month-old Erg+/Mld2 or wild-type mice (CD45.2) were mixed with an equal number of 10-week-old GFP+Erg+/Mld2 or GFP+Erg+/+ marrow cells (CD45.2), respectively, and then transplanted into irradiated CD45.1 recipient mice. In transplant recipients in which aged wild-type HSCs competed with young wild-type HSCs, the aged cells contributed to ∼ 50% of the donor-derived LSK cells, hematopoietic progenitor cells (Lin−Sca1−cKit+), myeloid, T, and B lymphoid cells (Figure 1E). A similar contribution was evident from 12-month-old Erg+/Mld2 HSCs competing against genotype-matched 10-week-old Erg+/Mld2 competitor HSCs (Figure 1E). These data suggest that, despite defects in the capacity for competitive repopulation of myeloablated recipients, Erg+/Mld2 HSCs are capable of steady-state self-renewal without significant loss of functional activity with age.
Defective long-term hematopoietic reconstitution from Erg+/Mld2 HSCs
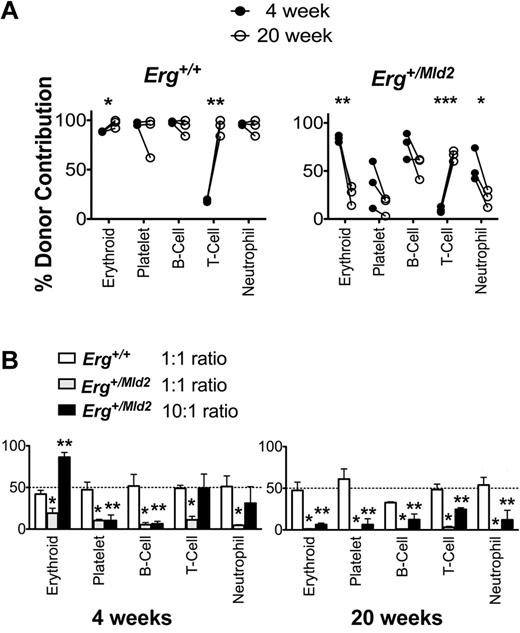
To better define the failure of Erg+/Mld2 HSC function in the setting of stress hematopoiesis, we characterized short- and long-term reconstitution in lethally irradiated recipients receiving Erg+/Mld2 or wild-type control bone marrow expressing green fluorescent protein (GFP+Erg+/+ or GFP+Erg+/Mld2). As expected, by 4 weeks after transplantation, wild-type GFP+Erg+/+ donor bone marrow contributed to 90%-100% of red blood cells, platelets, and peripheral blood B-lymphocytes and neutrophils. T-lymphoid contribution was low at this time, reflecting the requirement for thymic reconstitution before generation of circulating T cells30 ; by 20 weeks, contribution at high level to all peripheral blood lineages was evident (Figure 2A). GFP+Erg+/Mld2 bone marrow contributed significantly to erythroid, B-lymphocyte, and neutrophil reconstitution at 4 weeks after transplantation. Contribution to platelet production was low, reflecting the requirement for Erg in megakaryocytopoiesis.16,19 In contrast to wild-type marrow, by 20 weeks after transplantation of GFP+Erg+/Mld2 cells, the proportion of donor contribution to these lineages had declined significantly. These data suggest that, although retaining significant short-term repopulating potential, relative to wild-type cells, Erg-deficient stem cells are severely compromised in their capacity to sustain long-term hematopoiesis. In limiting dilution competitive repopulating assays,31,32 this deficit was manifest as 10-fold fewer long-term competitive reconstituting units in Erg+/Mld2 bone marrow compared with wild-type marrow (Table 1; supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Short-term hematopoietic reconstitution by Erg+/Mld2 bone marrow is not sustained. (A) Reconstitution of peripheral blood of lethally irradiated recipients by 1 × 106GFP+Erg+/+ or GFP+Erg+/mld2 unfractionated bone marrow cells. GFP+ donor contribution is shown; each point is the mean of 4 recipients with 3 independent donors included. *P < .05, **P < .001, ***P ≤ .0001: intragenotype comparison of reconstitution at 4 and 20 weeks after transplantation. (B) Competitive transplantation of GFP+Erg+/Mld2 or GFP+Erg+/+ unfractionated bone marrow cells mixed with competitor Erg+/+ bone marrow into lethally irradiated recipients. Peripheral blood reconstitution of mature lineages assessed at 4 weeks and 20 weeks after transplantation is shown for 1:1 ratio of GFP+Erg+/+ to Erg+/+ and 1:1 and 10:1 ratios of GFP+Erg+/Mld2 to Erg+/+ bone marrow cells. The mean ± SD of GFP+ donor contribution is shown from 3 independent experiments with 4 recipients per transplant. *P < .01, GFP+Erg+/Mld2 1:1 competitive donor ratio compared 1:1 GFP+Erg+/+:Erg+/+ ratio transplant. **P < .01, GFP+Erg+/Mld2 10:1 competitive donor ratio compared with 1:1 GFP+Erg+/+:Erg+/+ ratio transplant.
Short-term hematopoietic reconstitution by Erg+/Mld2 bone marrow is not sustained. (A) Reconstitution of peripheral blood of lethally irradiated recipients by 1 × 106GFP+Erg+/+ or GFP+Erg+/mld2 unfractionated bone marrow cells. GFP+ donor contribution is shown; each point is the mean of 4 recipients with 3 independent donors included. *P < .05, **P < .001, ***P ≤ .0001: intragenotype comparison of reconstitution at 4 and 20 weeks after transplantation. (B) Competitive transplantation of GFP+Erg+/Mld2 or GFP+Erg+/+ unfractionated bone marrow cells mixed with competitor Erg+/+ bone marrow into lethally irradiated recipients. Peripheral blood reconstitution of mature lineages assessed at 4 weeks and 20 weeks after transplantation is shown for 1:1 ratio of GFP+Erg+/+ to Erg+/+ and 1:1 and 10:1 ratios of GFP+Erg+/Mld2 to Erg+/+ bone marrow cells. The mean ± SD of GFP+ donor contribution is shown from 3 independent experiments with 4 recipients per transplant. *P < .01, GFP+Erg+/Mld2 1:1 competitive donor ratio compared 1:1 GFP+Erg+/+:Erg+/+ ratio transplant. **P < .01, GFP+Erg+/Mld2 10:1 competitive donor ratio compared with 1:1 GFP+Erg+/+:Erg+/+ ratio transplant.
Reduced frequency of competitive repopulating units (CRU) in Erg+/Mld2 bone marrow
| Donor bone marrow genotype . | CRU* . |
|---|---|
| Erg+/+ | 1 in 24 004 (47 174-12 214) |
| Erg+/Mld2 | 1 in 256 253 (412 654-159 130)† |
| Donor bone marrow genotype . | CRU* . |
|---|---|
| Erg+/+ | 1 in 24 004 (47 174-12 214) |
| Erg+/Mld2 | 1 in 256 253 (412 654-159 130)† |
Calculated frequency from limiting dilution assay (95% confidence interval in parentheses).
P < .0000005 for pairwise comparison of CRU frequency in Erg+/Mld2 and Erg+/+ bone marrow by χ2 analysis.
We next compared competitive 1:1 and 10:1 test to competitor bone marrow ratios in transplantation assays (Figure 2B). In a 1:1 ratio, short-term reconstitution (4 weeks) from GFP+Erg+/Mld2 test bone marrow was inferior in all blood cell lineages compared with wild-type marrow. A 10:1 excess of GFP+Erg+/Mld2 to wild-type competitor bone marrow cells compensated for the short-term competitive reconstitution disadvantage for erythroid, T-lymphoid, and neutrophil lineages, but reconstitution was not sustained long-term. These data suggest that, although the competitive short-term reconstitution disadvantage intrinsic to Erg+/Mld2 marrow can be overcome by increased cell dose, the competitive long-term repopulating deficit remains, even when using a 10-fold greater number of mutant bone marrow cells.
Erg+/Mld2 LSK cells have normal committed progenitor cell activity
Because short-term hematopoietic engraftment relies on the activity of the stem cell pool to rapidly generate multipotential and committed progenitor cells and ultimately mature blood cells, we examined the in vivo spleen colony (CFU-s) and in vitro hematopoietic colony-forming activity33,34 of flow cytometrically purified Erg+/Mld2 and wild-type LSK cell subsets. Comparison of Erg+/Mld2 and wild-type CD34+Flt3R− LSK cells, enriched for short-term HSCs,22 revealed no inferiority in the number of spleen colonies generated by an equivalent number of Erg+/Mld2 donor cells (Erg+/+: 14.5 ± 1.5 CFU-s per 400 cells, Erg+/Mld2: 20 ± 4.1, P = .046).
Similarly, in cytokine-stimulated semisolid cultures, there were no differences between Erg+/Mld2 and wild-type for colony production from long-term HSC-enriched CD34− LSK or short-term HSC-enriched CD34+Flt3R− LSK fractions (Table 2). Interestingly, however, the Erg+/Mld2 LSK fractions yielded significantly fewer pre-progenitor blast cell colonies, a cell type with a known self-renewal capacity35,36 (Table 2).
Colony-forming potential of purified population of Erg+/Mld2 bone marrow cells
| Cell population/genotype . | No. of colony-forming cells per 100 cells plated* . | |||||||
|---|---|---|---|---|---|---|---|---|
| Blast . | Granulocyte . | Granulocyte-macrophage . | Macrophage . | Eosinophil . | Megakaryocyte . | |||
| Total . | Multicentric . | Dispersed . | ||||||
| CD34− | ||||||||
| Erg+/+ | 18.4 ± 6.0 | 11.6 ± 4.6 | 6.9 ± 3.0 | 0.1 ± 0.4 | 0 | 3.9 ± 3.8 | 0 | 5.1 ± 6.2 |
| Erg+/Mld2 | 6.0 ± 3.8† | 4.0 ± 3.3† | 2.0 ± 1.9† | 0 | 0.1 ± 0.4 | 2.6 ± 2.4 | 0 | 8.1 ± 7.1 |
| CD34+Flt3R− | ||||||||
| Erg+/+ | 31.3 ± 8.1 | 27.3 ± 9.5 | 4.0 ± 1.7 | 3.9 ± 3.9 | 4.4 ± 3.8 | 4.0 ± 3.6 | 0 | 5.8 ± 2.0 |
| Erg+/Mld2 | 13.7 ± 10.5† | 12.3 ± 9.9† | 1.4 ± 2.1† | 3.3 ± 2.9 | 1.1 ± 0.9 | 0.7 ± 1.0 | 0.4 ± 0.8 | 5.1 ± 4.1 |
| Cell population/genotype . | No. of colony-forming cells per 100 cells plated* . | |||||||
|---|---|---|---|---|---|---|---|---|
| Blast . | Granulocyte . | Granulocyte-macrophage . | Macrophage . | Eosinophil . | Megakaryocyte . | |||
| Total . | Multicentric . | Dispersed . | ||||||
| CD34− | ||||||||
| Erg+/+ | 18.4 ± 6.0 | 11.6 ± 4.6 | 6.9 ± 3.0 | 0.1 ± 0.4 | 0 | 3.9 ± 3.8 | 0 | 5.1 ± 6.2 |
| Erg+/Mld2 | 6.0 ± 3.8† | 4.0 ± 3.3† | 2.0 ± 1.9† | 0 | 0.1 ± 0.4 | 2.6 ± 2.4 | 0 | 8.1 ± 7.1 |
| CD34+Flt3R− | ||||||||
| Erg+/+ | 31.3 ± 8.1 | 27.3 ± 9.5 | 4.0 ± 1.7 | 3.9 ± 3.9 | 4.4 ± 3.8 | 4.0 ± 3.6 | 0 | 5.8 ± 2.0 |
| Erg+/Mld2 | 13.7 ± 10.5† | 12.3 ± 9.9† | 1.4 ± 2.1† | 3.3 ± 2.9 | 1.1 ± 0.9 | 0.7 ± 1.0 | 0.4 ± 0.8 | 5.1 ± 4.1 |
Cells were cultured for 7 days in granulocyte colony-stimulating factor (10 ng/mL) + stem cell factor (10 ng/mL) for enumeration of blast colonies or stem cell factor (10 ng/mL) + IL-3 (10 ng/mL) + erythropoietin (2 U/mL) for other colony types.
P < .022 after adjustment for multiple testing; n = 8 independent sorted cell samples per genotype.
Defective stress-induced self-renewal by Erg+/Mld2 HSCs
To further define the defect in long-term repopulation from Erg+/Mld2 HSCs in vivo, lethally irradiated mice transplanted with GFP-positive donor Erg+/Mld2 or wild-type bone marrow were analyzed at 4 weeks and 6 months after transplantation to enumerate the LSK compartment. At both time points, nearly all LSK cells in recipients of GFP+Erg+/+ bone marrow were donor-derived, compared with one-third in recipients of GFP+Erg+/Mld2 bone marrow (Figure 3A). Moreover, although the number of donor LSK cells expanded several-fold in recipients of GFP+Erg+/+ bone marrow, little or no LSK cell expansion was evident from the GFP+Erg+/Mld2 graft (Figure 3A), despite robust GFP+Erg+/Mld2 contribution at 4 weeks to mature peripheral blood cell reconstitution (Figure 2A).
Failure of Erg+/Mld2 HSCs in stress-induced self-renewal. (A) Analysis of donor Lin−Sca+cKit+ HSC contribution to recipient bone marrow at 4 weeks and 6 months after transplantation of 1 × 106GFP+Erg+/+ or GFP+Erg+/Mld2 unfractionated bone marrow cells into lethally irradiated recipients. The mean ± SD of percentage GFP+ donor contribution to the recipient LSK compartment (left panel) and the number of LSK cells per femur per 1000 transplanted LSK cells (right panel) are shown for 3 independent experiments with 4 recipients per donor. GFP+Erg+/+ (open bars) and GFP+Erg+/Mld2 (closed bars). *P < .005. **P < .02. (B) Recovery of the LSK (left panel) and Lin−cKit+Sca1− committed myeloid progenitor cell fraction (right panel) after exposure of Erg+/+ mice (○) and Erg+/Mld2 (●) to 350 cGy sublethal irradiation. Data are mean ± SD of data from 3-6 mice per genotype per time point. *P ≤ .02. **P ≤ .0025. (C) Survival curve of lethally irradiated secondary transplant recipients receiving 5 × 105 to 1 × 106GFP+Erg+/+ (solid line) or GFP+Erg+/Mld2 (broken line) bone marrow cells from primary recipients 6 months after injection with 1 × 106 unfractionated bone marrow cells from GFP+Erg+/+ or GFP+Erg+/Mld2 primary donors. Data shown are from 3 independent experiments with 4 recipients per transplant. (D) Donor-derived erythroid reconstitution at 6 months after transplantation in surviving secondary recipients (2°) of bone marrow from primary (1°) GFP+Erg+/Mld2 recipients relative to GFP+Erg+/+ bone marrow transplanted mice. Data are mean ± SD. *P < .05.
Failure of Erg+/Mld2 HSCs in stress-induced self-renewal. (A) Analysis of donor Lin−Sca+cKit+ HSC contribution to recipient bone marrow at 4 weeks and 6 months after transplantation of 1 × 106GFP+Erg+/+ or GFP+Erg+/Mld2 unfractionated bone marrow cells into lethally irradiated recipients. The mean ± SD of percentage GFP+ donor contribution to the recipient LSK compartment (left panel) and the number of LSK cells per femur per 1000 transplanted LSK cells (right panel) are shown for 3 independent experiments with 4 recipients per donor. GFP+Erg+/+ (open bars) and GFP+Erg+/Mld2 (closed bars). *P < .005. **P < .02. (B) Recovery of the LSK (left panel) and Lin−cKit+Sca1− committed myeloid progenitor cell fraction (right panel) after exposure of Erg+/+ mice (○) and Erg+/Mld2 (●) to 350 cGy sublethal irradiation. Data are mean ± SD of data from 3-6 mice per genotype per time point. *P ≤ .02. **P ≤ .0025. (C) Survival curve of lethally irradiated secondary transplant recipients receiving 5 × 105 to 1 × 106GFP+Erg+/+ (solid line) or GFP+Erg+/Mld2 (broken line) bone marrow cells from primary recipients 6 months after injection with 1 × 106 unfractionated bone marrow cells from GFP+Erg+/+ or GFP+Erg+/Mld2 primary donors. Data shown are from 3 independent experiments with 4 recipients per transplant. (D) Donor-derived erythroid reconstitution at 6 months after transplantation in surviving secondary recipients (2°) of bone marrow from primary (1°) GFP+Erg+/Mld2 recipients relative to GFP+Erg+/+ bone marrow transplanted mice. Data are mean ± SD. *P < .05.
We further examined the early stages of stress-induced HSC self-renewal and progenitor formation in vivo by exposing Erg+/Mld2 and wild-type littermate controls to sublethal doses of γ-irradiation37 and then comparing regeneration of the LSK and Lin−Sca1−cKit+ myeloid progenitor cell pools. Relative to wild-type cells, HSCs from Erg+/Mld2 bone marrow exhibited a significant defect in postirradiation LSK expansion, whereas recovery expansion of Lin−Sca1−cKit+ myeloid progenitors was less severely affected (Figure 3B).
Erg+/Mld2 HSCs have a severe self-renewal defect in serial transplantation assays
We further tested Erg+/Mld2 HSC self-renewal potential in serial transplantation assays. GFP-positive bone marrow was harvested from primary wild-type recipients 24 weeks after transplantation of either GFP+Erg+/+ or GFP+Erg+/Mld2 marrow, and then transplanted into secondary recipients. Although GFP+Erg+/+ bone marrow was able to radioprotect and reconstitute all secondary recipients to 6 months after transplantation, 58% (7 of 12) of secondary recipients receiving GFP+Erg+/Mld2 bone marrow died rapidly after transplantation (Figure 3C). Two of 3 cohorts of surviving secondary recipients receiving bone marrow from independent Erg+/Mld2 donors had < 1% reconstitution from GFP+Erg+/Mld2 HSCs at 6 months compared with 42% ± 20% donor contribution in recipients of GFP+Erg+/+ marrow (Figure 3D). Together, these findings confirmed a self-renewal defect in Erg+/Mld2 HSCs.
Erg is required to maintain HSCs in the absence of Mpl signaling
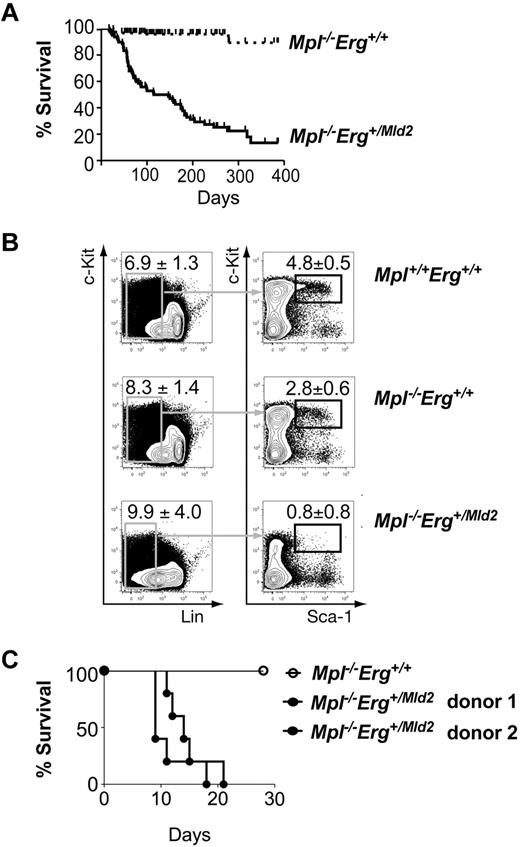
Thrombopoietin (TPO, OMIM ID 600044) is a cytokine regulator of HSC quiescence that acts via it cell surface receptor, the c-Mpl protein (OMIM ID 159530): disruption of the TPO/Mpl pathway is associated with increased HSC cycling.38,39 The Mld2 mutation was originally detected in a sensitized ENU mutagenesis screen using Mpl-deficient mice, and Erg+/Mld2Mpl−/− mice have more severe peripheral blood cell deficiencies relative to Erg+/Mld2Mpl+/+ mice.16 We further characterized the Erg+/Mld2Mpl−/− phenotype with the hypothesis that the severity of the hematopoietic anomalies in Erg+/Mld2Mpl−/− mice may be the result of a more profound HSC defect, reflecting interaction between Erg and TPO signaling in HSC regulation. Unlike Mpl+/+Erg+/Mld2 and Mpl−/−Erg+/+ mice, which retain general good health, Mpl−/−Erg+/Mld2 compound mutant mice became moribund at a median of 115 days of age (Figure 4A). Moribund Mpl−/−Erg+/Mld2 mice had pale organs consistent with a severe anemia evident in peripheral blood analysis (red cell count: 5.0 ± 1.0 × 1012/L, n = 6). Severe thrombocytopenia (platelet count: 31 ± 17 × 109/L, n = 6) was accompanied by cerebral, pulmonary, intraperitoneal, and lymph node hemorrhage, and examination of sternal sections revealed severe bone marrow aplasia (supplemental Figure 1). None of these histologic features was evident in healthy Mpl−/−Erg+/+ control mice. Taken together, these findings suggested that adult Mpl−/−Erg+/Mld2 mice died of complications arising from primary bone marrow failure.
Bone marrow failure in Mpl−/−Erg+/Mld2 mice. (A) Survival of Mpl−/−Erg+/+ (broken line, n = 172) and Mpl−/−Erg+/Mld2 (solid line, n = 58) mice. P < .0001 by log-rank analysis, with median survival of 115 days for Mpl−/−Erg+/Mld2 mice. (B) Representative flow cytometric analysis of the LSK fraction from Mpl+/+Erg+/+ (n = 6), Mpl−/−Erg+/+ (n = 6), and Mpl−/−Erg+/Mld2 (n = 6) mice at 7-10 weeks of age. (Left panels) The number (mean ± SD) of Lin− cells as a percentage of viable bone marrow cells. (Right panels) The number of Kit+ Sca-1+ cells as a percentage of Lin− cells. (C) Survival of mice transplanted with of 3 × 106 unfractionated bone marrow cells from Mpl−/−Erg+/+ (○) and Mpl−/−Erg+/Mld2 (●, 2 independent donors) mice, with 5 recipients per donor.
Bone marrow failure in Mpl−/−Erg+/Mld2 mice. (A) Survival of Mpl−/−Erg+/+ (broken line, n = 172) and Mpl−/−Erg+/Mld2 (solid line, n = 58) mice. P < .0001 by log-rank analysis, with median survival of 115 days for Mpl−/−Erg+/Mld2 mice. (B) Representative flow cytometric analysis of the LSK fraction from Mpl+/+Erg+/+ (n = 6), Mpl−/−Erg+/+ (n = 6), and Mpl−/−Erg+/Mld2 (n = 6) mice at 7-10 weeks of age. (Left panels) The number (mean ± SD) of Lin− cells as a percentage of viable bone marrow cells. (Right panels) The number of Kit+ Sca-1+ cells as a percentage of Lin− cells. (C) Survival of mice transplanted with of 3 × 106 unfractionated bone marrow cells from Mpl−/−Erg+/+ (○) and Mpl−/−Erg+/Mld2 (●, 2 independent donors) mice, with 5 recipients per donor.
Healthy Mpl−/−Erg+/Mld2 mice at 7-10 weeks of age contained significantly fewer bone marrow LSK cells compared with Mpl−/−Erg+/+ littermates (Figure 4B). These findings suggested that bone marrow failure in Mpl−/−Erg+/Mld2 mice was secondary to an exacerbated HSC defect. Unfractionated bone marrow from Mpl−/−Erg+/Mld2 or Mpl−/−Erg+/+ mice was then transplanted into lethally irradiated recipients to test HSC function. Whereas all recipients of Mpl−/−Erg+/Mld2 bone marrow failed to survive after transplantation (Figure 4C), Mpl−/−Erg+/+ bone marrow was able to radioprotect and reconstitute lethally irradiated recipients as previously reported.40
Distinct genetic programs are regulated by Erg and Mpl signaling in LSK cells
Although the extremely low number of LSK cells in Mpl−/−Erg+/Mld2 mice precluded analysis of HSC cell cycle status, incorporation of BrdU into Mpl+/+Erg+/Mld2 stem cells was indistinguishable from wild-type (supplemental Figure 2), suggesting that the long-term HSC defect in Mpl+/+Erg+/Mld2 mice was not the result of aberrant cell cycling and inferring that further deregulation of HSC quiescence may not underpin the exacerbated Mpl−/−Erg+/Mld2 defect. To correlate our biologic findings with gene expression changes induced by reduced Erg activity or loss of Mpl signaling, we compared the published lists of differentially regulated genes in the LSK fraction of Erg+/Mld2 mice16 with those deregulated in the LSK fraction of Mpl−/− mice.25 Of 240 genes differentially expressed in the LSK fraction of either Erg+/Mld2 or Mpl−/− mice, only 2 were concordantly deregulated in both Erg+/Mld2 and Mpl−/− LSK cells (Figure 5). Furthermore, the expression changes undergone by differentially expressed genes in Mpl−/− and Erg+/Mld2 LSK cells were generally uncorrelated, with no tendency to be in the same direction (P = .3). Thus, the LSK gene signatures from Mpl−/− and Erg+/Mld2 mice are remarkably distinct, suggesting that the effect of reduced Erg activity occurs via molecular mechanisms independent from those associated with TPO/Mpl regulation of HSCs.
Distinct gene expression profiles in Erg+/Mld2 and Mpl−/− LSK cells. (A) Heat map of differentially expressed genes in the LSK compartments of Erg+/Mld2 and Mpl−/− mice (false discovery rate value set at 0.2). Genes that were up-regulated compared with the wild-type are shown in red; genes that were down-regulated compared with the wild-type are shown in blue. (B) Venn diagram of concordantly differentially expressed genes from LSK cells of each mutant, showing direction of differential expression. Numbers of up-regulated probes shown in red, and down-regulated probes in blue. Only 2 deregulated genes were shared by Erg+/Mld2 and Mpl−/− LSK cells, Fgd5 and Robo4.41,42
Distinct gene expression profiles in Erg+/Mld2 and Mpl−/− LSK cells. (A) Heat map of differentially expressed genes in the LSK compartments of Erg+/Mld2 and Mpl−/− mice (false discovery rate value set at 0.2). Genes that were up-regulated compared with the wild-type are shown in red; genes that were down-regulated compared with the wild-type are shown in blue. (B) Venn diagram of concordantly differentially expressed genes from LSK cells of each mutant, showing direction of differential expression. Numbers of up-regulated probes shown in red, and down-regulated probes in blue. Only 2 deregulated genes were shared by Erg+/Mld2 and Mpl−/− LSK cells, Fgd5 and Robo4.41,42
Discussion
Adult long-term HSCs are predominantly quiescent at steady state but can be induced into cell cycle by myelotoxic stress or cytokine treatment when rapid mature blood cell production is required.43 During stress hematopoiesis, HSC division initiates lineage-committed progenitor formation and the first wave of short-term hematopoietic reconstitution, as well as self-renewal of HSCs to reliably sustain long-term hematopoietic reconstitution. The balance of this differentiation versus self-renewal fate decision must be closely regulated to ensure timely mature blood cell recovery without HSC exhaustion and failure of sustained hematopoietic reconstitution.44,45
Previous studies have demonstrated that mice heterozygous for a loss of function mutation in the Erg gene produce HSCs incapable of competing with normal cells for long-term hematopoietic reconstitution of myeloablated transplant recipients.16 The data presented here suggest that Erg is an essential transcriptional regulator of self-renewal in HSCs during stress hematopoiesis. Transplantation of HSCs haploinsufficient for functional Erg results in adequate short-term repopulation but severely impaired long-term donor-derived reconstitution. Erg+/Mld2 bone marrow can radioprotect and, on transplantation or endogenously in sublethally irradiated mice, permits expansion of committed progenitor cells with kinetics and magnitude not dramatically different from wild-type marrow. In contrast, stress-induced expansion of the LSK stem cell compartment was profoundly defective in these experiments. Thus, in the context of stress hematopoiesis, Erg+/Mld2 HSCs appear to favor committed progenitor cell formation over self-renewal of HSCs and early multipotential progenitors within the LSK compartment.
Significantly, at steady state, Erg+/Mld2 HSCs were capable of maintaining hematopoiesis without exhaustion or significant reduction in the numbers of cells in the HSC pool with aging. Thus, stress-induced HSC self-renewal and steady-state HSC self-renewal may be separate and uniquely regulated processes; indeed, gene expression signatures in proliferating and quiescent HSCs are clearly distinct46 ; and in addition to Erg, mutations in other genes, including those encoding Ube1L (OMIM ID 191325) and SLUG (OMIM ID 602150), appear to differentially affect steady state and stress-induced HSC behavior.47,48 Alternatively, molecular controls may be similar in stem cells in normal or stress contexts, but the impact of the imbalance in HSC self-renewal and differentiation caused by Erg haploinsufficiency may be largely imperceptible at steady state, when the call on the HSC pool is low, and only obvious in emergencies that challenge the full capacity of the stem cell reserve.
Although the steady-state numbers and function of HSCs in Erg+/Mld2 mice appear not to deteriorate with aging, it is clear from the data here and from previous work16 that Erg+/Mld2 mice contain significantly fewer prospectively identifiable HSCs than wild-type mice. This implies that, although steady-state maintenance of the HSC pool, once established, may be largely intact in Erg+/Mld2 mice, the process of hematopoietic development fails to generate a replete HSC pool. Indeed, mice homozygous for the ErgMld2 allele fail to survive beyond mid-gestation with hematopoietic failure, which is the result of failure of definitive HSC maintenance rather than hematopoietic specification or differentiation.16,17 Because, unlike steady-state adult hematopoiesis, developmental hematopoiesis and stress hematopoiesis in the adult both involve extensive HSC cycling, these observations further support the role for Erg in maintaining the balance of self-renewal and differentiation cell divisions during HSC proliferation.
Lineage-specific anomalies were also noted during hematopoietic reconstitution from Erg+/Mld2 bone marrow after transplantation. Poor platelet reconstitution from Erg+/Mld2 donor marrow compared with wild-type transplants was particularly evident. These findings suggest that, in addition to the role of Erg in HSC self-renewal, Erg is required for megakaryopoiesis. Indeed, Erg+/Mld2 mice have fewer platelets at steady state compared with wild-type,16 the Mld2 mutation cooperates with Fli-1 haploinsufficiency to synergistically perturb megakaryocyte differentiation,19 and trisomy of Erg leads to megakaryocytosis, thrombocytosis, and myeloproliferation.15 Conversely, erythroid reconstitution from Erg+/Mld2 bone marrow was superior to platelet reconstitution. This suggests that Erg may regulate the balance of erythroid-megakaryocyte lineage specification from bipotential megakaryocyte-erythroid progenitors, as has recently been suggested for Fli-1.49 Although reconstitution of B-cells was sensitive to haploinsufficiency of functional Erg, T-cell reconstitution from Erg+/Mld2 bone marrow paralleled wild-type reconstitution. Importantly, as early lymphoid progenitors must first seed the thymus before donor-derived mature T cells can emerge,30 this observation may be biased by the lag of mature T-cell reconstitution behind other lineages. Further definitive study of Erg function in differentiated blood cells will require models in which the Erg gene has been conditionally mutated in specific mature lineages and early committed progenitors.
Although mice functionally compromised for either Erg or Mpl, the receptor for TPO, exhibit significant HSC defects, Erg+/Mld2 and Mpl−/− mice maintain sufficient steady-state HSC function to sustain hematopoiesis with aging.16,50 However, Mpl−/−Erg+/Mld2 compound mutant mice displayed exacerbated stem cell deficiencies and died of bone marrow failure within the first year of life. TPO, acting through c-Mpl, is required to maintain HSC quiescence, and in mice lacking TPO or Mpl, HSCs exhibit higher than normal levels of cell cycling.38,39 Our data suggest that Mpl signaling and Erg cooperate to sustain adult HSC function and support a model in which, in the context of increased HSC cycling caused by Mpl deficiency, the additional loss of Erg function in Mpl−/−Erg+/Mld2 compound mutant mice deregulates HSC self-renewal, leading to HSC exhaustion and hematopoietic failure. Consistent with distinct molecular actions of TPO/Mpl and Erg, comparison of differentially expressed genes within the LSK compartment of Erg+/Mld2 and Mpl−/− mice revealed that unique sets of genes were deregulated by loss of Mpl signaling and haploinsufficiency of Erg.
Thus, through analysis of steady-state hematopoiesis as well as 3 independent models of hematopoietic stress (bone marrow transplantation, endogenous hematopoietic recovery from myelosuppressive irradiation, and Mpl deficiency), we have defined Erg as a critical regulator of HSCs during stress hematopoiesis, essential for maintaining self-renewal at times of high HSC cycling. The central role for Erg in self-renewal of normal stem cells raises the hypothesis that overexpression or activation of Erg in proliferative diseases, such as prostate cancer and leukemia, may promote disease by favoring self-renewal of cycling cancer or leukemic stem cells.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Shauna Ross, Kelly Trueman, Jackie Gilbert, Stephanie Green, and Chris Evans for excellent animal husbandry and Jason Corbin and Janelle Lochland for automated peripheral blood analysis.
This work was supported by the Australian National Health and Medical Research Council (program grant 461219; project grant 516726; fellowships, W.S.A., B.T.K., A.P.N.; and Independent Research Institutes Infrastructure Support Scheme Grant 361646), the Australian Research Council (fellowship; B.T.K.), the Sylvia and Charles Viertel Charitable Foundation (fellowship; B.T.K.), the Cancer Council, Victoria (Carden Fellowship Fund, D.M.; and Sydney Parker Smith Postdoctoral Research Fellowship, C.A.d.G.), the Australian Department of Education, Science and Training (scholarship; S.J.L., C.A.d.G.), Australian Stem Cell Center (Postgraduate Supplementary Scholarship; C.A.d.G.), Haematology Society of Australia and New Zealand/AMGEN (New Investigator Scholarship; A.P.N.), the Australian Cancer Research Fund, and the Victorian State Government (Operational Infrastructure Support grant).
Authorship
Contribution: All authors were responsible for the whole work, including the conception, design, and conduct of the study, analysis and interpretation of the data, drafting of the paper, discussion and revision of the paper, and gave their permission for the final version submitted for publication.
Conflict-of-interest disclosure: D.J.H., B.T.K., and W.S.A. hold shares in and have received research support from MuriGen Pty Ltd. D.J.H. and W.S.A. have received research support from CSL Ltd. The remaining authors declare no competing financial interests.
Correspondence: Ashley P. Ng, Walter and Eliza Hall Institute of Medical Research, 1G Royal Parade, Parkville, Victoria, 3052, Australia; e-mail: ang@wehi.edu.au.