Abstract
Previous work has demonstrated that both rapamycin (RAPA) and IL-2 enhance CD4+CD25+Foxp3+ regulatory T-cell (Treg) proliferation and function in vitro. We investigated whether the combination of RAPA plus IL-2 could impact acute GVHD induction after bone marrow transplantation (BMT). RAPA plus IL-2 resulted in improved survival and a reduction in acute GVHD lethality associated with an increased expansion of donor type CD4+Foxp3+ Tregs and reduced CD4+CD25− conventional T cells (Tcons). RAPA plus IL-2, but not either drug alone, increased both expansion of donor natural Tregs and conversion of induced Tregs from donor CD25− Tcons while IL-2 alone increased conversion of Tregs from CD25− Tcon. RAPA plus IL-2 treatment resulted in less production of IFN-γ and TNF, cytokines known to be important in the initiation of acute GVHD. These studies indicate that the pharmacologic stimulation of T cells with IL-2 and the suppression of Tcon proliferation with RAPA result in a selective expansion of functional Tregs and suppression of acute GVHD.
Introduction
Rapamycin (Sirolimus; RAPA), a macrolide antibiotic produced by Streptomyces hygroscopicus, has been used for the prophylaxis and treatment of several immune reactions including GVHD and solid organ rejection.1-3 RAPA has also been used in vitro for the expansion of CD4+CD25+Foxp3+ regulatory T cells (Tregs)4-7 and preserves the in vivo suppressive effects of Treg while inhibiting CD4+CD25− and CD8+ T cells.8 The differential response of Tregs and effector T cells to RAPA results from the use of different signaling pathways by these 2 populations of CD4+ T cells. RAPA functions by binding to the mammalian target of RAPA (mTOR) FK506 binding protein complex leading to inhibition of mTOR protein kinase activity, characteristic of conventional CD4+ T-cell activation.9 In contrast, activation of Tregs with IL-2 results in phosphorylation of STAT3 and STAT5, with limited use of the PI3K/mTOR pathway, while CD4+CD25− conventional T cells (Tcons) are inhibited via the mTOR/PI3K pathway by RAPA eventually resulting in apoptotic cell death.10
IL-2 is a 4-helix cytokine, produced primarily by activated CD4+ T cells and exerts its biologic activity by binding to the high-affinity IL-2 receptor. IL-2 is known to be a major cytokine for T-cell proliferation and differentiation based on its effects on T-cell growth in vitro. However, IL-2 can also have immune suppressive effects.11,12 Mice deficient in IL-2 or the IL-2 receptor have reduced numbers of Tregs both in the thymus and in the periphery,13,14 and develop a lethal phenotype characterized by T-cell hyperproliferation.15-17 IL-2 signaling is required for both thymic development and peripheral expansion/maintenance of CD4+CD25+ Treg cells.2 There is evidence that IL-2 can modulate Treg suppressive activity in vitro18-20 and also activate Tregs in vivo.21 In vivo, IL-2 administration not only expands Tregs, but also induces IL-10 production and suppressive activity.22
Recently, an in vitro study demonstrated that the addition of RAPA to cultures containing IL-2 increased the frequency and absolute number of functional CD4+CD25+Foxp3+ Tregs from CD25−Foxp3− T cells. However, it is not known whether these agents have cooperative effects on the expansion of Tregs in vivo or have functional implications, for example, in the prevention of GVHD by the expanded Tregs. In this study we show that in vivo administration of RAPA plus IL-2 results in the expansion of adoptively transferred donor type CD4+CD25+Foxp3+ Tregs and reduces acute GVHD in a MHC mismatched murine model of BMT.
Methods
Mice
C57BL/6 (H-2kb and Thy-1.2), and BALB/c (H-2kd and Thy-1.2) mice were purchased from The Jackson Laboratory and used at age 8-12 weeks. Only female-to-female combinations were used for transplant experiments. Luciferase-expressing (luc+) C57BL/6 (H-2kb) animals were bred by backcrossing FVB/N L2G85 animals into the C57BL/6 strain for > 14 generations.23,24 Thy-1.1+CD45.1+ or green fluorescent protein (GFP)+ Thy-1.1+ C57BL/6 mice were bred in our animal facility. Animal protocols were approved by the Institutional Animal Care and Use Committee at Stanford University.
Flow cytometry
The following antibodies were used for flow cytometric analysis: anti-CD4 (GK1.5 or RM4-5), CD8α (53-6.7), CD25 (PC61.5), H-2Kb (AF6-88.5), Foxp3 (FJK-16s) and Thy-1.1 (HIS51) from Biolegend and eBiosciences. Foxp3 staining was performed using the intracellular Foxp3 staining kit (antibody: FJK-16s; eBiosciences). Intracellular IFN-γ and TNF-α were determined using Leukocyte Activation Cocktail with GolgiPlug (BD Pharmingen). All assays were performed according to the manufacturer's instructions. Briefly, the cocktail was rapidly thawed at 37°C in a water bath and 2 μL of cocktail added for every 1 mL of cell culture and mixed thoroughly. The cells were cultured in a 37°C humidified CO2 incubator for 4-6 hours. After activation, the cells were harvested and washed with FACS Staining Buffer for use in antibody staining protocols. Propidium iodide (PI; Sigma-Aldrich) was added before analysis to exclude dead cells. Live/dead fixable Aqua dead stain kit (Invitrogen) was used in studies of intracellular Foxp3 staining. All analytical flow cytometry was done on a modified dual laser or 4 laser LSRScan (BD Immunocytometry Systems) in the Shared FACS Facility, Center for Molecular and Genetic Medicine at Stanford University using FlowJo software (TreeStar) for data analysis.
Cell isolation and sorting
Cervical lymph nodes (LNs), axillary LNs, inguinal LNs, mesenteric LNs, and splenocytes were harvested from donor mice and processed in PBS (Invitrogen) supplemented with 2% FCS (Invitrogen) into single-cell suspensions. After red blood cells (RBC) lysis, cells were blocked with normal rat serum (Caltag Laboratories/Invitrogen), stained with anti-CD25 PE (eBioscience) and anti-PE magnetic beads, and positive selection for the CD25+ population was performed by manual MACS column selection (Miltenyi Biotec). CD25+ cells were then stained with anti-CD4 APC and PI, and sorted on a BD FACS Aria cell sorter (BD Biosciences) for the CD4+CD25high population (15%-20% of the enriched CD25+ cells). This purification protocol yielded > 95% purity of CD4+CD25+Foxp3+ cells. T cell–depleted bone marrow (TCD BM) was obtained through negative depletion using anti-CD4 and anti-CD8 magnetic beads (Miltenyi Biotech). For transplantation of Tcons, splenic and LN single-cell suspensions from C57BL/6 mice were enriched with CD4/CD8-conjugated magnetic beads or Thy-1.2 magnetic beads using the manual MACS selection (Miltenyi Biotech). After enrichment, CD4+ and CD8+ T cells were > 90% pure. CD25-depleted (CD25−) Tcons were obtained through negative depletion using anti-PE magnetic beads after staining with anti-CD25 PE (eBioscience).
Cytokine measurements
Serum was collected at day 7 after BMT and was immediately stored at −20°C freezer until the assay day. Up to 20 different cytokines were analyzed in a multiplex assay system using fluorescently labeled microsphere beads (Cytokine Mouse 10 or 20-plex Panel LMC0001M and LMC0006 Invitrogen by Life Technologies). The assay was performed according to the manufacturer's instructions in duplicates. Cytokine levels were quantitated using the Luminex 200 system (Luminex)
Acute GVHD model
Acute GVHD was induced as described previously.8 Briefly, BALB/c recipient mice were lethally irradiated with 800 cGy, given in 2 split doses of 400 cGy at least 4 hours apart, on day 0 followed by intravenous injection of 5 × 106 C57BL/6 donor TCD-BM cells. To induce GVHD CD4+/CD8+ Tcons (0.5-1 × 106 cells per animal) were injected intravenously on day 0. In specific experiments, CD25− Tcons were used to induce acute GVHD. Tregs (5 × 105 per animal) were purified as described8 and injected intravenously on day 0 as indicated. In some experiments Tcons from luc+ C57BL/6 donors were used to track T-cell proliferation and trafficking by bioluminescence imaging (BLI) where indicated. Mice were kept in autoclaved cages and on antibiotic water (sulfamethoxazole and trimethoprim; Hi-Tech Pharmacal) for a minimum of 30 days after transplantation. Animals were monitored and weighed 2-3 times per week. Clinical evidence of GVHD was evaluated and scored as previously described.25
In vivo RAPA or IL-2 treatment
For the in vivo studies, RAPA (Sigma-Aldrich) was dissolved in carboxymethylcellulose sodium salt (C-5013; Sigma-Aldrich) and polysorbate 80 (P-8074; Sigma-Aldrich). RAPA stock solution was stored at 4°C in the dark in distilled water according to the manufacturer's instructions. Intraperitoneal injections were administered once daily starting on day 0. Dosage was adjusted to the body weight every other day. The RAPA dosage used in vivo was 0.5 mg/kg as described previously.24 Immunosuppressive treatment using RAPA was continued until 7 or 14 days after BMT. IL-2 was administrated as described.26,27 Briefly, mice were injected intraperitoneally with 5 × 104 IU/mouse of recombinant human IL-2 (Proleukin; Chiron) in sterile PBS before BMT and then twice daily thereafter for 3 or 7 days as indicated.
In vivo BLI
In vivo BLI was performed as described previously.28 Briefly, mice were injected with luciferin (10 μg/g of body weight, intraperitoneally). Ten minutes later, the mice were imaged using an IVIS200 charge-coupled device (CCD) imaging system (Xenogen) for 5 minutes. Imaging data were analyzed and quantified with Living Image Software (Xenogen) and IgorProCarbon (WaveMetrics).
Statistical analysis
Differences in proliferation of luc transgenic T cells in vivo, mean fluorescence intensity (MFI), body weight, and GVHD scores were analyzed using the 2-tailed Student t test. A P value < .05 was considered statistically significant.
Results
Impact of the RAPA, IL-2, and combination of RAPA plus IL-2 on conventional and regulatory CD4+ T cell subsets
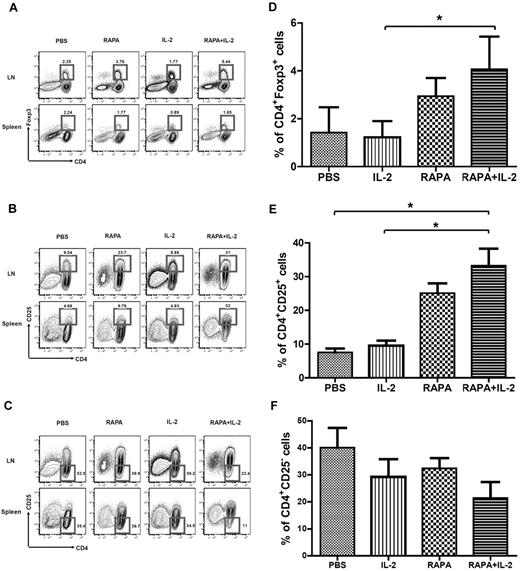
To investigate the impact of the combination of RAPA plus IL-2 on different subsets of CD4+ T cells, we analyzed CD4+CD25+Foxp3+, CD4+CD25+, and CD4+CD25− T-cell populations on day 7 after BMT after infusion of Tcons alone among mouse groups receiving PBS, RAPA, and IL-2 alone as well as the combination of RAPA plus IL-2. RAPA alone and the combination of RAPA plus IL-2 but not IL-2 alone increased the frequency of CD4+Foxp3+ T cells in LNs (Figure 1A) and CD4+CD25+ T cells in LNs and splenocytes (Figure 1B). RAPA or IL-2 alone as well as the combination of RAPA plus IL-2 decreased the frequency of CD4+CD25− T cells in LNs, while only the combination of RAPA plus IL-2 resulted in a decrease in the percentage of CD4+CD25− T cells in the spleen (Figure 1C). Overall, RAPA alone or the combination of RAPA plus IL-2 resulted in an increase in the expansion of donor type CD4+Foxp3+ T cells, CD4+CD25+ T cells and a reduction in the frequency of CD4+CD25− T cells in LNs (Figure 1D-F).
Combination of RAPA + IL-2 results in the expansion of donor-type CD4+Foxp3+ Tregs in the LNs, CD4+CD25+ T cells in peripheral tissues, and reduces CD4+CD25− T cells. BALB/c mice (Thy-1.2 and H-2kd) were injected with 5 × 106 TCD-BM cells (Thy-1.2 and H-2kb) and 1 × 106 Tcons (Thy-1.1 and H-2kb) from C57BL/6 mice after lethal irradiation with 800 cGy on day 0. Mice in each group received PBS, RAPA, IL2 or a combination of RAPA plus IL-2 from day 0 to day 7 after BMT. Flow cytometry of the CD4 T-cell population and intracellular staining of Foxp3 (A) or CD25 (B,C) in total lymphocytes by gating Thy-1.1+ from LNs and spleen of BALB/c mice on day 7 after BMT was performed (3 mice in each group). The bar graphs compare the proportion of CD4+Foxp3+ cells (D), CD4+CD25+ T cells (E) and CD4+CD25− T cells (F) in LNs of each group. Data are derived from 3 independent experiments. *P < .05. Results were shown as mean ± SD.
Combination of RAPA + IL-2 results in the expansion of donor-type CD4+Foxp3+ Tregs in the LNs, CD4+CD25+ T cells in peripheral tissues, and reduces CD4+CD25− T cells. BALB/c mice (Thy-1.2 and H-2kd) were injected with 5 × 106 TCD-BM cells (Thy-1.2 and H-2kb) and 1 × 106 Tcons (Thy-1.1 and H-2kb) from C57BL/6 mice after lethal irradiation with 800 cGy on day 0. Mice in each group received PBS, RAPA, IL2 or a combination of RAPA plus IL-2 from day 0 to day 7 after BMT. Flow cytometry of the CD4 T-cell population and intracellular staining of Foxp3 (A) or CD25 (B,C) in total lymphocytes by gating Thy-1.1+ from LNs and spleen of BALB/c mice on day 7 after BMT was performed (3 mice in each group). The bar graphs compare the proportion of CD4+Foxp3+ cells (D), CD4+CD25+ T cells (E) and CD4+CD25− T cells (F) in LNs of each group. Data are derived from 3 independent experiments. *P < .05. Results were shown as mean ± SD.
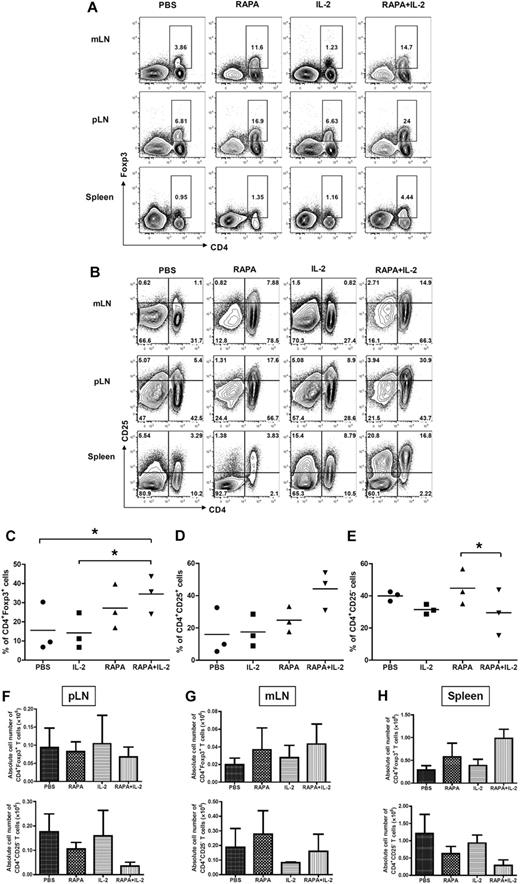
To determine whether the combination of RAPA plus IL-2 results in the expansion of Tregs in vivo from donor CD4+CD25high Tregs, which are part of the infused Tcons, or results in an increase in the conversion of Foxp3+ cells from CD25− Tcons, mice were injected with donor Tregs together with donor CD25-depleted Tcons after lethal irradiation on day 0. Congenic mouse strains were used to separate these 2 populations of T cells on day 7 after BMT. CD25− Tcons were obtained from Thy1.1+ mice whereas Tregs were isolated from Thy1.2+ mice. The animals received PBS, RAPA, IL-2, and RAPA plus IL-2 from day 0 to 7 after BMT. RAPA alone or the combination of RAPA plus IL-2 resulted in an increased frequency of donor-type CD4+Foxp3+ T cells in mesenteric and peripheral LNs as well as the spleen (Figure 2A,C). However, the combination of RAPA plus IL-2 had more prominent effects than RAPA alone. Similarly, there was an increase in the frequency of CD4+CD25+ T cells in these organs (Figure 2B-D) and a reduced frequency of CD4+CD25− T cells (Figure 2B-E) originating from infused donor CD4+CD25high Tregs and CD25− Tcons. The combination of RAPA plus IL-2 resulted in an expansion of CD4+Foxp3+ T cells (2.9 ± 0.5 fold) and CD4+CD25+ T cells (4.2 ± 1.1 fold), and a reduction in the frequency of CD4+CD25− T cells (0.4 ± 0.2 fold) compared with control animals that just received PBS. The combination of RAPA plus IL-2 was more potent than RAPA or IL-2 alone in expanding Treg populations.
Combination of RAPA + IL-2 has additive effects on the expansion of CD4+Foxp3+ and CD4+CD25+ cells after infusion of donor Tregs and CD25 depleted Tcons. BALB/c mice (Thy1.2 and H-2kd) were injected with 5 × 106 TCD-BM cells from GFP transgenic C57BL/6 mice (Thy1.1 and H-2kb), 5 × 105 Treg from wild-type C57BL/6 mice (Thy1.2 and H-2kb) and 5 × 105 CD25− Tcons (CD4+CD8+CD25− T cells) from congenic Thy1.1 C57BL/c mice (Thy1.1 and H-2kb) after lethal irradiation with 800 cGy on day 0. Mice received either PBS alone, RAPA, IL-2 or a combination of RAPA + IL-2 from day 0 to day 7 after BMT (5 mice per group). Cells were reisolated from mLNs, pLNs, and spleen and analyzed by flow cytometry after staining with CD4, CD25, and intracellular Foxp3 on day 7 after BMT. Cells from each lymphoid organ were stained with (A) anti-CD4 and anti-Foxp3 intracellular staining, and (B) anti-CD4 and anti-CD25; data shown are gated on H-2kb+ lymphocytes. These data are representative of 3 separate experiments. The combination of RAPA + IL-2 increased the expansion of donor-type CD4+Foxp3+ T cells and CD4+CD25+ T cells, and reduced donor-type CD4+CD25− T cells from donor-type Tregs and CD25 depleted Tcons. The graphs compare the proportion of (C) CD4+Foxp3+ cells, (D) CD4+CD25+ T cells, and (E) CD4+CD25− T cells from peripheral LNs in each group (PBS alone, ●; IL-2, ■; RAPA, ▴; RAPA + IL-2, ▾). Absolute cell number of CD4+Foxp3+ T cells and CD4+CD25− T cells in pLNs, mLNs cells and splenocytes from each treatment group are shown (F-H). Data are derived from 3 separate experiments. Results are displayed as the mean ± SD. *P < .05.
Combination of RAPA + IL-2 has additive effects on the expansion of CD4+Foxp3+ and CD4+CD25+ cells after infusion of donor Tregs and CD25 depleted Tcons. BALB/c mice (Thy1.2 and H-2kd) were injected with 5 × 106 TCD-BM cells from GFP transgenic C57BL/6 mice (Thy1.1 and H-2kb), 5 × 105 Treg from wild-type C57BL/6 mice (Thy1.2 and H-2kb) and 5 × 105 CD25− Tcons (CD4+CD8+CD25− T cells) from congenic Thy1.1 C57BL/c mice (Thy1.1 and H-2kb) after lethal irradiation with 800 cGy on day 0. Mice received either PBS alone, RAPA, IL-2 or a combination of RAPA + IL-2 from day 0 to day 7 after BMT (5 mice per group). Cells were reisolated from mLNs, pLNs, and spleen and analyzed by flow cytometry after staining with CD4, CD25, and intracellular Foxp3 on day 7 after BMT. Cells from each lymphoid organ were stained with (A) anti-CD4 and anti-Foxp3 intracellular staining, and (B) anti-CD4 and anti-CD25; data shown are gated on H-2kb+ lymphocytes. These data are representative of 3 separate experiments. The combination of RAPA + IL-2 increased the expansion of donor-type CD4+Foxp3+ T cells and CD4+CD25+ T cells, and reduced donor-type CD4+CD25− T cells from donor-type Tregs and CD25 depleted Tcons. The graphs compare the proportion of (C) CD4+Foxp3+ cells, (D) CD4+CD25+ T cells, and (E) CD4+CD25− T cells from peripheral LNs in each group (PBS alone, ●; IL-2, ■; RAPA, ▴; RAPA + IL-2, ▾). Absolute cell number of CD4+Foxp3+ T cells and CD4+CD25− T cells in pLNs, mLNs cells and splenocytes from each treatment group are shown (F-H). Data are derived from 3 separate experiments. Results are displayed as the mean ± SD. *P < .05.
The absolute number of CD4+FoxP3+ and CD4+CD25–T cells was evaluated in recipient tissues (pLNs, mLNs, and spleen) 7 days after BMT (Figure 2F-H). RAPA alone resulted in an increase in the number of total CD4+Foxp3+ T cells in mLNs and spleen and reduced CD4+CD25− T cells in pLNs and spleen. The combination of RAPA plus IL-2 resulted in a further increase in the number of CD4+Foxp3+ T cells in mLNs and spleen and a reduction in CD4+CD25− T cells in pLNs and spleen. However, the differences did not reach statistical significance.
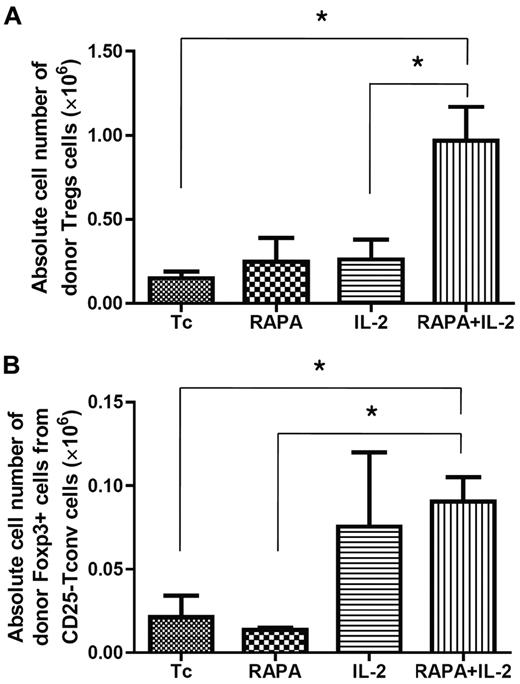
To investigate the origin of donor-type CD4+Foxp3+ cells, BALB/c mice were injected with CD4+CD25high Tregs from wild-type C57BL/6 mice and CD25-depleted Tcons from congenic Thy1.1 C57BL/c mice at the same time after lethal irradiation on day 0. To distinguish between the infused CD4+CD25high Tregs and the BM derived Tregs, TCD-BM was isolated from GFP transgenic mice. The animals received PBS, RAPA, IL-2, and RAPA plus IL-2 from day 0 to 7 after BMT. Donor-type CD4+Foxp3+ Tregs were reisolated from each organ and analyzed by flow cytometry on day 7 after BMT. The combination of RAPA plus IL-2 resulted in an increase in both the number of CD4+Foxp3+ Tregs from donor CD4+CD25high Tregs (Figure 3A) and the number of converted Tregs from donor CD25-depleted Tcons (Figure 3B). In contrast, treatment with IL-2 alone induced an increase only in the number of Tregs derived from donor CD25-depleted Tcons (Figure 3B).
Origin of CD4+Foxp3+cells. BALB/c mice (Thy1.2 and H-2kd) were injected with 5 × 106 TCD-BM cells from GFP transgenic C57BL/6 mice (Thy1.1 and H2-kb), 5 × 105 Treg from wild-type C57BL/6 mice (Thy1.2 and H-2kb) and 5 × 105 CD25-depleted Tcons (CD4+CD8+CD25− T cells) from congenic Thy1.1 C57BL/c mice (Thy1.1 and H2-kb) after lethal irradiation with 800 cGy on day 0. Mice of each group received PBS alone, RAPA, IL-2, or a combination of RAPA + IL-2 from day 0 to day 7 after BMT. Cells were reisolated from mLNs, pLNs, and spleen on day 7 after BMT and analyzed by flow cytometry. The combination of RAPA + IL-2 had a synergistic effect resulting in an increase in the frequency and absolute number of adoptively transferred donor-type Tregs in each lymphoid organ. The absolute cell number of CD4+Foxp3+ cells by expansion of adoptively transferred donor CD4+CD25highFoxP3+ Tregs (A) and by increased conversion from CD25− Tcons (B) in each treatment group. Results were shown as mean ± SD using horizontal column bar graphs. Data are derived from 3 independent experiments. *P < .05.
Origin of CD4+Foxp3+cells. BALB/c mice (Thy1.2 and H-2kd) were injected with 5 × 106 TCD-BM cells from GFP transgenic C57BL/6 mice (Thy1.1 and H2-kb), 5 × 105 Treg from wild-type C57BL/6 mice (Thy1.2 and H-2kb) and 5 × 105 CD25-depleted Tcons (CD4+CD8+CD25− T cells) from congenic Thy1.1 C57BL/c mice (Thy1.1 and H2-kb) after lethal irradiation with 800 cGy on day 0. Mice of each group received PBS alone, RAPA, IL-2, or a combination of RAPA + IL-2 from day 0 to day 7 after BMT. Cells were reisolated from mLNs, pLNs, and spleen on day 7 after BMT and analyzed by flow cytometry. The combination of RAPA + IL-2 had a synergistic effect resulting in an increase in the frequency and absolute number of adoptively transferred donor-type Tregs in each lymphoid organ. The absolute cell number of CD4+Foxp3+ cells by expansion of adoptively transferred donor CD4+CD25highFoxP3+ Tregs (A) and by increased conversion from CD25− Tcons (B) in each treatment group. Results were shown as mean ± SD using horizontal column bar graphs. Data are derived from 3 independent experiments. *P < .05.
Reduced production of IFN-γ and TNF-α in the mice receiving RAPA plus IL-2
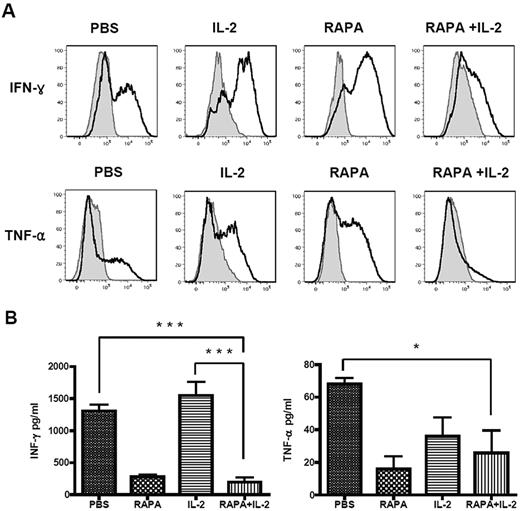
A range of cytokines and chemokines were assayed within serum and by intracellular cytokine analysis of T cells in the mice receiving PBS, RAPA, IL-2, or RAPA plus IL-2 at day 7 after transplantation. We were particularly interested in cytokines that have a known role in the induction of GVHD such as IFN-γ and TNF-α. In control animals there was abundant IFN-γ and TNF-α in serum and produced by splenocytes isolated 7 days after transplantation (Figure 4A-B). Treatment with IL-2 alone resulted in slightly increased production of IFN-γ whereas TNF-α production remained high. RAPA alone resulted in reduction in the intracellular production of IFN-γ and TNF-α by splenocytes at day 7 after transplantation and a reduction in the concentration of these cytokines in the serum of animals at this timepoint. RAPA plus IL-2 resulted in the greatest reduction in the production of both IFN-γ and TNF-α assessed by intracellular cytokine production and reduction in the concentration of both of these cytokines in serum of animals (Figure 4A-B)
Combination of RAPA plus IL-2 reduced production of IFN-γ and TNF-α. BALB/c mice (Thy1.2 and H-2kd) were injected with 5 × 106 TCD-BM cells from GFP transgenic C57BL/6 mice (Thy1.1 and H2-kb), 5 × 105 Tregs from wild-type C57BL/6 mice (Thy1.2 and H-2kb) and 5 × 105 CD25-depleted Tcons (CD4+CD8+CD25− T cells) from congenic Thy1.1 C57BL/c mice (Thy1.1 and H2-kb) after lethal irradiation with 800 cGy on day 0. Mice of each group received PBS, RAPA or IL-2 alone, or a combination of RAPA + IL-2 from day 0 to day 7 after BMT. Intracellular cytokines (A) were determined from splenocytes of each mice group, and serum was collected and cytokines (B) were analyzed in a multiplex assay system on day 7 after BMT. RAPA plus IL-2 resulted in the greatest reduction in the production of both IFN-γ and TNF-α assessed by intracellular cytokine production and reduction in the concentration of both of these cytokines in serum of animals. Results were shown as mean ± SD using horizontal column bar graphs (5 mice were included at each group). *P < .05, *** P < .001.
Combination of RAPA plus IL-2 reduced production of IFN-γ and TNF-α. BALB/c mice (Thy1.2 and H-2kd) were injected with 5 × 106 TCD-BM cells from GFP transgenic C57BL/6 mice (Thy1.1 and H2-kb), 5 × 105 Tregs from wild-type C57BL/6 mice (Thy1.2 and H-2kb) and 5 × 105 CD25-depleted Tcons (CD4+CD8+CD25− T cells) from congenic Thy1.1 C57BL/c mice (Thy1.1 and H2-kb) after lethal irradiation with 800 cGy on day 0. Mice of each group received PBS, RAPA or IL-2 alone, or a combination of RAPA + IL-2 from day 0 to day 7 after BMT. Intracellular cytokines (A) were determined from splenocytes of each mice group, and serum was collected and cytokines (B) were analyzed in a multiplex assay system on day 7 after BMT. RAPA plus IL-2 resulted in the greatest reduction in the production of both IFN-γ and TNF-α assessed by intracellular cytokine production and reduction in the concentration of both of these cytokines in serum of animals. Results were shown as mean ± SD using horizontal column bar graphs (5 mice were included at each group). *P < .05, *** P < .001.
The combination of RAPA plus IL-2 had a protective effect against acute GVHD-related morbidity and mortality by reducing alloreactive T-cell expansion in vivo
To investigate whether the combination of RAPA plus IL-2 impacted the incidence and severity of acute GVHD and survival after transplantation groups of mice were treated with PBS, RAPA, IL-2 alone and the combination of RAPA plus IL-2 after donor luc+ Tcon infusion on day 0. The acute GVHD score was reduced significantly in mice receiving RAPA plus IL-2 compared with those receiving RAPA (P < .01) or IL-2 alone (P < .01) or PBS alone (all of the animals died within 10-14 days; P = .07), respectively (Figure 5A). To evaluate the impact of these different drug treatments on the proliferation of donor-derived T cells total body photons/second/animal were evaluated by BLI. The combination of RAPA and IL-2 resulted in decreased T-cell proliferation compared with PBS (P = .01), RAPA (P = .15) or IL-2 alone (P < .01) over the initial 30 days after transplantation (Figure 5B). Images of representative animals at days 4, 9 and 21 after transplantation are shown (Figure 5C). RAPA alone improved overall survival compared with PBS or IL-2 alone, and the addition of IL-2 to RAPA further enhanced overall survival indicating that the combination of RAPA plus IL-2 resulted in the best survival compared with RAPA or IL-2 alone (RAPA plus IL-2 vs PBS, P < .0001; RAPA plus IL-2 vs RAPA, P = .13; RAPA plus IL-2 vs IL-2, P < .01; Figure 5D).
Combination of RAPA and IL-2 protects animals from acute GVHD by reducing alloreactive T-cell expansion in vivo. BALB/c mice were treated with irradiation alone (800 cGy; ●) or injected with 5 × 106 TCD-BM cells alone (■) or together with 1 × 106luc+ Tcons after lethal irradiation. Each group of mice received PBS (▴), RAPA (▾), IL-2 alone (♦) or a combination of RAPA plus IL-2 (○). Treatment of RAPA was continued until 14 days after BMT and IL-2 was administrated intraperitoneally before BMT and then twice a day thereafter for 3 days. Mice receiving the combination of RAPA + IL-2 had lower acute GVHD score than those receiving RAPA or IL-2 alone (A). (B) Expansion of luciferase-expressing T cells as quantified by total emitted photons per mouse at serial time points after BMT. Signal intensity is significantly lower in mice receiving a combination of RAPA + IL-2 compared with PBS (P < .05) or IL-2 alone (P < .01). (C) Single time points showing the expansion of luc+ donor T cells in BALB/c mice in the different groups. T-cell expansion was reduced in mice receiving the combination of RAPA + IL-2 compared with RAPA alone at day 21 after BMT or IL-2 alone at day 9 and day 21 after BMT. (D) Survival of mice in the different groups (n = 15 per group). Lethal acute GVHD induced by C57BL/6 Tcons in BALB/c recipients was reduced in animals treated with the combination of RAPA + IL-2 (○ versus ▴, P < .0001; ○ versus ♦, P = .007). Error bars represent SEM.
Combination of RAPA and IL-2 protects animals from acute GVHD by reducing alloreactive T-cell expansion in vivo. BALB/c mice were treated with irradiation alone (800 cGy; ●) or injected with 5 × 106 TCD-BM cells alone (■) or together with 1 × 106luc+ Tcons after lethal irradiation. Each group of mice received PBS (▴), RAPA (▾), IL-2 alone (♦) or a combination of RAPA plus IL-2 (○). Treatment of RAPA was continued until 14 days after BMT and IL-2 was administrated intraperitoneally before BMT and then twice a day thereafter for 3 days. Mice receiving the combination of RAPA + IL-2 had lower acute GVHD score than those receiving RAPA or IL-2 alone (A). (B) Expansion of luciferase-expressing T cells as quantified by total emitted photons per mouse at serial time points after BMT. Signal intensity is significantly lower in mice receiving a combination of RAPA + IL-2 compared with PBS (P < .05) or IL-2 alone (P < .01). (C) Single time points showing the expansion of luc+ donor T cells in BALB/c mice in the different groups. T-cell expansion was reduced in mice receiving the combination of RAPA + IL-2 compared with RAPA alone at day 21 after BMT or IL-2 alone at day 9 and day 21 after BMT. (D) Survival of mice in the different groups (n = 15 per group). Lethal acute GVHD induced by C57BL/6 Tcons in BALB/c recipients was reduced in animals treated with the combination of RAPA + IL-2 (○ versus ▴, P < .0001; ○ versus ♦, P = .007). Error bars represent SEM.
Discussion
Prior studies from our laboratory and others have demonstrated that conventional CD4+ T cells and CD4+CD25+ Tregs use different signaling pathways after IL-2 stimulation where conventional CD4+CD25− T cells primarily signal through the mTOR pathway whereas Tregs use stat5-mediated signaling pathways.29-31 Further, it is well-recognized that Tregs require IL-2 for activation and stimulation yet do not produce this cytokine. Therefore, we hypothesized that stimulation of T-cell proliferation with IL-2 in the presence of an mTOR inhibitor such as RAPA could preferentially enhance Treg expansion. In the current study, we tested this hypothesis by treating recipient animals with either PBS alone, RAPA, IL-2, or the combination of RAPA plus IL-2 after allogeneic transplantation in a well-established major-MHC mismatch model of BMT. We demonstrated that the in vivo administration of RAPA plus IL-2 had additive effects on the expansion of donor derived CD4+CD25+ and CD4+Foxp3+ Treg populations, and inhibited the proliferation of effector donor CD4+ T cells that are known to induce acute GVHD. In circumstances of concomitant infusion of both donor CD4+CD25high Treg and CD25-depleted Tcon cells the combination of RAPA plus IL-2 as well as RAPA alone also increased the frequency of donor CD4+Foxp3+ Tregs. The combination of RAPA plus IL-2 was more effective and resulted in ∼ 3 times more CD4+Foxp3+ Tregs as control animals. RAPA plus IL-2 as well as IL-2 alone reduced the frequency of CD4+CD25− effector Tcon cells. Further, the combination of RAPA plus IL-2 and RAPA alone expanded the Treg population in mLNs and spleen. Although it is not clear why the effects of RAPA plus IL-2 were more pronounced in mLNs compared with pLNs, it is speculated that Treg cells induced by RAPA plus IL-2 were primed in the mLN located near the gut that was inflamed because of the preparative regimen or the acute GVHD reaction. The combination of RAPA plus IL-2 resulted in potent suppression of effector Tcon cell proliferation in almost all tissues including mLNs, pLNs, and spleen.
The effects of IL-2 on Tregs are mediated by activation of the Janus/STAT pathway, whereas RAPA-sensitive downstream targets of PI3K are not activated.10 Therefore, Kopf et al suggested that RAPA did not block IL-2 signaling leading to the de novo generation of Tregs from naive CD4 cells because TGF-β–induced expression of Foxp3 was not inhibited by this compound.32 On the contrary, CD4+CD25− Tcons responding to TCR and IL-2 stimulation signals via the PI3K pathway are sensitive to RAPA-induced inhibition of proliferation. In the current study, the combination of RAPA plus IL-2 had potent effects on Treg expansion and reduced effector CD4+ T cells. We hypothesize that exogenous IL-2 induced Treg proliferation via the stat5 pathway while RAPA inhibited effector Tcon proliferation via the PI3K/Akt/mTOR pathway.
There are 2 different Treg subsets, namely natural Tregs (nTregs) that are released from the thymus and induced Tregs (iTregs) that are converted in peripheral lymphoid tissues by the activation of mature CD4+ T cells via specific antigenic and cytokine stimulation.33 nTreg and iTreg cells are indistinguishable by their cell surface markers,34 but these 2 populations differ predominantly in their stability and potency of immunosuppression. nTreg cells are more stable and potent than iTreg cells in maintaining self-tolerance and preventing autoimmunity.35 In mice, the regulatory regions of the Foxp3 gene are more widely demethylated in nTregs than in TGF-β–induced iTregs, suggesting functional instability of the latter cell population. In addition, unlike mice, naive T cells in humans readily express Foxp3 on TCR stimulation, although the expression is generally much lower and more transient than in nTregs.36 There are at least 2 mechanisms to increase the adoptively transferred donor Treg cell population. One is an expansion of donor nTregs that are contained within the adoptively transferred donor Tcon cells. The other is an increased conversion of Foxp3+ iTregs from CD4+CD25− Tcons. In the current study, we demonstrated that RAPA plus IL-2 resulted in an increase in donor-derived CD4+FoxP3+ Treg populations via both the expansion of donor CD4+CD25highFoxp3+ nTregs as well as increased conversion of CD4+Foxp3+ iTregs from CD4+CD25− donor Tcons. The expansion of donor nTregs was most remarkable in the mice treated with the combination of RAPA plus IL-2 compared with RAPA or IL-2 alone. Interestingly, IL-2 alone resulted in an increase in the conversion of iTregs from donor CD25− Tcon cells while this cytokine did not expand donor nTregs. It has been known that IL-2 signaling is required to maintain Treg cells in vivo37 and IL-2 and TGF-β play a central role in the maintenance of nTreg cells and in the conversion of iTreg cells in vitro. Moreover, IL-2 plays a critical role in the maintenance and increased frequency of Foxp3 expression of Tregs in vivo.29,38 This study suggests that RAPA selectively promotes expansion of Tregs by inducing cell death of contaminating CD4+CD25− Tcons. In vitro study with human peripheral blood demonstrated Tregs cultured in the presence of RAPA plus IL-2 had regulatory function.39
The exact mechanism through which the combination of RAPA plus IL-2 results in Treg expansion is not known with certainty and whether suppression of CD4+CD25− donor Tcons resulted from direct inhibition by expanded donor Tregs by this combination. Further studies using selective depletion of Foxp3 expressing Tregs is in progress to address this issue. Nevertheless, RAPA plus IL-2 down-regulated IFN-γ and TNF-α production that are proinflammatory cytokines that play important roles in the GVHD pathophysiology.40 RAPA leads to significant reduction of IL-12–induced IFN-γ expression by dendritic cells (DCs), the most potent antigen-presenting cells, which play a critical role in the initiation and regulation of acute GVHD.41 RAPA also is known to interfere with DC function, impairing immune reactivity at both the immature and mature stages. Moreover, mature DCs after RAPA treatment exert some immunosuppressive effects on allogeneic T lymphocytes.42 These effects are other possible mechanism(s) that RAPA itself could decrease acute GVHD. In addition, the increased benefit of adding IL-2 to RAPA suggests that the expansion of Tregs by this combination may also play an important role in reducing acute GVHD lethality.
CD4+CD25high nTreg cells have been shown to have remarkable functional activity in several different model systems where they are capable of reducing the severity of GVHD, autoimmune disease, and induce tolerance of solid organ transplants. Therefore, there is great interest in the clinical application of Treg biology and several clinical trials have begun with supportive initial findings.43,44 A significant challenge for the use of nTregs in the clinic is the difficulty in isolating sufficient numbers of Tregs for clinical application that are present in very low numbers (∼ 2%-10%) of the peripheral CD4+ T cells in humans.45,46 There have been several in vitro methods to expand nTreg cells using anti-CD3 and anti-CD28 monoclonal antibodies and IL-2 together with TGF-β, DCs, and RAPA. However, these methods are challenging to apply for human clinical trials because of several issues including that nonTreg cells might be expanded during in vitro cell cultures, that the expanded cells may have different homing and survival properties as well as the regulatory requirements. Nonetheless, clinical trials have been initiated with expanded Tregs in the setting of umbilical cord blood transplantation.47 Therefore, in vivo expansion of nTregs or conversion of iTregs is another alternative option to expand sufficient donor derived Treg cells after HCT to prevent acute GVHD or possibly in other clinical settings. Several studies aimed at the in vivo expansion of Tregs have been published; however, the expansion of Tregs by RAPA alone has been somewhat disappointing24,48,49 ; one study showed affirmative findings.50 While RAPA alone can reduce GVHD by interfering with antigen-presenting cells or secretion of proinflammtory cytokines including IL-12 and IFN- γ, or by expansion of Tregs, the combination of RAPA plus IL-2 may be more effective in the prevention or suppression of autoimmune disorders and acute GVHD by in vivo expansion of nTregs and inhibition of effector CD4+CD25− T cells. However, in our model of GVHD further experimentation is required to determine the precise impact of Treg expansion after RAPA plus IL-2 on GVHD reduction and whether other or alternative mechanisms are causing the observed improvement in survival we have observed. These studies will have important implications for the treatment of patients to prevent acute GVHD or possibly treat chronic GVHD.51
In conclusion, we have shown that the combination of RAPA plus IL-2 was effective in expanding donor-derived Tregs from both expansion of nTreg populations and an enhanced conversion of iTregs in vivo. While RAPA alone also reduces acute GVHD by several potential mechanisms, the addition of IL-2 to RAPA reduced the expansion of alloreactive Tcon cells, which resulted in reduced GVHD-related mortality. These results are readily translatable to the clinic in future clinical trials in an effort to expand Treg populations preferentially in clinical settings where expansion of Tregs could result in clinical use such as for the prevention or treatment of GVHD, autoimmunity, or solid organ tolerance induction.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: H.S. designed the study and, with D.B.L., E.I.S., and J.B., performed the research, analyzed the data and wrote the manuscript; A.T.S. took care the experiment animals; and R.S.N. interpreted the data and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Robert S. Negrin, MD, Clinical Sciences Research Bldg, Rm 2205, 269 Campus Dr, Stanford, CA 94305; e-mail: negrs@stanford.edu.
References
Author notes
H.-J.S. and J.B. contributed equally to this article.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal