Abstract
We evaluated a novel alemtuzumab-based conditioning regimen in HSCT for acquired severe aplastic anemia (SAA). In a multicenter retrospective study, 50 patients received transplants from matched sibling donors (MSD; n = 21) and unrelated donors (UD; n = 29), using fludarabine 30 mg/m2 for 4 days, cyclophosphamide 300 mg/m2 for 4 days, and alemtuzumab median total dose of 60 mg (range:40-100 mg). Median age was 35 years (range 8-62). Overall survival at 2 years was 95% ± 5% for MSD and 83% for UD HSCT (p 0.34). Cumulative incidence of graft failure was 9.5% for MSD and 14.5% for UD HSCT. Full-donor chimerism (FDC) in unfractionated peripheral blood was 42%; no patient achieved CD3 FDC. Acute GVHD was observed in only 13.5% patients (all grade I-II) and only 2 patients (4%) developed chronic GVHD. A low incidence of viral infections was seen. Factors influencing overall survival were HSCT comorbidity 2-year index (92% with score 0-1 vs 42% with score ≥ 2, P < .001) and age (92% for age < 50 years vs 71% ≥ 50 years, P < .001). Our data suggest that the use of an alemtuzumab-based HSCT regimen for SAA results in durable engraftment with a low incidence of chronic GVHD.
Introduction
Allogeneic HSCT offers the chance of long-term cure for patients with severe aplastic anemia (SAA),1-5 but chronic GVHD represents a current major challenge that impacts on quality of life as well as survival.6 The ideal conditioning regimen for SAA is one that results in sustained engraftment, minimal regimen-related toxicity, and absence of both acute and chronic GVHD. There is no advantage for any degree of chronic GVHD in AA, in contrast to the beneficial impact of GVL effect on HSCT for myeloid malignancies.
Over the past few decades, survival after HSCT for acquired SAA has improved greatly. HLA-matched sibling donor (MSD) HSCT results in long-term survival in at least 80% of patients.1,2,4,5,7,8 The standard of care conditioning for young patients undergoing MSD BM transplantation (BMT) for AA is high-dose cyclophosphamide (CY) 200 mg/kg and ATG, with cyclosporin (CSA) and methotrexate (MTX) as post-graft immunosuppression. Long-term survival occurs in 80%-90% of patients, although this is age-dependent, with only 50% survival above the age of 40-50 years.3,9,10 Graft rejection occurs in 5%-10%, and acute GVHD in 12%-30%. Notably, chronic GVHD remains a major problem for 30%-40% of patients. In addition, incremental impact of increasing age is observed on the cumulative incidence of acute and chronic GVHD.3 Unrelated donor (UD) HSCT had previously been reserved for those patients who fail to respond to 2 courses of immunosuppressive therapy (IST), but outcomes after UD HSCT have improved significantly during the past decade.11-15 A prospective pediatric study from Japan comparing repeat course of IST with UD HSCT showed benefit in favor of early UD HSCT in AA.16 UD HSCT is now considered after failing 1 course of IST.17,18 Improved outcomes are likely because of improved HLA-matching techniques, better supportive care, and improved conditioning regimens. For example, the use of fludarabine, low-dose CY, and ATG (FCATG) conditioning regimen for young patients, and FCATG with low-dose (2 Gy) total body irradiation (TBI) for older patients, results in 75% and 79% overall survival (OS) at 5 years, respectively. Graft rejection occurs in 17% of patients. Although acute GVHD grade II-IV occurs in only 13% of patients, chronic GVHD is observed in 27% of young patients and 50% in the older age group.12
We have previously evaluated the CD52 mAbs Campath-1G and the humanized Ab Campath 1-H (alemtuzumab), in pilot studies for MSD and UD HSCT for SAA.19-23 The rationale was to develop a conditioning regimen that provided good anti-GVHD prophylaxis with the addition of Campath to CSA, without the necessity for methotrexate, thereby reducing hepatotoxicity and mucositis. At the same time, to achieve long-term hematopoietic engraftment, additional immunosuppression would be provided by the addition of CSA. In the early studies, high-dose CY was used with Campath and CSA for MSD HSCT and demonstrated good survival, with a low risk of acute and chronic GVHD. A high incidence of graft failure was associated with the use of Campath given both pre- and peritransplantation, and reduced when given only pretransplantation.20 For UD HSCT, alemtuzumab was used with low-dose CY (300 mg/m2 × 4) and fludarabine.21
In this retrospective multicenter study from 5 centers, we have analyzed outcomes of 50 patients with acquired SAA who have been transplanted with a minimal-intensity conditioning regimen using alemtuzumab with fludarabine and CY 300 mg/m2 × 4 fludarabine cyclophosphamide campath (FCC). We confirm that the use of alemtuzumab in this setting is the first reported maneuver to result in a much lower incidence of chronic GVHD compared with previously reported conditioning regimens used in SAA. We also show that this regimen is associated with a low incidence of infections, low toxicity, sustained engraftment, and excellent long-term survival. In addition, we show that survival after UD HSCT is not significantly different from MSD HSCT.
Methods
Patients
From 1999 to 2009, 50 adults and children with acquired SAA received transplantations at 5 centers (King's College Hospital, London; St George's Hospital, London; Bristol; Nottingham; and Princess Margaret Hospital, Toronto). The diagnosis of aplastic anemia and assessment of disease severity were established according to published criteria.24-26 Fanconi anemia was excluded in all cases by chromosome breakage studies on cultured PBLs with diepoxybutane. Eight patients included in this study were previously reported.21,23
Patient characteristics are shown in Table 1. The median follow-up of patients was 18.2 months (range: 2.3-118.2). Twenty-one patients received transplantations from an HLA MSD, and 29 from an UD. UD HSCT was performed after failure to respond to at least 1 course of immunosuppressive therapy. All donor-recipient pairs were matched for HLA-A, -B, -C, -DRB1, and DQB1 using high-resolution DNA typing, apart from 2 patients who received a 9 of 10 Ag-matched UD. Each local institutional research ethics committee approved the study and informed consent was obtained from all participating patients in accordance with the Declaration of Helsinki.
Patient characteristics
| Characteristic . | No. . | % . |
|---|---|---|
| Median age, y (range) | 35 (8-62) | |
| Patients younger than 50 | 38 | 76 |
| Patients 50 or older | 12 | 24 |
| Sex | ||
| Male | 26 | 52 |
| Female | 24 | 48 |
| Disease severity | ||
| Very severe AA | 15 | 30 |
| Severe AA | 25 | 50 |
| Nonsevere AA | 10 | 20 |
| AA aetiology | ||
| Idiopathic | 39 | 78 |
| Drug-induced | 5 | 10 |
| Hepatitis | 4 | 8 |
| Pregnancy | 2 | 4 |
| Median interval Dx-HSCT, mo (range) | ||
| SIB | 6 (2-252) | |
| UD | 10 (4-137) | |
| Prior ATG treatment | ||
| Yes | 33 | 66 |
| No | 14 | 28 |
| Unknown | 3 | 6 |
| Donor type | ||
| Matched sibling | 21 | 42 |
| Unrelated | 29 | 58 |
| Stem cell source | ||
| BM | 24 | 48 |
| G-BM | 7 | 14 |
| PBSC | 14 | 27 |
| BM + PBSC | 5 | 10 |
| Transplantation period | ||
| 1999-2004 | 6 | 12 |
| 2005-2010 | 44 | 88 |
| Sorror score | ||
| 0-1 | 36 | 72 |
| 2 or more | 6 | 12 |
| Unknown | 8 | 16 |
| PNH clone at time of HSCT | 9/34 | 26 |
| CMV status | ||
| Donor+/recipient+ | 14 | 28 |
| Donor-/recipient- | 24 | 48 |
| Donor+/recipient- | 6 | 12 |
| Donor-/recipient+ | 6 | 12 |
| Total alemtuzumab dose | ||
| > 60 mg | 20 | 40 |
| ≤ 60 mg | 30 | 60 |
| Characteristic . | No. . | % . |
|---|---|---|
| Median age, y (range) | 35 (8-62) | |
| Patients younger than 50 | 38 | 76 |
| Patients 50 or older | 12 | 24 |
| Sex | ||
| Male | 26 | 52 |
| Female | 24 | 48 |
| Disease severity | ||
| Very severe AA | 15 | 30 |
| Severe AA | 25 | 50 |
| Nonsevere AA | 10 | 20 |
| AA aetiology | ||
| Idiopathic | 39 | 78 |
| Drug-induced | 5 | 10 |
| Hepatitis | 4 | 8 |
| Pregnancy | 2 | 4 |
| Median interval Dx-HSCT, mo (range) | ||
| SIB | 6 (2-252) | |
| UD | 10 (4-137) | |
| Prior ATG treatment | ||
| Yes | 33 | 66 |
| No | 14 | 28 |
| Unknown | 3 | 6 |
| Donor type | ||
| Matched sibling | 21 | 42 |
| Unrelated | 29 | 58 |
| Stem cell source | ||
| BM | 24 | 48 |
| G-BM | 7 | 14 |
| PBSC | 14 | 27 |
| BM + PBSC | 5 | 10 |
| Transplantation period | ||
| 1999-2004 | 6 | 12 |
| 2005-2010 | 44 | 88 |
| Sorror score | ||
| 0-1 | 36 | 72 |
| 2 or more | 6 | 12 |
| Unknown | 8 | 16 |
| PNH clone at time of HSCT | 9/34 | 26 |
| CMV status | ||
| Donor+/recipient+ | 14 | 28 |
| Donor-/recipient- | 24 | 48 |
| Donor+/recipient- | 6 | 12 |
| Donor-/recipient+ | 6 | 12 |
| Total alemtuzumab dose | ||
| > 60 mg | 20 | 40 |
| ≤ 60 mg | 30 | 60 |
Study protocol
The transplantation conditioning regimen used (FCC) comprised fludarabine 30 mg/m2 IV daily for 4 days given from day −7 to −4, CY 300 mg/m2 IV daily for 4 days on day −7 to −4. Alemtuzumab (Genzyme Therapeutics) was administered IV/SC pre-HSCT at a total dose of 40-100 mg (median 60 mg) between days −7 and −3: 100 mg (n = 9), 75 mg (n = 11), 60 mg (n = 21), 50 mg (n = 8), and 40 mg (n = 1). Each center used their approved regimen for alemtuzumab (see supplemental Data for each institutional protocol, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Stem cell source was BM in 24 (49%), G-CSF–primed BM in 7 (14%), PBSC in 14 (27%), and BM + PBSC in 5 (10%) of patients. Posttransplantation GVHD prophylaxis was achieved with CSA (2.5 mg/kg IV; BD Biosciences) from day −1–titrated to plasma trough levels of 200-300 ng/mL. Oral CSA was substituted when a good oral intake was achieved, and continued for 12 months with tapering at 9 months only in the presence of stable hematologic parameters and without declining mixed donor chimerism. Acute and chronic GVHD were graded using standard criteria.27
Supportive care
Study definitions
Neutrophil engraftment was defined as the first of 3 consecutive days with an absolute neutrophil count (ANC) ≥ 0.5 × 109/L unsupported by G-CSF. Platelet engraftment was defined as the first of 3 consecutive days with a platelet count ≥ 20 × 109/L without platelet transfusion support for 7 preceding days. Patients were evaluable for engraftment if they survived > 21 days after transplantation. Primary graft failure was defined as the absence of neutrophil count ≥ 0.5 × 109/L on 3 consecutive days and late graft failure as recovery followed by recurrent pancytopenia with a hypocellular BM in the absence of severe GVHD. Platelet refractoriness was defined as a platelet count increment of < 10 × 109/L at 1 hour following random donor platelet transfusion, in the absence of fever or bleeding. GVHD was diagnosed on clinical grounds with histopathologic biopsy where possible and graded according to published criteria. The HSCT comorbidity index (HSCT-CI) was used to assign a pretransplantation comorbidity score to patients.30
Analysis of chimerism
Chimerism assessments were scheduled for days 30, 60, and 100; 6 months; and then yearly. Chimerism was assessed in unfractionated BM samples as well as fractionated peripheral blood CD3+ T-cell and CD15+ granulocyte populations whenever possible.31 Thirty-nine patients had serial unfractionated BM chimerism samples available for interpretation, and 21 patients had additional peripheral blood CD3+ chimerism data.
Full donor chimerism (FDC) was defined as the presence of > 95% donor hematopoietic cells, while mixed donor chimerism (MDC) was defined as 5%-95% donor cells posttransplantation.32 Progressive mixed chimerism was defined as ≥ 15% increase in recipient cells over 3 months and stable mixed chimerism as < 5% fluctuation in the percentage of recipient cells over time. Patients who showed mixed donor-recipient chimerism but who subsequently reverted to full donor chimerism were classified as having transient mixed chimerism.33 Declining donor chimerism was defined as a 10% or greater increase in recipient chimerism on 2 consecutive measurements.
Statistical analysis
Overall survival (OS) was measured from day 0 to death from any cause or last known follow-up. Failure-free survival (FFS) was defined as survival with sustained engraftment, death, and graft failure were categorized as treatment failure. FFS was measured from day 0 to the first indicator of graft failure, death of any cause, or last known follow-up. Univariate comparisons and multivariate analysis used the Cox proportional hazards regression model. Variables analyzed included: recipient age (≥ or < 50 years), severity of AA (nonsevere vs severe vs very severe), donor status (MSD vs UD), stem cell source, HSCT-CI (0-1 vs ≥ 2), and time from diagnosis to HSCT (≥ or < 12 months). All data were censored as of the March 5, 2010.
Results
Patient characteristics
Table 1 summarizes the patient characteristics. Of the 50 patients, 29 received transplantations using an UD and 21 a MSD. The median age was 35 years (range 8-62), of whom 38 patients (76%) were aged younger than 50 years and 12 (24%) 50 years or older at time of HSCT. Eighty-eight percent (44/50) of transplants were performed after 2005. The median interval from diagnosis to HSCT was 6 months (range 2-252) for MSD and 10 months (range 4-137) for UD (P = .75). A paroxysnal nocturnal hemoglobinuria (PNH) clone was detected by flow cytometry, with or without fluorescently labeled inactive toxin aerolysin (FLAER), in 9 (18%) patients at time of SCT. The median size (and range) of the clone was 0.07% (0%-3.1%) for red cells, 0.4% (0%-97.1%) for granulocytes and 2% (0%-52.8%) for monocytes. In 2 patients, the size of the granulocytic clone was > 10%.
Graft failure
The median time to neutrophil recovery was 17 days (range: 2-50) and platelet recovery was 19 days (range: 10-57). Graft failure occurred in 6 patients (12%), of whom 3 had early graft failure following UD HSCT and 3 had late graft failure on days +32, 86, and 126, 2 after MSD and 1 after UD HSCT. All patients had been on immunosuppressive therapy at time of graft failure. Of these 6 patients, 4 had received BM, 1 G-BM and 1 PBSCs. The median cell dose (and range) was 1.68 CD34 × 106/kg (1.2-6.35) compared with 3.19 CD34 × 106/kg (1.10-7.83) for patients who had sustained engraftment (P = .24). Two patients died of complications related to graft failure, 2 patients had autologous reconstitution following graft failure with improvement in hematologic parameters, and 2 patients proceeded to a second rescue allograft.
The cumulative incidence of graft failure was 9.5% for MSD and 14.5% for UD HSCT. As all patients in the cohort had persistent mixed donor chimerism, it was unsurprising that preceding donor chimerism had no correlation with subsequent graft failure of these 6 patients. There was no difference in engraftment according to HSCT-CI score, with 92% engraftment for a score of 0-1 and 80% for ≥ 2 (P = .5). At last follow-up, 29 patients in the cohort were at least 1 year posttransplantation, of which 22 patients had stopped their immunosuppression. There have been no episodes of last onset graft failure/rejection or acute GVHD in the cohort to date.
Chimerism data
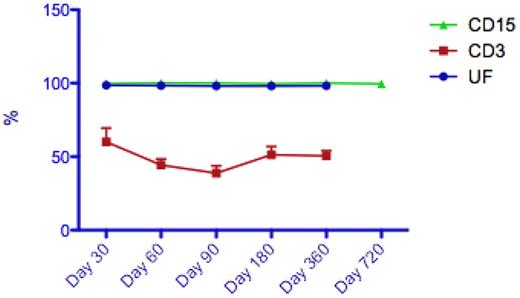
Thirty-nine patients (78%) had sequential data on unfractionated whole peripheral blood chimerism available, of whom 21 patients had additional sublineage CD3 (lymphocyte) chimerism data available for analysis. At day 100 posttransplantation, 21 patients had attained FDC in the unfractionated PB. However, at the same time point, no patients had attained CD3 FDC, with a median CD3 chimerism level of 45% (range: 9%-94%). Likewise, at 1-year posttransplantation, none of the 16 evaluable patients had attained CD3 FDC (see Figure 1). Of note, no patients received donor lymphocyte infusions, and all patients were blood and platelet independent 1 year posttransplantation. Only 1 of the patients who had subsequently developed GVHD (acute) had available chimerism for assessment (CD3 chimerism was 29% at time of GVHD).
Representative median peripheral blood chimerism results in patients following FCC conditioning (UF indicates unfractionated; [n = 33], CD3 [n = 16], and CD15 [n = 16]).
Representative median peripheral blood chimerism results in patients following FCC conditioning (UF indicates unfractionated; [n = 33], CD3 [n = 16], and CD15 [n = 16]).
GVHD
Acute GVHD was observed in 7 (13.7%) patients, all grade I-II. Only 2 patients (4%) developed chronic GVHD, one limited and one extensive. The cumulative incidence of acute and chronic GVHD at 1 year was 16.5% ± 8% and 7% ± 4%, respectively. Chronic GVHD contributed to death of 1 patient (on day +427) who also had CMV disease.
Infections
EBV viremia was seen in 4 of 49 patients (8.2%), of whom 2 patients had posttransplantation lymphoproliferative disease (PTLD). One patient was successfully treated with 1 course of R-CHOP, but another patient died of progressive PTLD. There were 9 cases of CMV reactivation but only 1 case of CMV disease, who unfortunately died from complications associated with GVHD and CMV. Seven of 42 patients (16.7%) had adenovirus viremia.
Fertility
While the fertility status of patients was not uniformly assessed in all patients posttransplantation, of 14 female patients in the age group 16-40 years at HSCT, 1 patient delivered a full-term healthy baby at 31 months post-HSCT and another had a medical termination for unwanted pregnancy.
Overall survival
The actuarial OS at 2 years for all patients is 88% ± 5%, with a 2-year FFS of 80% ± 6%. At time of last follow-up, a total of 5 patients had died, 4 following UD HSCT. The individual causes of death were chronic GVHD with CMV disease (day +427), presumed invasive pulmonary fungal infection (day +14), graft failure in 2 patients (days +137 and +199) and PTLD (day +180). There was no significant difference in 2-year OS between MSD with UD HSCT, 95% and 83%, respectively (Figure 2A). The following factors were shown to significantly influence OS.
Overall survival (OS) curves. (A) OS curves for the cohort stratified by donor type (MDS indicates matched sibling donor; and UD, unrelated donor). (B) OS curves for the cohort stratified by transplantation comorbidity index (HSCT-CI; HSCT-CI 0-1 vs HSCT-CI ≥ 2). (C) OS curves for the cohort stratified by recipient age (recipient age < 50 years vs ≥ 50 years). (D) OS curves for the cohort stratified by stem cell source (PBSC indicates peripheral blood progenitor cells).
Overall survival (OS) curves. (A) OS curves for the cohort stratified by donor type (MDS indicates matched sibling donor; and UD, unrelated donor). (B) OS curves for the cohort stratified by transplantation comorbidity index (HSCT-CI; HSCT-CI 0-1 vs HSCT-CI ≥ 2). (C) OS curves for the cohort stratified by recipient age (recipient age < 50 years vs ≥ 50 years). (D) OS curves for the cohort stratified by stem cell source (PBSC indicates peripheral blood progenitor cells).
Comorbidity index (Figure 2B).
For patients with a Sorror score of 0-1 (n = 37), the 2-year OS was 95% compared with only 42% with a score of ≥ 2 (n = 6; P < .001). Likewise, 2-year FFS was 88% ± 7% for HSCT-CI 0-1 versus 44% ± 22% for HSCT-CI ≥ 2 (P = .02).
Age (Figure 2C).
Advanced recipient age (≥ 50 years) adversely impacted on the OS of the cohort. HSCT was performed in 12 patients aged 50 years or older, with an OS of 71% compared with 92% for patients younger than 50 years old (P = .02). Three patients were aged 60 years or older who received UD HSCT, of whom 2 are alive; 1 had grade I acute GVHD, the second had fungal pneumonia at time of HSCT, died on day +14 from progression of fungal infection. Two-year FFS was 86.5% ± 6% (< 50 years) vs 72.9% ± 13.5% (≥ 50 years), P = .50.
Stem cell source (Figure 2D).
While there was no significant difference on the 2-year OS between all stem cell sources, with PBSCs (n = 14) 73% ± 13%, BM (n = 24) 93% ± 6%, GCSF-BM (n = 7) 86% ± 13%, and BM + PBSC: 100% (P = .72), there was a significant difference on pairwise log-rank analysis in OS between patients who received BM versus PBSCs (P = .03). Of note, there was no difference in 2-year FFS based on stem cell source, BM 88% ± 7% versus PBSC 70% ± 13% (P = .36).
Time from diagnosis to HSCT.
The time from initial diagnosis to transplantation had no significant impact on OS in this study, with those receiving transplants within 12 months having a 2-year OS of 95.8% ± 4% versus those ≥ 12 months 75.7% ± 11% (P = .07). Two-year FFS for those receiving transplantation within 12 months of diagnosis was 90% ± 6% versus those ≥ 12 months: 65% ± 13% (P = .09).
Impact of alemtuzumab dose
Because of the retrospective nature of this study, there was a degree of variability in the dosage of alemtuzumab used between centers. Twenty patients (40%) received a total dose of > 60 mg of alemtuzumab while 30 (60%) received a total dose of 60 mg or less. There was no significant difference in donor type (MSD vs MUD), stem cell source, or stem cell dose, between patients who received > or ≤ 60 mg of alemtuzumab. In terms of transplantation outcomes, there was no difference in OS (> 60 mg: 81% ± 9% vs ≤ 60 mg: 96% ± 4%, P = .23) or FFS (> 60 mg: 74% ± 9% vs ≤ 60 mg: 88% ± 7%, P = .41) between groups, likewise, there was no significant difference in CMV, EBV, or GVHD incidence.
Discussion
We have shown that the use of alemtuzumab in combination with fludarabine and low-dose CY (FCC) in MSD and UD HSCT for acquired AA is associated with a very low risk of chronic GVHD (4%), a low risk and low grade of acute GVHD (14%, and all cases grade I or II) and excellent OS. The incidence of graft failure is comparable with conditioning regimens using ATG instead of alemtuzumab, and infection rates were low.
We first explored an alternative approach to reduce GVHD initially using the mAb Campath-1G during the 1980s, and later the humanized mAb Campath-1H (alemtuzumab) when it became available in 1999.19-21 The aim was to develop a conditioning regimen for HSCT in SAA that produced sustained engraftment, with minimal toxicity and GVHD. Pharmacokinetic studies have shown that alemtuzumab is detectable in the plasma for several weeks after administration, resulting in depletion of recipient autoreactive lymphocytes and prevention of GVHD by depletion of donor alloreactive T cells.34,35 For MSD HSCT, Campath-1G or alemtuzumab was used instead of ATG, with CY 200 mg/kg and CSA.21 Avoidance of MTX as GVHD prophylaxis helps reduce regimen-related toxicity. Excellent 5-year survival of 81% was associated with a very low incidence of GVHD (14% grade II-IV acute GVHD and 4% chronic GVHD), limited in all cases, but graft rejection was high at 24%. The high graft rejection was associated with the initial use of Campath-1G given both pre- and peritransplantation in the first 21 patients from day −8 to +5, in an attempt to achieve maximum GVHD prophylaxis. Subsequent patients received Campath mAb pretransplantation only, resulting in a reduction in graft rejection to 11%, while maintaining a low incidence of GVHD. The high survival of early patients with early graft failure was in part because of a high incidence of autologous recovery, in ∼ 50% of patients.19,22 We previously used alemtuzumab with fludarabine-based conditioning for UD HSCT and reported excellent engraftment in a small series of patients with both acquired and inherited SAA.21
Risk factors for chronic GVHD after HSCT for SAA include acute GVHD, older age, and donor chimerism. The use of peripheral blood stem cells (PBSCs) in MSD HSCT using ATG-containing regimens, results in a high incidence of chronic GVHD among younger patients and worse survival for both young and older patients.36 A low incidence of chronic GVHD using alemtuzumab instead of ATG in HSCT for SAA was also observed separately from one of the centers participating in this study.23 In addition to deleting alloreactive donor T cells, alemtuzumab targets host Ag-presenting dendritic cells which trigger the alloreactive response.37 In our study, the use of alemtuzumab was associated with a very low incidence of GVHD. Acute GVHD occurred in 8 patients, 4 who received BM and 4 who received PBSCs. Of the 2 cases of chronic GVHD, one with extensive chronic GVHD received BM, the other with limited disease received PBSC.
Mixed donor chimerism occurs frequently after HSCT for SAA.33,38-40 Progressive mixed chimerism carries a high risk of graft rejection but stable mixed chimerism is associated with absence of chronic GVHD and excellent survival. Analysis of chimersim showed that while the majority of patients attain FDC in the unfractionated BM by day 100, none of the patients achieve CD3 FDC even at 1-year post-HSCT. Persistent mixed CD3 donor chimerism, particularly in the backdrop of T-cell depletion with alemtuzumab, has been shown to be associated with a low incidence of GVHD. The data presented here suggest that using an FCC protocol, patients are able to maintain sustained mixed donor chimerism even after the cessation of immunosuppression, with maintenance of clinical remission and haematologic parameters. Similar observations have been reported using alemtuzumab with TBI (3 Gy) and sirolimus in HSCT for sickle cell disease, with complete absence of both acute and chronic GVHD in association with a high incidence of stable mixed chimerism.41 The proposed advantage of using sirolimus instead of CSA in that study was that sirolimus promotes differentiation of T regulatory cells and T helper cells which may have contributed to the absence of GVHD. However, sirolimus is associated with toxicity from hyperlipidaemia, arthralgia, pneumonitis, hyperglycemia, and myelosuppression.
A low stem cell dose is associated with an increased risk of graft failure in HSCT for AA.42,43 We have recently shown that infusion of < 2.0 × 106 CD34 cells/kg is associated with increased graft failure, increased incidence of bacterial infections and delay in neutrophil engraftment, although neutrophil engraftment is more dependent on stem cell source.28 One study reported more chronic GVHD when > 3.7 × 108 nucleated cells/kg were infused, but data on CD34 cells were lacking.44 We have shown in this study that the use of PBSC with alemtuzumab-based conditioning permits collection of an adequate cell dose without causing significant chronic GVHD.
Concerns have been raised that the use of alemtuzumab may increase the risk of viral infections post-HSCT. As in our previously reported studies in HSCT for SAA,19,20 and for treatment of refractory autoimmune cytopenias,45 we confirmed a low incidence of viral infections and we observed only 2 cases of EBV PTLD. We have previously shown that there is a strong association of EBV PTLD post-HSCT for SAA when ATG is used as IST for AA before HSCT.22
We observed no expansion of PNH clones post-HSCT. Lymphocytes deficient in the expression of GPI-anchored proteins may emerge after treatment with alemtuzumab, mimicking a PNH clone, as a result of immune selection by the anti-CD52 Ab.46 A PNH clone was present in 9 of 34 patients before HSCT, but only detectable post-HSCT in 2 patients, which disappeared at 6 months in 1 patient and detectable in only 0.1% granulocytes and monocytes at 4 months in the other patient. In the remaining 7 patients, the PNH clones disappeared post-HSCT. However, we did not examine expression of GPI-anchored proteins on PBLs.
There is much debate concerning the upper age limit for HSCT in SAA. Using standard CY 200 mg/kg with ATG, OS of 65% with median follow-up of 9.1 years, was recently reported among 23 patients aged 43-68 years from Seattle.47 However, there are concerns about cardiac toxicity of CY at this dose in older patients; fluid overload was reported in 6 patients in that series, which contributed to cause of death in 4 patients.47 A recent study from the Center for International Blood and Marrow Transplantation Research (CIBMTR) showed worse survival among patients older than 40 years of age transplanted for SAA from MSD. Older patients were more likely to (1) have a worse performance status, (2) receive IST before HSCT, (3) have an interval from diagnosis to HSCT of > 3 months, and (4) have received a PBSC graft. With the use of fludarabine-based conditioning regimens, improved outcomes have been reported in older patients.9 Previously, first-line MSD HSCT was recommended for patients younger than 40 years of age because of worse outcome after SCT in patients older than 40 years old. Our institution at King's College Hospital has pioneered the use of alemtuzumab with reduced intensity conditioning for allogeneic HSCT in myelodysplastic syndrome (MDS).29,48 Furthermore, in a retrospective study from the EBMT, patients older than 60 years transplanted for MDS show similar nonrelapse mortality to patients aged 50-60 years.49 Another CIMBTR study in MDS showed that older age has no impact on OS, disease-free survival in addition to nonrelapse mortality.50 In our study, we show excellent outcomes in a small subgroup of patients older than 50 years of age (range 50-62) with 71% survival. The low toxicity of FCC, as well as low risk of GVHD and infections, makes this an especially attractive regimen for older patients, and further studies in older patients are now indicated.
An important prognostic factor for survival in our study was the CI, as assessed by the Sorror score, and this emphasizes the importance of careful selection of patients before planned HSCT. A score of > 2 in SAA indicated a worse outcome with OS of only 42% after FCC HSCT, and this may be especially important in decision planning in older patients. Patients receiving BM as the stem cell source had an improved OS compared with those receiving peripheral blood stem cells (93% vs 73%, P = .03). Given the possible confounding factors influencing the outcomes in our study, larger studies will be required to truly establish the influence of stem cell source on outcomes of patients receiving the FCC regimen.
The excellent outcome of UD HSCT using FCC adds further support to the current recommendation of early HSCT after failure of only 1 course of IST, instead of waiting until failure after a second course.17,18 Patients who received transplantations within 12 months of diagnosis had a more favorable 2-year OS (96% vs 76%, P = .07). In conclusion, the use of alemtuzumab in SCT for acquired AA is the first reported change in conditioning regimen to result in a major reduction in chronic GVHD compared with previously reported studies.
Presented in abstract form at the 42nd annual meeting of the American Society of Hematology, New Orleans, LA, November 5-8, 2009.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge the nursing and medical staff that looked after the patients at each center.
Authorship
Contribution: J.C.M., V.G., Z.L., G.J.M., and A.P. contributed to the study design; J.C.M., V.G., Z.L., A.Y.H., R.I., N.R., D.I.M., G.J.M., and A.P. contributed to the writing of the paper; V.G., Z.L., R.I., J.H., V.P., M.B.K., M.S.I., N.R., and D.I.M. contributed to the data collection; V.G., Z.L., V.P., M.B.K., N.R., and D.I.M. contributed to patient recruitment; Z.L. analyzed the results and made the figures; J.C.M. served as the principal investigator for this study; and all authors reviewed the paper.
Conflict-of-interest disclosure: J.C.M. held a consultancy with Genzyme from May 2008 to May 2009 and from June 2009 to July 2009, and received research funding in 2010 from Genzyme. The remaining authors declare no competing financial interests.
Correspondence: Prof Judith C. Marsh, Department of Hematological Medicine, King's College Hospital, Denmark Hill, London, SE59RS, United Kingdom; e-mail: judith.marsh@nhs.net.
References
Author notes
G.J.M. and A.P. are joint last authors.

![Figure 1. Representative median peripheral blood chimerism results in patients following FCC conditioning (UF indicates unfractionated; [n = 33], CD3 [n = 16], and CD15 [n = 16]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/8/10.1182_blood-2010-12-327536/4/m_zh89991172980001.jpeg?Expires=1767708465&Signature=T31zMD-mD0A5fZVQEy5rdivyUxhadl2IgJvAp4ycgZsGHiLORLyp9yKfyuUJaThv8qZa8OT-rSzQgu~sGEBFHWMzyRwgQWkTGCQX2pzvMJv-M0Z1Gi9EFb58Je4ciWRH0OyJRCRsOnYqdRELFMB-Kjsmhe9NGnBFpqfQJCHUCs972o-Sl~Sv2snAwqgk-TdizeqnijO8ANDMfXT5wdXqF4pCq4-zidLCe~QS6y0v6JZKerq4cZnl5TfM5WGwcj~ycLsJbtVuf6LQHLuDmtNw3NoZUsWiyM8WzuTqKop40lOQdC07c~imI-RmSLDh3AalsgfwIhGLdylHbnFWCgy7gw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal