Abstract
Forodesine and nelarabine (the pro-drug of ara-G) are 2 nucleoside analogues with promising anti-leukemic activity. To better understand which pediatric patients might benefit from forodesine or nelarabine (ara-G) therapy, we investigated the in vitro sensitivity to these drugs in 96 diagnostic pediatric leukemia patient samples and the mRNA expression levels of different enzymes involved in nucleoside metabolism. Forodesine and ara-G cytotoxicities were higher in T-cell acute lymphoblastic leukemia (T-ALL) samples than in B-cell precursor (BCP)–ALL and acute myeloid leukemia (AML) samples. Resistance to forodesine did not preclude ara-G sensitivity and vice versa, indicating that both drugs rely on different resistance mechanisms. Differences in sensitivity could be partly explained by significantly higher accumulation of intracellular dGTP in forodesine-sensitive samples compared with resistant samples, and higher mRNA levels of dGK but not dCK. The mRNA levels of the transporters ENT1 and ENT2 were higher in ara-G–sensitive than –resistant samples. We conclude that especially T-ALL, but also BCP-ALL, pediatric patients may benefit from forodesine or nelarabine (ara-G) treatment.
Introduction
Leukemia is the most common childhood malignancy, and the general incidence in both adults and children of acute lymphoblastic leukemia (ALL) or acute myeloid leukemia (AML) is approximately 1 per 100 000 and 2-3 per 100 000, respectively. Although overall cure rates have been improved over the last decades, approximately 20% of children with ALL and 40% of children with AML still eventually die from their disease.1,2 In adults, the prognosis is worse with a survival below 60% in ALL3 and 50% in AML,4 indicating that there is still a great need for better therapy. Currently, purine nucleosides analogues are in clinical trials for different types of leukemia including clofarabine, forodesine (BCX-1777/Immucillin H), and nelarabine (506U78/Arranon/Atriance) the latter being the pro-drug for 9-β-D-arabinofuranosylguanine (ara-G).
Forodesine is a noncleavable inosine analog developed to bind and inhibit the purine nucleoside phosphorylase (PNP) enzyme.5 PNP normally degrades excess of intracellular deoxyguanosine (dGuo) into guanosine and deoxyribose-1-phosphate through phosphorylysis. dGuo is continuously produced in the body as the result of DNA degradation during cellular turnover. Inhibition of PNP by forodesine results in the intracellular accumulation of dGuo. DGuo is rapidly phosphorylated to dGTP in the purine salvage pathway leading to dGTP accumulation.6,7 High intracellular levels of dGTP cause cell death through mechanisms that are still not fully understood, but which may likely involve imbalance in the deoxynucleotide pool and/or inhibition of ribonucleotide reductase8 resulting in inhibition of DNA synthesis and/or by activation of a p53-induced cell-cycle arrest and apoptosis.9 Whereas most nucleoside analogues depend on DNA incorporation to exert their toxic effect, this is not the case for forodesine. T cells seem to be especially sensitive to PNP inhibition as SCID patients with PNP deficiency have increased plasma levels of dGuo10,11 and a severe depletion of T cells compared with other cell types.12,13 In contrast to SCID, however, severe opportunistic infections are not seen in treatment with forodesine, as there seems to be a selective toxicity toward leukemic cells.7
Ara-G is an arabinosylguanine analog that is resistant to PNP-mediated phosphorylysis. Accumulated intracellular ara-G is rapidly converted to ara-GTP which results in cell death through inhibition of ribonucleotide reductase and incorporation of ara-GTP in the DNA which blocks further DNA synthesis.14,15 In contrast to various other arabinonucleoside compounds including ara-C, selective T-cell toxicity has only been demonstrated for ara-G.14-17 However, the use of ara-G is limited because of its poor water solubility. Therefore nelarabine, a pro-drug of ara-G that is 8-fold more water soluble,18 is used in clinical settings. In vivo nelarabine is rapidly converted into ara-G through demethoxylation by adenosine deaminase.
Forodesine has been tested in clinical phase 1/2 trials in relapsed or refractory patients with T-cell ALL or lymphoblastic lymphoma,7,19 B-cell precursor ALL (BCP-ALL) and chronic lymphocytic leukemia20 (reviewed in Al-Kali et al21 ). Forodesine treatment resulted in an overall response in 32% of the T-cell leukemia patients, with 21% of the having a complete response. Forodesine administration resulted in an increase in plasma dGuo and intracellular dGTP levels. Adverse affects were mild, with only grade 3 thrombocytopenia and leukopenia.19,21 For BCP-ALL patients, forodesine treatment resulted in complete responses in 17% of the patients.21
Nelarabine, the pro-drug of ara-G, has been tested in clinical phase 1/2 trials in adults22 and children23,24 with refractory or relapsed T-ALL or T-cell lymphoblastic lymphoma (T-LBL) and is an approved drug for T-cell disease in both the United States and Europe. Thirty-one percent of adult T-ALL and T-LBL patients achieved a complete remission with an overall response rate in 41% of the patients. Median disease-free survival (DFS) and overall survival (OS) were 20 weeks, with 28% of the patients surviving 1 year. Principal toxicity was a grade 3 or 4 neutropenia and thrombocytopenia.22 For pediatric T-ALL patients at first relapse, complete responses were documented for 55% of the patients. For patients in second relapse or for patients with extramedullary relapses, response rates ranged from 14%-33%. However, 18% of the patients had a ≥ grade 3 neurologic adverse event.23
To better predict which patients might benefit from forodesine or nelarabine treatment, we investigated the in vitro sensitivity to forodesine or ara-G in pediatric ALL and acute myeloid leukemia (AML) diagnostic patient samples. Forodesine toxicity was investigated in relation to intracellular accumulation of dGTP levels. We also investigated potential mechanisms that may be responsible for differences in drug sensitivity among patient samples. To this end, we measured mRNA expression levels of proteins that are involved in purine metabolism and uptake (Figure 1). In addition, we tested whether forodesine had a synergistic or antagonistic effect with 7 commonly used drugs in leukemia treatment.
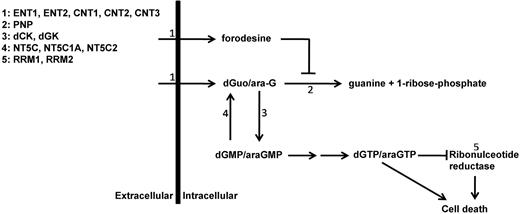
Purine metabolism overview. Schematic overview of main enzymes and transporters involved in purine conversion and uptake. ENT1-2 indicates equilibrative nucleoside transporter 1-2; CNT1-3, concentrative nucleoside transporter 1-3; PNP, purine nucleoside phosphorylase; dCK, deoxycytidine kinase; dGK, deoxyguanine kinase; NT5C, cytosolic 5′ nucleotidase 1A; NT5C1A, cytosolic 5′ nucleotidase 1A; NT5C2, cytosolic 5′ nucleotidase; and RRM1 and RRM2, ribonucleotide reductase subunit 1 and subunit 2.
Purine metabolism overview. Schematic overview of main enzymes and transporters involved in purine conversion and uptake. ENT1-2 indicates equilibrative nucleoside transporter 1-2; CNT1-3, concentrative nucleoside transporter 1-3; PNP, purine nucleoside phosphorylase; dCK, deoxycytidine kinase; dGK, deoxyguanine kinase; NT5C, cytosolic 5′ nucleotidase 1A; NT5C1A, cytosolic 5′ nucleotidase 1A; NT5C2, cytosolic 5′ nucleotidase; and RRM1 and RRM2, ribonucleotide reductase subunit 1 and subunit 2.
Methods
Patient material
Fresh or viably frozen BM or peripheral blood samples from a total of 96 de novo, untreated pediatric acute leukemia patients were used, comprising 36 T-ALL, 43 BCP-ALL, and 17 AML samples. All samples were tested for forodesine cytotoxicity, whereas additional assays were performed on the same samples based on the availability of material. The patients' parents or legal guardians provided informed consent to use leftover diagnostic patient biopsies for research in accordance with the Institutional Review Board of the Erasmus MC Rotterdam and in accordance with the Declaration of Helsinki. Leukemic cells were isolated and enriched as previously described.25 All resulting samples contained ≥ 90% leukemic cells, as determined morphologically by May-Grünwald-Giemsa–stained cytospins (Merck) and were viably frozen in liquid nitrogen as described earlier.25
Cell lines
T-ALL cell lines (CCRF-CEM, LOUCY, BE-13, MOLT-4, PEER, KARPAS-45, MOLT-3, JURKAT, HPB-ALL, PF-382) were purchased from the German Collection of Microorganisms and Cell Cultures (DSMZ), and cultured under recommended conditions.
Assessment of PNP inhibition by forodesine (dGuo measurements)
The efficacy of forodesine to inhibit phosphorylysis of dGuo into guanosine and deoxyribose-1-phosphate by PNP was assessed in 4 pediatric T-ALL and 2 pediatric BCP-ALL patient samples. For this, the decrease in dGuo concentration was measured over time in the supernatant of cell cultures that were treated with varying concentrations of forodesine. Cells were cultured in RPMI 1640 Dutch modification without l-glutamine, 20% FCS, 2mM l-glutamine (Invitrogen), 5 μg/mL insulin, 5 μg/mL transferrin, 5 ng/mL sodium selenite (ITS media supplement; Sigma-Aldrich), 100 IU/mL penicillin, 100 μg/mL streptomycin, 0.125 μg/mL fungizone, and 0.2 mg/mL gentamycin (Invitrogen) at a concentration of 1.6 × 106 cells/mL. Forodesine (provided by Mundipharma Research Ltd) was added to final concentrations of 1, 3, or 10μM, or replaced by dH2O in the control. dGuo (Sigma-Aldrich) was added to all cultures to a final concentration of 10μM. Cells were plated in triplicate in 96-well plates (Bioplastics) for each condition (320 × 103 cells/well). After 0, 4, 24, 48. and 96 hours, cells were pelleted by centrifugation and the supernatant was collected for dGuo measurement and stored at −80°C until further analysis. dGuo levels were analyzed by HPLC (or LC) with tandem mass spectrometry detection (MS/MS) as previously described.19 Briefly, dGuo was extracted from the supernatant using a Waters Oasis “HLB” affinity solid-phase extraction (SPE) cartridge. The mass of dGuo plus H+ (268.1 m/z) was monitored in quadrupole one (Q1). The dGuo product ion 157.0 m/z was monitored in quadrupole 3 (Q3). The concentrations of dGuo were determined by weighted (1/x) quadratic regression analysis of peak areas produced from the standard curve.
In vitro forodesine, ara-G and ara-C cytotoxicity (MTT assay)
Forodesine (36 T-ALL, 43 BCP-ALL, and 17 AML samples), ara-G (28 T-ALL, 35 BCP-ALL, and 17 AML samples), and ara-C (28 T-ALL samples) cytotoxicities were determined using the MTT assay as described previously.26 Ara-G is the active metabolite of the pro-drug nelarabine. We measured cell viability in the presence of 1μM forodesine and 6 concentrations (0.01, 0.1, 1, 3, 10, and 50μM) of dGuo, after an incubation period of 4 days. As control, samples were incubated with the same range of dGuo concentrations in the absence of forodesine. Additional controls were 1μM forodesine in the absence of dGuo, and vehicle only. dGuo is added to the culture to mimic the natural variable presence of dGuo in the blood, as this compound mediates forodesine cytotoxicity. For ara-G (Carbosynth Limited), the following concentrations were used: ara-G 0.01, 0.1, 1, 3, 10, 50μM. The concentrations used in the MTT assay for ara-C were: 0.01, 0.04, 0.16, 0.625, 2.5, and 10μM.
Combination cytotoxicity assay
Using the MTT assay as previously described,26 we screened for potential antagonistic or synergistic effects in forodesine-mediated cytotoxicity for 7 compounds that are used in ALL treatment, comprising ara-C, ara-G, 6MP (Sigma-Aldrich), asparaginase (Medac), daunorubicin (cerubidine; Sanofi-Aventis), prednisolone (BUFA BV), and vincristine (TEVA Pharmachemie). Four to 9 T-ALL and 6 to 8 BCP-ALL pediatric patient samples were tested for each drug combination. Before this, the median concentration that is lethal to 10% (LC10) and to 30% (LC30) of cells were determined for dGuo in the presence of 1μM forodesine on the basis of in vitro forodesine cytotoxicity assay results for 10 T-ALL and 10 BCP-ALL patient samples. The T-ALL and BCP-ALL median LC10 or LC30 concentrations were used in the combination assay for T-ALL and BCP-ALL samples, respectively. Forodesine (1μM) and the median LC10 or LC30 concentrations of dGuo were then combined with a range of each of the 7 drugs (ara-C: 0.01, 0.04, 0.16, 0.625, 2.50, 10.0μM; ara-G: 0.01, 0.10, 1.0, 3.0, 10, 50μM; 6-mercaptopurine [6MP]: 0.016, 0.031, 0.063, 0.125, 0.50, 1.0 mg/mL; asparaginase: 0.003, 0.016, 0.08, 0.40, 2.0, 10.0 IE/mL; daunorubicin: 0.002, 0.008, 0.031, 0.125, 0.5, 2.0 μg/mL; prednisolone: 0.008, 0.06, 0.49, 3.9, 31.3, 250 μg/mL; and vincristine: 0.05, 0.20, 0.78, 3.1, 12.5, 50.0 μg/mL). The controls were: 1μM forodesine in combination with the median LC10 or LC30 value of dGuo. Previous experiments on T-ALL cell lines (JURKAT, HPB-ALL, LOUCY, and PF-382) showed no effect of addition of the median LC30 values of dGuo on the cytotoxicity of the 7 drugs in the absence of forodesine (data not shown). Because 6MP solutions give a background signal in the MTT assay, varying concentrations of 6MP in culture medium were included as an additional control. For each patient and each concentration of compound tested, a hypothetical maximal additive effect of either LC10 or LC30 forodesine/dGuo treatment in combination with the other compound was calculated by the following formula: ([100 − A] × B/100) + A, where A and B are the percentages of cell death caused by each compound individually. We performed a t test to analyze for each drug concentration whether the median calculated hypothetical values were significantly different from the actual measured median values obtained by combining the drugs, that is, whether the results differed significantly from the hypothetical maximum additive effect. When a significant difference was observed, we performed another t test to analyze whether the median cell survival measured with drug only increased significantly by addition of forodesine/dGuo, that is, whether an antagonistic effect was present.
dGTP measurement
Accumulation of dGTP was calculated using a polymerase assay as previously described27 in 22 T-ALL, 6 BCP-ALL, and 2 AML samples. Ten million cells were cultured for 24 hours in 5 mL of culture medium in the presence of 3μM dGuo and 1μM forodesine. The control reaction comprised 3μM dGuo. Proliferation and apoptosis were measured with Trypan blue staining and counting in a Bürker-Türk counting chamber. Cells were washed twice with PBS and spinned down by centrifugation. The cell pellet was resuspended in 1 mL of 60% methanol (−20°C) and stored at −20°C. The samples were centrifuged and supernatants were dried in a TurboVap. Dried extracts were stored at −20°C until further analysis. Extracts were suspended in 25 μL of buffer (20mM Hepes-NaOH, pH 7.3; 2mM MgCl2) and 20 μL was used in the assay. dGTP standards were used at 0, 0.5, 1, 5, 10, and 50 pmol. Reactions contained 20 μL of extract or standard, 100mM HEPES-NaOH (pH 7.3), 10mM MgCl2, 50nM primer, 2.5μM [3H]-dATP, 0.5 U of Klenow Exo-Free DNA Polymerase I, and dH2O to 100 μL of final volume. Reactions were incubated in U-bottom 96-well tissue-culture plates at room temperature for 1 hour. Samples were harvested onto Whatman DE81 DEAE cellulose paper using a Packard cell harvester, washed 3 times with 5% Na2PO4, once with dH2O, once with 95% ethanol and then air-dried and counted on a Packard Matrix-9600 β counter. A standard curve was generated (cpm vs dGTP concentration) for each experiment and the amounts of dGTP present in the extracts were calculated using the standard curve.
Real-time quantitative PCR
cDNA was available for 25 T-ALL samples, 24 BCP-ALL samples, and 1 AML patient sample. RNA extraction and cDNA synthesis were performed as previously described.25 Real-time quantitative PCRs (RQ-PCR) were performed in 1× DyNAmo HS SYBR Green mastermix (Finnzymes), 1× ROX (Finnzymes), 8.3 pmol forward primer, 8.3 pmol reverse primer, 20 ng of cDNA, and 4mM MgCl2 in a final volume of 27.5 μL. RQ-PCR was performed on a 9700HT Fast Real-Time PCR system (Applied Biosystems) starting with DNA polymerase heat activation at 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. A melting curve was recorded during a heating step from 25°C to 95°C during a 10-minute period. We performed cycle threshold analysis for each reaction using SDS2.3 analysis software (Applied Biosystems) and expression levels were quantified relative to the endogenous housekeeping gene GAPDH using the ΔCt method.28 All reactions were performed in duplicate. Primer sequences for deoxycytidine kinase (dCK), cytosolic 5′ nucleotidase 1A (PNI/NT5C/P5N2), equilibrative nucleoside transporter 1 (ENT1/SLC29A1), ribonucleotide reductase subunit 1 (RRM1) and subunit 2 (RRM2) and GAPDH have been described elsewhere.25,29 Other primer combinations are listed in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). cDNA of a T-ALL cell line pool (CCRF-CEM, LOUCY, BE-13, MOLT-4, PEER, KARPAS-45, MOLT-3, and JURKAT) was used as positive control for these targets.
Statistical analysis
Differences in the distribution of continuous variables were analyzed using the Mann-Whitney U test. Analyses of proportional differences were performed by the χ2 test or the Fisher exact test. The Student t test was used to analyze whether differences in cell survival differed significantly from zero. Statistical tests were performed at a 2-tailed significance level of .05.
Results
In vitro forodesine and ara-G cytotoxicity levels
To explore the efficacy of purine nucleosides analogues as a potential therapeutic drug for ALL, we tested in vitro toxicity levels of forodesine and ara-G on pediatric ALL and AML samples. Forodesine toxicity depends on the plasma availability of dGuo and its conversion into dGTP, and we first tested the ability of forodesine to block the degradation of dGuo into guanosine and deoxyribose-1-phosphate by PNP. These measurements were performed in the presence of dGuo and increasing forodesine concentrations. Without forodesine, dGuo levels in the culture media are rapidly being depleted as consequence of PNP-mediated degradation to nearly undetectable levels within 24 hours in 5 of 6 patient samples. For all samples tested, 1μM forodesine was sufficient to block PNP activity (supplemental Figure 1) resulting in the complete stabilization of dGuo levels in the culture supernatants. This dose of forodesine was then chosen in subsequent cellular cytotoxicity experiments.
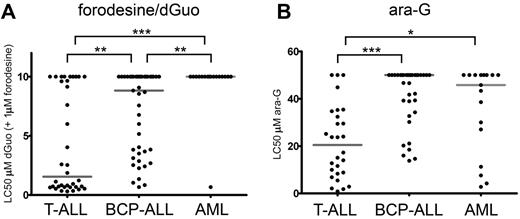
We then measured the cellular toxicity to 1μM forodesine in 96 pediatric primary leukemia samples in the presence of varying concentrations of dGuo (Figure 2). In our assay, dGuo itself elicited no cellular toxicity up to concentrations of 10μM as it is rapidly being degraded by PNP (data not shown). Forodesine (1μM) in the absence of dGuo had no effect on survival (data not shown). However, in the presence of forodesine and subsequent blockage of PNP activity, T-ALL samples were more sensitive to dGuo levels (median LC50 = 1.6μM dGuo) than BCP-ALL (median LC50 = 8.8μM dGuo, P = .001) and AML (median LC50 > 10μM, P < .001) samples (Figure 2A). Only 1 of 17 AML samples reached an LC50 in our assay.
Forodesine/dGuo and ara-G sensitivity in pediatric leukemia. (A) LC50 values for forodesine/dGuo for T-ALL, BCP-ALL, and AML leukemia samples. When no LC50 was reached, a value of 10μM was assigned. (B) LC50 values for ara-G for T-ALL, BCP-ALL, and AML leukemias. When no LC50 was reached a value of 50μM was assigned. Median LC50 values are indicated by gray horizontal lines. Significance levels are indicated by asterisks: *P < .05; **P < .01; ***P < .001.
Forodesine/dGuo and ara-G sensitivity in pediatric leukemia. (A) LC50 values for forodesine/dGuo for T-ALL, BCP-ALL, and AML leukemia samples. When no LC50 was reached, a value of 10μM was assigned. (B) LC50 values for ara-G for T-ALL, BCP-ALL, and AML leukemias. When no LC50 was reached a value of 50μM was assigned. Median LC50 values are indicated by gray horizontal lines. Significance levels are indicated by asterisks: *P < .05; **P < .01; ***P < .001.
Ara-G cytotoxicity was measured in 28 T-ALL, 35 BCP-ALL, and 17 AML pediatric patient samples. Again, T-ALL samples were most sensitive to treatment (median LC50 = 20.5μM) compared with BCP-ALL (median LC50 > 50μM, P < .001) or AML (median LC50 = 45.8μM, P = .012) samples (Figure 2B).
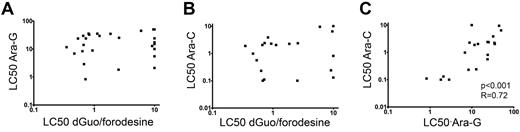
As conversion of dGuo and ara-G rely on the same enzymatic pathways, we investigated potential cross-resistance toward dGuo/forodesine and ara-G in T-ALL patient samples. patient who require drug concentrations higher than 10μM dGuo (at 1μM forodesine) or 50μM ara-G as LC50 values in our assay were regarded as resistant. We did not find any correlation between dGuo/forodesine and ara-G cytotoxicities, nor between dGuo/forodesine and the pyrimidine equivalent of the ara-G drug, that is, ara-C (Figure 3A-B). For T-ALL patients, 2 of 3 samples that were resistant to ara-G were sensitive to forodesine/dGuo exposure whereas 6 of 7 forodesine/dGuo-resistant samples remained sensitive for ara-G. For all patient samples tested, 10 of 30 ara-G–resistant samples remained sensitive to forodesine/dGuo exposure and 19 of 39 forodesine/dGuo-resistant samples were still sensitive to ara-G exposure. Therefore, resistance to ara-G exposure did not preclude sensitivity to forodesine/dGuo exposure and vice versa, and suggests that the modes of cytotoxicity or resistance between forodesine and ara-C or ara-G are different. In contrast, LC50 values for ara-C and ara-G cytotoxicities strongly correlated (P < .001, R = 0.72; Figure 3C), indicating that the cytotoxic mechanisms are the same for ara-G and ara-C compounds.
Relation between LC50 values of forodesine/dGuo, ara-G and ara-C in T-ALL. Relationship between (A) ara-G and forodesine/dGuo LC50 values, (B) between ara-C and forodesine/dGuo LC50 values, and (C) between ara-G and ara-C LC50 values. LC50 values for ara-G and ara-C were available for 28 and 21 T-ALL patients, respectively.
Relation between LC50 values of forodesine/dGuo, ara-G and ara-C in T-ALL. Relationship between (A) ara-G and forodesine/dGuo LC50 values, (B) between ara-C and forodesine/dGuo LC50 values, and (C) between ara-G and ara-C LC50 values. LC50 values for ara-G and ara-C were available for 28 and 21 T-ALL patients, respectively.
dGTP accumulation
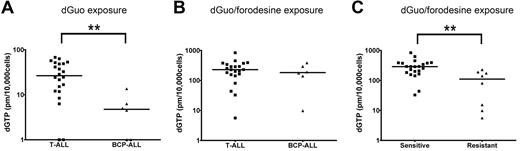
To investigate whether differences in forodesine sensitivity levels could be attributed to differences in intracellular accumulation of dGTP, we analyzed dGTP levels among patient samples in the absence or presence of forodesine. After 24 hours, no significant differences were found in proliferation rate or the number of apoptotic cells between forodesine/dGuo-treated or dGuo-treated control cells (not shown). Without blocking PNP activity, T-ALL patient samples accumulated higher basal intracellular dGTP levels within 24 hours than BCP-ALL samples (P = .004; Figure 4A), so BCP-ALL cells may have a higher intrinsic ability to degrade dGuo levels than T-ALL cells or have a slower conversion rate of dGuo into dGTP. On blockage of PNP by forodesine, total intracellular dGTP levels increased 10- to 100-fold within 24 hours (Figure 4B). No difference was observed between T-ALL and BCP-ALL samples indicating that both ALL types are equally efficient to convert dGuo into dGTP. Intracellular dGTP accumulation was significantly higher for forodesine-sensitive cells than for resistant cells (P = .001; Figure 4C). So, resistant patients may convert less dGuo into dGTP or resistant patients more efficiently consume (toxic) dGTP levels.
dGTP accumulation. (A) Basal dGTP levels after 24 hours of 10μM dGuo exposure and (B) dGTP accumulation after 24 hours of 10μM dGuo and 1μM forodesine exposure in 22 T-ALL and 6 BCP-ALL patient samples. Undetectable dGTP levels have been assigned a value of 1. (C) Intracellular dGTP levels after 24 hours 10μM dGuo and 1μM forodesine exposure in forodesine-sensitive versus -resistant patients (22 T-ALL, 6 BCP-ALL, and 2 AML samples). Horizontal lines represent median values. **P < .01.
dGTP accumulation. (A) Basal dGTP levels after 24 hours of 10μM dGuo exposure and (B) dGTP accumulation after 24 hours of 10μM dGuo and 1μM forodesine exposure in 22 T-ALL and 6 BCP-ALL patient samples. Undetectable dGTP levels have been assigned a value of 1. (C) Intracellular dGTP levels after 24 hours 10μM dGuo and 1μM forodesine exposure in forodesine-sensitive versus -resistant patients (22 T-ALL, 6 BCP-ALL, and 2 AML samples). Horizontal lines represent median values. **P < .01.
Gene expression
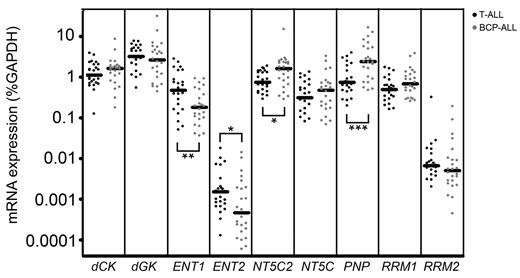
To find potential explanations for differences in forodesine or ara-G sensitivity levels, we determined mRNA expression levels of different transporters and enzymes that are involved in the purine metabolism (Figure 1). Of the 13 genes investigated, 4 genes (CNT1, CNT2, CNT3, NT5C1A) were expressed at low to undetectable levels in most of our patient samples and were therefore excluded from further analyses. ENT1 and ENT2 were both expressed at higher levels in T-ALL samples than in BCP-ALL samples (P = .007 and P = .036, respectively) while levels of the nucleotidase NT5C2/PNT5 and PNP were expressed at lower levels (P = .016 and P < .001, respectively; Figure 5).
Gene expression in leukemia subtypes. mRNA expression of 9 genes in T-ALL and BCP-ALL patients. Each dot represents a measurement in 1 patient sample. cDNA was available for 25 T-ALL samples, 24 BCP-ALL samples. *P < .05; **P < .01; ***P < .001.
Gene expression in leukemia subtypes. mRNA expression of 9 genes in T-ALL and BCP-ALL patients. Each dot represents a measurement in 1 patient sample. cDNA was available for 25 T-ALL samples, 24 BCP-ALL samples. *P < .05; **P < .01; ***P < .001.
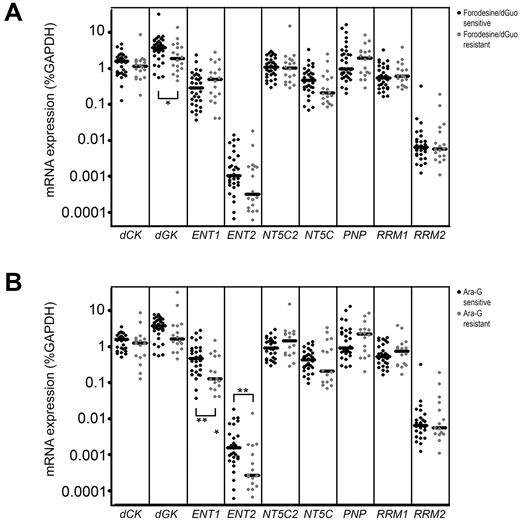
Patient samples sensitive to forodesine/dGuo expressed higher levels of dGK (P = .039; Figure 6A), and may more efficiently convert dGuo into dGMP as a first activation step in the conversion of dGuo into dGTP. ENT1 and ENT2 levels were significantly higher in ara-G–sensitive patients than in resistant patients (P = .010 and P = .009, respectively; Figure 6B) permitting a higher uptake of ara-G. ENT1 expression levels strongly correlated with ara-G sensitivity levels (P = .005 R = −0.503). Also for T-ALL samples, we found a correlation between ENT1 levels and ara-C sensitivity (P = .011 R = −0.60). Strikingly, ENT1 and ENT2 levels were not related to forodesine sensitivity, indicating that cellular uptake of forodesine may be facilitated by another transporter.
Gene expression in relation to forodesine/dGuo or ara-G sensitivity. mRNA expression of 9 genes in forodesine/dGuo (A) or ara-G (B) –sensitive and –resistant patient samples. Each dot represents a measurement in one patient sample. *P < .05, **P < .01.
Gene expression in relation to forodesine/dGuo or ara-G sensitivity. mRNA expression of 9 genes in forodesine/dGuo (A) or ara-G (B) –sensitive and –resistant patient samples. Each dot represents a measurement in one patient sample. *P < .05, **P < .01.
Combination studies
In the treatment of leukemia, multiple drugs are administered simultaneously or administered sequentially. It is therefore important to test for drug interactions. To this end we explored the presence of synergistic, additive or antagonistic effects between forodesine/dGuo and 7 other compounds that are currently used in ALL treatment protocols. Leukemic cells were incubated with a concentration range of these 7 compounds with or without the LC10 or LC30 cytotoxic dGuo concentrations (0.02μM and 0.48μM for T-ALL and 0.5μM and 3.5μM for BCP-ALL, respectively) in the presence of 1μM forodesine. As controls, samples were incubated with LC10 or LC30 concentrations of dGuo with 1μM forodesine only. For prednisone, vincristine, and asparaginase, no significant synergistic or antagonistic effects were found in combination with forodesine/dGuo. To our surprise, no antagonism was observed between forodesine/dGuo and ara-G or ara-C although these drugs depend on the same enzymes of the guanosine salvage pathway. For daunorubicin, addition of LC10 forodesine/dGuo levels resulted in an increase of cellular viability, both for T-ALL as well as for BCP-ALL samples (42% vs 61% for T-ALL (P = .009) and 45% vs 66% for BCP-ALL (P = .018). This effect was only observed at a daunorubicin concentration of 0.125 μg/mL (supplemental Figure 2A-B), but not at other daunorubicin concentrations. In addition, no antagonistic effect was measured for any of the daunorubicin concentrations combined with the LC30 forodesine/dGuo level. For various concentrations of 6MP combined with the LC10 or LC30 concentrations forodesine/dGuo, synergistic toxicity was observed for T-ALL samples (supplemental Figure 2C-D).
Discussion
In this study, we have demonstrated selective toxicity of forodesine/dGuo treatment for pediatric T-ALL compared with BCP-ALL and AML samples. The median forodesine/dGuo LC50 value was > 5-fold lower for T-ALL than for BCP-ALL samples. Only 1 of 17 AML patients reached an LC50 below 10μM. This patient was also a Down syndrome patient, a syndrome known to display increased sensitive to a wide range of drugs and these patients are highly susceptibility toward toxic side effects.30,31 High sensitivity of pediatric T-ALL patients toward forodesine/dGuo exposure is in line with expectations, as natural occurring PNP deficiency is known to result in T-cell lymphopenia,12,13 and provided the rationale to develop PNP inhibitors for treatment of T-cell malignancies. Forodesine is a very potent inhibitor of PNP that inhibits PNP activity in the picomolar range in biochemical experiments.32 Cytotoxic effects of forodesine were shown on T-ALL cell lines before,7 and a clinical response has been documented in a phase 1 trial for advanced T-cell malignancies.19 Our in vitro studies indicated that 1μM forodesine is sufficient to inhibit PNP activity in a cellular system, which is well within clinical achievable plasma concentrations. Steady-state forodesine levels that range between 4 and 8μM were documented in the plasma of patients after IV infusion of 40 mg/m2 of forodesine.19 In this clinical phase 1 trial, elevated dGuo levels up to 34μM in plasma were documented. As the median LC50 dGuo levels (in the presence of 1μM forodesine) in our study for forodesine responsive T-ALL samples was estimated on 1.6μM (range 0.31-10μM), this indicates that forodesine may be a promising compound in future clinical trials for nearly 75% of pediatric T-ALL patients.
In the present study, we demonstrate that nearly half of all BCP-ALL patient samples responded to dGuo/forodesine with dGuo LC50 values that ranged between 0.67 and 10μM. Again, this is well within clinical achievable plasma dGuo levels after forodesine infusion, suggesting that forodesine treatment may be effective for nearly 50% of BCP-ALL samples.
Selective T-cell toxicity was also demonstrated for the arabinoguanosine derivative compound ara-G. Primary T-ALL patient samples had a median LC50 value of 20.5μM ara-G whereas approximately half of BCP-ALL or AML samples did not reach an LC50 within the limits of our assay. T-cell selective toxicity of ara-G is in line with previous studies,14-17,33 and nelarabine is an approved drug for T-cell malignancies.23 One of the explanations for selective T-cell toxicity by forodesine/dGuo or ara-G treatment is the finding that T-ALL samples express less PNP, which is in line with our previous finding that T-ALL cells have lower PNP activity compared with BCP-ALL cells.34 In addition, the expression of cytosolic purine 5-prime nucleotidase NT5C2 was lower in T-ALL cells than in BCP-ALL cells, so T-ALL cells have a reduced capacity to revert phosphorylation of dGuo. The expression of the equilibrative nucleoside transporters ENT1 and ENT2 was higher for T-ALL than for BCP-ALL cells, possibly resulting in enhanced cellular uptake of dGuo and ara-G. Lower expression levels of PNP and NT5C2 but higher expression of ENT1 and ENT2 transporters in T-ALL cells are in line with our finding of higher basal intracellular dGTP levels after exposure to dGuo in T-ALL patient samples than in BCP-ALL samples. However, after inhibition of PNP activity by forodesine, both responding T-ALL and BCP-ALL samples seem equally efficient to accumulate comparable levels of intracellular dGTP. So, differential sensitivity for T-ALL and B-ALL cells toward forodesine may not be because of differences in the dGuo to dGTP activation steps in the purine salvage pathway, but may be because of differential cytotoxic effects of accumulated dGTP levels on ribonucleotide reductase activity and inhibition of DNA synthesis, or intrinsic differences in the apoptotic thresholds between T cells and B cells.
Although dGuo-mediated toxicity through forodesine and ara-G toxicity depends on stepwise phosphorylation steps in the purine salvage pathway, no relationship could be demonstrated between forodesine/dGuo sensitivity and ara-G sensitivity. This was further supported by the fact that resistance to ara-G exposure did not preclude sensitivity for forodesine/dGuo or vice versa. In contrast, sensitivity levels toward ara-G strongly correlated with ara-C sensitivity levels. Although T-ALL samples have different expression levels of enzymes and transporters that favor preferential phosphorylation of dGuo or ara-G in T-ALL cells compared with BCP-ALL cells, our results imply that toxicity levels for both compounds are determined by different components in the purine salvage pathway. For this, dCK has been suggested as an important and rate-limiting factor in the phosphorylation of pyrimidine and purine deoxynucleosides9 that has been associated with ara-C resistance35-38 or relapse.39,40 However, we did not observe differences in dCK expression levels between forodesine/dGuo-sensitive and -resistant patients, nor between ara-G–sensitive and –resistant patients. In our previous study on infant BCP-ALL, a 2-fold lower expression in dCK levels was identified despite a 3.3-fold higher sensitivity levels toward ara-C compared with noninfant ALL patients.25 This indicates that dCK is not a major contributer to ara-C, ara-G, or forodesine/dGuo toxicity, even when nonphysiologic high levels of deoxycytidine can block ara-G toxicity.14,17
We observed significant differences in the mitochondrial deoxyguanosine kinase (dGK) expression levels between forodesine/dGuo-sensitive and -resistant patient samples, but not between ara-G–sensitive and –resistant patients. This finding is completely in line with previous findings by Gandhi and coworkers who demonstrated that dGuo is predominantly phosphorylated by dGK but not by dCK, whereas ara-G can be phosphorylated by both enzymes with dGK as preferential enzyme at limiting ara-G concentrations.41 Ara-G resistance could be associated with significant lower expression levels of the ENT1 and ENT2 transporters. These transporters have been shown important for the import of ara-C,42 and elevated ENT1 levels have been reported to explain the high ara-C sensitivity of infant ALL, and a strong correlation was observed between ENT1 expression levels and ara-C sensitivity.25 Lower ENT1 expression levels have been related to ara-C resistance in childhood AML.29 Previous work by Huang et al43 on the T-ALL cell line CCRF-CEM demonstrated that while the cellular uptake of forodesine was dependent on ENT1 and ENT2, forodesine toxicity was not. This is in agreement with our data, and ENT1 and ENT2 expression levels were not related to forodesine toxicity levels. These data therefore suggest that forodesine import and subsequent PNP inhibition seems not limited in leukemia cells but may depend on the import and activation of dGuo. Import of dGuo has been reported to occur via concentrative nucleoside transporters.43 Although the observed differences in dGTP levels and ENT1-2 and dGK expression may contribute to forodesine/dGuo or araG resistance, exact resistance mechanisms are not yet clear. For CLL blasts, forodesine/dGuo effectiveness has been related to basal levels of MCL1 and BIM, elevated phospho-dCK to dCK ratios after treatment, and induction of p73 that may up-regulate BIM via the FOXO1 and FOXO3A transcription factors.6 A recent study provided an alternative mechanism of forodesine resistance as marrow stromal cells were shown to antagonize forodesine-enforced apoptosis in CLL cells.44
The combination cytotoxicity assays revealed no antagonistic or synergistic effect of forodesine/dGuo combined with prednisone, vincristine, or asparaginase. For daunorubicin, we observed an antagonistic effect, but only at a single concentration combined with the LC10, but not with the concentration of LC30 forodesine/dGuo. We found no antagonistic effect for forodesine/dGuo with either the purine analog ara-G, nor with the pyrimidine analog ara-C. Moreover forodesine/dGuo had a synergistic effect in T-ALL with another purine analog, 6MP, at multiple concentrations combined with the LC10 and LC30 dGuo/forodesine concentrations. The molecular basis of these differences in combined effects remains elusive.
We conclude that forodesine and ara-G have cytotoxic effects on T-ALL and to a lesser extent on BCP-ALL cells in vitro and could therefore have potential beneficial therapeutic effects in both types of leukemia, possibly in a combined therapy approach. In AML patients, forodesine treatment is expected to result in little response. Our study gives no indication of clear antagonistic effects of forodesine/dGuo when combined with any of the 7 drugs as currently used in leukemia therapy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by research funding from Mundipharma International Ltd (J.P.P.M. and C.M.Z.). I.H. is financed by the Dutch Cancer Society (KWF-EMCR 2006-3500). W.K.S. is financed by the Stichting Kinderen Kankervrij (KiKa; grant KiKa 2008-029).
Authorship
Contribution: I.H. wrote the manuscript and performed experiments; C.M.Z. wrote the manuscript and designed experiments; C.Y.M., S.B., and F.H. designed experiments; C.P. and W.K.S. performed experiments; R.P. designed experiments and wrote the manuscript; and J.P.P.M. was principal investigator, designed the study, and wrote the manuscript.
Conflict-of-interest disclosure: C.P. and S.B. are employees of Biocryst Pharmaceuticals Inc. C.Y.M. and F.H. are employees of Mundipharma International Ltd. J.P.P.M and C.M.Z. received research funding from Mundipharma International Ltd for this study. The remaining authors declare no competing financial interests.
Correspondence: Jules P. P. Meijerink, PhD, Department of Pediatric Hemato-Oncology, Erasmus MC Rotterdam–Sophia Children's Hospital, Dr Molewaterplein 60 Sp2456, 3015 GJ Rotterdam, The Netherlands; e-mail: j.meijerink@erasmusmc.nl.