Abstract
The prognostic value of MRD in large series of childhood T-ALL has not yet been established. Trial AIEOP-BFM-ALL 2000 introduced standardized quantitative assessment of MRD for stratification, based on immunoglobulin and TCR gene rearrangements as polymerase chain reaction targets: Patients were considered MRD standard risk (MRD-SR) if MRD was negative at day 33 (time point 1 [TP1]) and day 78 (TP2), analyzed by at least 2 sensitive markers; MRD intermediate risk (MRD-IR) if positive either at day 33 or 78 and < 10−3 at day 78; and MRD high risk (MRD-HR) if ≥ 10−3 at day 78. A total of 464 patients with T-ALL were stratified by MRD: 16% of them were MRD-SR, 63% MRD-IR, and 21% MRD-HR. Their 7-year event-free-survival (SE) was 91.1% (3.5%), 80.6% (2.3%), and 49.8% (5.1%) (P < .001), respectively. Negativity of MRD at TP1 was the most favorable prognostic factor. An excellent outcome was also obtained in 32% of patients turning MRD negative only at TP2, indicating that early (TP1) MRD levels were irrelevant if MRD at TP2 was negative (48% of all patients). MRD ≥ 10−3 at TP2 constitutes the most important predictive factor for relapse in childhood T-ALL. The study is registered at http://www.clinicaltrials.gov; “Combination Chemotherapy Based on Risk of Relapse in Treating Young Patients With Acute Lymphoblastic Leukemia,” protocol identification #NCT00430118 for BFM and #NCT00613457 for AIEOP.
Introduction
T immunophenotype was considered to be an unfavorable prognostic feature in childhood acute lymphoblastic leukemia (ALL) until the late 1980s,1-5 and T-ALL was generally treated with high-risk protocols. Since the 1990s, more intensive chemotherapy approaches have allowed to achieve results in T-ALL that are similar to those obtained in B-cell precursor ALL (pB-ALL).6-8
In the last 2 decades, the prognostic impact of minimal residual disease (MRD) in childhood ALL has been extensively investigated.9-14 The bulk of data, however, concerns pB-ALL, whereas data in T-ALL are scarce and based on small numbers of patients. PCR-MRD results obtained with 2 clonal Ig/TCR targets, in a series of 71 childhood T-ALL cases treated in ALL-BFM 90, AIEOP-ALL 91, and DCLSG-ALL-8 studies, identified a slower clearance of leukemic cells in T-ALL compared with pB-ALL. MRD levels at week 5 and 12 of treatment were available in 43 cases and identified subgroups of T-ALL with very different prognoses.15
This paper reports the largest series of children with T-ALL (n = 464), in whom MRD response has been evaluated prospectively at 2 time points; this was performed as part of the cooperative AIEOP-BFM ALL 2000 study (carried out in Austria, Germany, Italy, and Switzerland).16 Although the results obtained in this study on pB-ALL have been recently reported,14 here we describe the dynamics of molecular response, its prognostic relevance, compared with that of traditional prognostic features, and the relationship with maturational stage for the T-ALL subpopulation.
Methods
Patients
From July 1, 2000 (September 1, 2000 for AIEOP) to July 31, 2006 (June 30, 2006 for BFM), 4741 children between 1 and 18 years of age with Ph− ALL were eligible to the AIEOP-BFM ALL 2000 study, conducted in 127 centers in Austria, Germany, Italy, and Switzerland. T-ALL was diagnosed in 627 patients (13.2%); 464 of them were eligible and fully evaluable for stratification by MRD, according to study design, and are here described. The remaining patients could not be stratified by MRD because of: early patient's death (n = 16) or relapse before day 78 (n = 2), lack of follow-up material (n = 6), only 1 sensitive marker available (n = 65), and lack of sensitive markers (n = 80). Features and outcome of these 145 patients were very similar to those of patients stratified by MRD (data not shown).
Diagnostic studies
Diagnosis of ALL was performed using cytomorphology and cytochemistry when ≥ 25% of lymphoblastic cells were present in the bone marrow. T-cell origin of ALL was defined by a blast cell immunophenotype CD3+ (either on surface or cytoplasmic) and CD19−. European Group for the Immunologic Characterization of Leukemias definition was routinely used in the central laboratories of each country to classify T-ALL in the following subtypes: early, cortical (or intermediate), and mature.17,18 Early T-cell precursor (ETP, early T with stem cell or myeloid markers19 ) subtype was not screened routinely but was available for a patient subset.
Complete remission (CR) was defined as: absence of physical signs of leukemia, bone marrow with active hematopoiesis and < 5% leukemia blast cells (identified morphologically), and normal cerebrospinal fluid. Patients who did not achieve CR at the end of induction phase IA were treated according to the high risk (HR) chemotherapy schedule and evaluated at a later time point; resistance was defined as failure to achieve CR by the end of the third HR block (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Molecular target identification and MRD analysis
The organization of the study, cell sample isolation, and the identification of the target for MRD evaluation were recently reported.16 Briefly, genomic DNA samples obtained at the time of diagnosis were screened by PCR amplification using the BIOMED-1 primer sets for complete and incomplete TCR-δ, TCRD (Vd-(Dd)-Jd1, Dd2-Jd1, Vd2-Dd3, Dd2-Dd3), TCR-γ, TCRG (Vg-Jg1.3/2.3, Vg-Jg1.1/2.1) rearrangements, and SIL-TAL deletion.20 Complete and incomplete IGH rearrangements (VH-(DH)-JH, DH-JH) were identified using 5 VH and 6 DH family primers in combination with one JH consensus primer21,22 ; whereas for complete and incomplete TCRB (Vb-(Db)-Jb, Db-Jb) rearrangements, the respective BIOMED-2 multiplex PCR primer sets were used.23 PCR products obtained from Ig/TCR gene rearrangements were further examined by heteroduplex or gene scanning analysis to discriminate between amplifications derived from monoclonal or polyclonal lymphoid cell populations.
Junctional regions of clonal PCR products were sequenced, and patient-specific junctional region sequences of potential MRD PCR targets identified.16 Allele-specific oligonucleotide primers were designed complementary to the junctional region sequence of each target, either manually or using the Primer Express Version 3.0 software (Applied Biosystems).
MRD PCR targets were tested for specificity and sensitivity to enable on-time monitoring of remission samples, and real-time quantitative PCR analysis was performed and interpreted according to the guidelines developed within the “European Study Group for MRD detection in ALL” (ESG-MRD-ALL).16,23,24
MRD-derived risk group classification and final stratification
Patients were defined at MRD standard risk (MRD-SR) if MRD was found negative at both time points, days 33 (time point 1 [TP1]) and 78 (time point 2 [TP2]), using at least 2 molecular targets with a sensitivity of ≤ 10−4. If MRD levels differed between the 2 markers, the highest MRD level was chosen for the final MRD assessment. Patients were considered at intermediate risk (MRD-IR) when MRD was positive at one or both TPs, but at a level < 10−3 at TP2 with at least 2 markers. If MRD levels differed between the 2 markers, the highest MRD level was chosen for the final MRD classification, provided that the selected markers had a sensitivity of at least 10−3. Patients with MRD ≥ 10−3 at TP2 were classified at MRD-HR, independently of the sensitivity and the number of markers (provided that at least 1 marker had a sensitivity of 10−3). EuroMRD guidelines were used to reduce the risk for false-negative and false-positive results. Final stratification reflected the MRD-based stratification, although patients with either prednisone poor-response (PPR, ≥ 1000 circulating blasts/μL on day 8) or failure to achieve CR after induction phase IA were all allocated to the HR arm, independently of their MRD results.
Treatment protocol
Treatment outlines, details, and differences between AIEOP and BFM are shown in supplemental Table 1. In the induction phase, all patients were given a prephase of 7 days, including steroid therapy (prednisone) and one intrathecal dose of methotrexate (MTX), followed by induction protocol IA and induction consolidation protocol IB; on day 8, patients were randomized to continue steroid treatment with either prednisone (60 mg/m2 per day) or dexamethasone (10 mg/m2 per day) until day 28 with subsequent tapering doses. In protocol M and reinduction phases, SR and IR patients received 4 courses of HD-MTX (5 g/m2, protocol M), together with oral 6-mercaptopurine and intrathecal therapy. At the beginning of the reinduction phase, all patients were eligible for the second randomization: either protocol II or reduced-intensity protocol III in SR group; either protocol II or reduced-intensity protocol III given twice in IR group; 3 blocks of non–cross-resistant drugs followed by protocol III given 3 times versus either 3 blocks followed by protocol II given twice (in AIEOP), or 6 blocks followed by protocol II (in BFM) in the HR group. Maintenance therapy consisted of daily 6-mercaptopurine together with weekly MTX until 24 months from diagnosis. With central nervous system-directed therapy, in AIEOP it consisted of intrathecal MTX administration during each treatment phase, including the continuation phase in patients not irradiated; cranial radiotherapy was given (dosage by age; supplemental Table 1) to patients non-HR with white blood cell (WBC) count at diagnosis ≥ 100 000/μL, or at HR or with central nervous system involvement at diagnosis. In BFM, it consisted of intrathecal MTX administration during each treatment phase (except for maintenance therapy), triple intrathecal therapy during the HR blocks, and cranial radiotherapy to all patients (the dosage was adjusted according to patient's age; supplemental Table 1). Ethical approval for this trial was obtained locally by each participating organization.
Statistical analysis
Event-free survival (EFS) and survival were calculated from date of diagnosis to date of events, which, for EFS, were resistance, relapse, death, or second malignant neoplasm, whichever occurred first, and, for survival, death from any cause. Of note, only patients alive by TP2 are, by definition of MRD stratification, included in this study population. EFS and survival curves were estimated according to Kaplan-Meier with Greenwood SE (always indicated in parentheses) and compared according to log-rank test. Cumulative incidence curves for relapse were estimated adjusting for competing risks of other events and were compared with Gray test.25 A one-step multivariate Cox model, stratified by group (AIEOP and BFM), was applied to analyze the cause-specific hazard of relapse.26 The proportional hazard assumption was tested by graphical checks. Tests were 2-sided, with .05 significance level. Follow-up was updated at December 2009. Analyses were carried out using SAS Version 9.1.
The study is registered at the United States National Institutes of Health Web site (http://www.clinicaltrials.gov; “Combination Chemotherapy Based on Risk of Relapse in Treating Young Patients With Acute Lymphoblastic Leukemia,” protocol identification #NCT00430118 for BFM and #NCT00613457 for AIEOP).
Results
For the whole cohort of 464 patients, the 7-year estimates of EFS (SE) and survival (SE) were 75.9% (2.0) and 80.7% (1.9), respectively, with a median follow-up of 5.6 years (Figure 1).
Overall EFS and survival of 464 T-ALL patients enrolled in the AIEOP-BFM-ALL 2000 study, and stratified by MRD.
Overall EFS and survival of 464 T-ALL patients enrolled in the AIEOP-BFM-ALL 2000 study, and stratified by MRD.
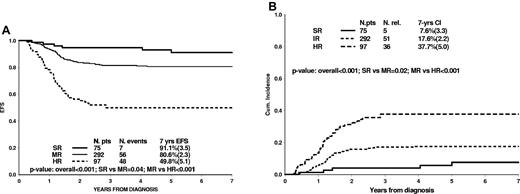
According to the results of MRD study, only 16% of patients tested negative at both time points and were thus at standard risk (MRD-SR). The majority of patients (63%) had detectable levels of MRD at one or both points and were identified as intermediate risk (MRD-IR), with the only exception of those patients who had higher levels of MRD at TP2 (≥ 10−3), then defined as MRD-HR (21%). These 3 subgroups had significantly different outcomes (P < .001), with 7-year-EFS estimates of 91.1% (3.5), 80.6% (2.3), and 49.8% (5.1), respectively (Figure 2A). In particular, in the MRD-HR group, the 7-year EFS becomes 40.5% (8.1) when EFS time is censored at transplantation in first CR. This results from a lower rate of relapse after allogeneic stem cell transplantation (SCT; 13 of 55 patients) compared with nontransplanted MRD-HR patients (23 relapses over 42 patients). A total of 8 patients died of SCT complications (2 patients died in complete continuous remission (CCR) in the nontransplanted group). Another 31 patients not MRD-HR underwent transplant, and 4 of them relapsed.
Treatment outcome in risk groups. EFS (A) and cumulative incidence of relapse (B) according to PCR-based MRD classification in 464 patients.
Treatment outcome in risk groups. EFS (A) and cumulative incidence of relapse (B) according to PCR-based MRD classification in 464 patients.
Types of events are reported in Table 1; 13 patients died in CR (8 of them among the 86 patients who underwent SCT in first CR), and 5 had a secondary malignant neoplasm. Leukemia relapse was the most frequent cause for treatment failure; the 7-year cumulative incidence of relapse was significantly different among PCR-MRD subgroups: 7.6% (3.3) for MRD-SR, 17.6% (2.2) for MRD-IR, and 37.7% (5.0) for MRD-HR patients (Figure 2B).
Distribution of events in T-ALL patients according to MRD classification
| . | MRD-SR . | MRD-IR . | MRD-HR . | Total . | ||||
|---|---|---|---|---|---|---|---|---|
| N . | % . | N . | % . | N . | % . | N . | % . | |
| Total | 75 | 16.2 | 292 | 62.9 | 97 | 20.9 | 464 | — |
| Resistant* | 0 | — | 0 | — | 1 | 1.0 | 1 | 0.2 |
| Relapses | 5 | 6.7 | 51 | 17.5 | 36 | 37.1 | 92 | 19.8 |
| BM | 3 | 4.0 | 23 | 7.9 | 18 | 18.5 | 44 | 9.5 |
| CNS | 2 | 2.7 | 10 | 3.4 | 7 | 7.2 | 19 | 4.1 |
| Testis | 0 | — | 0 | — | 2 | 2.1 | 2 | 0.4 |
| BM + other | 0 | — | 15 | 5.2 | 6 | 6.2 | 21 | 4.5 |
| Other | 0 | — | 3 | 1.0 | 3 | 3.1 | 6 | 1.3 |
| Death in CCR | 1 | 1.3 | 2 | 0.7 | 10 | 10.4 | 13 | 2.8 |
| After chemo | 1 | 1.3 | 2 | 0.7 | 2 | 2.1 | 5 | 1.1 |
| After SCT | 0 | — | 0 | — | 8 | 8.3 | 8 | 1.7 |
| SMN† | 1 | 1.3 | 3 | 1.0 | 1 | 1.0 | 5 | 1.1 |
| CCR | 68 | 90.7 | 236 | 80.8 | 49 | 50.5 | 353 | 76.1 |
| . | MRD-SR . | MRD-IR . | MRD-HR . | Total . | ||||
|---|---|---|---|---|---|---|---|---|
| N . | % . | N . | % . | N . | % . | N . | % . | |
| Total | 75 | 16.2 | 292 | 62.9 | 97 | 20.9 | 464 | — |
| Resistant* | 0 | — | 0 | — | 1 | 1.0 | 1 | 0.2 |
| Relapses | 5 | 6.7 | 51 | 17.5 | 36 | 37.1 | 92 | 19.8 |
| BM | 3 | 4.0 | 23 | 7.9 | 18 | 18.5 | 44 | 9.5 |
| CNS | 2 | 2.7 | 10 | 3.4 | 7 | 7.2 | 19 | 4.1 |
| Testis | 0 | — | 0 | — | 2 | 2.1 | 2 | 0.4 |
| BM + other | 0 | — | 15 | 5.2 | 6 | 6.2 | 21 | 4.5 |
| Other | 0 | — | 3 | 1.0 | 3 | 3.1 | 6 | 1.3 |
| Death in CCR | 1 | 1.3 | 2 | 0.7 | 10 | 10.4 | 13 | 2.8 |
| After chemo | 1 | 1.3 | 2 | 0.7 | 2 | 2.1 | 5 | 1.1 |
| After SCT | 0 | — | 0 | — | 8 | 8.3 | 8 | 1.7 |
| SMN† | 1 | 1.3 | 3 | 1.0 | 1 | 1.0 | 5 | 1.1 |
| CCR | 68 | 90.7 | 236 | 80.8 | 49 | 50.5 | 353 | 76.1 |
— indicates not applicable; BM, bone marrow; CNS, central nervous system; CCR, complete continuous remission; and SMN, secondary malignant neoplasm.
Only patients alive by TP2 are included in this cohort; consequently, induction deaths in protocol IA and IB are not included.
Resistant patients are those who did not achieve CR by the end of the third HR block of chemotherapy.
Brain tumor (n = 1, MRD-SR, at 7.7 years from diagnosis of T-ALL), myelodysplastic syndrome (n = 1, MRD-IR at 2.1 years), glioblastoma (n = 1, MRD-IR at 4.2 years), mucoepidermoid carcinoma (n = 1, MRD-IR at 2.3 years), and malignant histiocytosis (n = 1, MRD-HR at 1.2 years from diagnosis of T-ALL).
The distribution of disease and patient characteristics by PCR-MRD stratification is shown in Table 2, with the corresponding 5-year EFS values. Patients with WBC count < 100 000/μL had a higher probability of being at SR than patients with higher WBC count, but the discrimination of prognosis by MRD was very similar in the 2 subgroups. MRD-HR was more frequent in PPR than in prednisone good-response patients (P < .001); among MRD-HR patients, those with PPR tended to have a worse outcome compared with prednisone good-response patients (although not significant, P = .21).
Clinical-biologic features and related outcome (EFS estimated [SE] at 5 years from diagnosis) in 464 T-ALL patients according to MRD classification
| . | MRD-SR . | MRD-IR . | MRD-HR . | Total N . | |||
|---|---|---|---|---|---|---|---|
| N (%) . | 5-year EFS (SE) . | N (%) . | 5-year EFS (SE) . | N (%) . | 5-year EFS (SE) . | ||
| Total | 75 (16.2) | 93.0 (3.0) | 292 (62.9) | 80.6 (2.3) | 97 (20.9) | 49.8 (5.1) | 464 |
| Sex | |||||||
| Male | 52 (15.2) | 90.1 (4.2) | 219 (63.8) | 79.6 (2.8) | 72 (21.0) | 53.6 (5.9) | 343 |
| Female | 23 (19.0) | 100.0 | 73 (60.3) | 83.5 (4.3) | 25 (20.7) | 39.0 (9.9) | 121 |
| Age, y | |||||||
| 1-9 | 36 (14.6) | 88.7 (5.3) | 160 (64.8) | 84.2 (2.9) | 51 (20.6) | 48.2 (7.1) | 247 |
| 10-17 | 39 (18.0) | 97.4 (2.5) | 132 (60.8) | 76.4 (3.7) | 46 (21.2) | 51.7 (7.4) | 217 |
| WBC count, /μL | |||||||
| < 100 000 | 58 (21.3) | 94.8 (2.9) | 170 (62.5) | 82.9 (2.9) | 44 (16.2) | 49.5 (7.6) | 272 |
| ≥ 100 000 | 17 (8.9) | 87.8 (8.1) | 122 (63.5) | 77.6 (3.8) | 53 (27.6) | 50.2 (6.9) | 192 |
| Response to prednisone | |||||||
| PGR | 71 (23.8) | 94.1 (2.9) | 194 (65.1) | 83.9 (2.6) | 33 (11.1) | 59.1 (8.8) | 298 |
| PPR | 4 (2.5) | — | 94 (59.1) | 74.0 (4.6) | 61 (38.4) | 44.3 (6.4) | 159 |
| Not known | 0 | — | 4 (57.1) | — | 3 (42.9) | — | 7 |
| Final stratification AIEOP-BFM 2000 | |||||||
| SR | 71 (100.0) | 94.1 (2.9) | 0 | — | 0 | — | 71 |
| IR | 0 | — | 198 (100.0) | 83.8 (2.6) | 0 | — | 198 |
| HR | 4* (2.1) | — | 94† (48.2) | 74.0 (4.6) | 97 (49.7) | 49.8 (5.1) | 195 |
| T-ALL subtype | |||||||
| Early | 6 (5.7) | — | 68 (64.1) | 65.6 (5.9) | 32 (30.2) | 46.9 (8.8) | 106 |
| Cortical | 66 (24.2) | 92.1 (3.4) | 171 (62.6) | 85.9 (2.7) | 36 (13.2) | 50.8 (8.6) | 273 |
| Mature | 2 (2.8) | — | 42 (59.2) | 80.8 (6.1) | 27 (38.0) | 48.2 (9.6) | 71 |
| Not specified | 1 (7.1) | — | 11 (78.6) | — | 2 (14.3) | — | 14 |
| . | MRD-SR . | MRD-IR . | MRD-HR . | Total N . | |||
|---|---|---|---|---|---|---|---|
| N (%) . | 5-year EFS (SE) . | N (%) . | 5-year EFS (SE) . | N (%) . | 5-year EFS (SE) . | ||
| Total | 75 (16.2) | 93.0 (3.0) | 292 (62.9) | 80.6 (2.3) | 97 (20.9) | 49.8 (5.1) | 464 |
| Sex | |||||||
| Male | 52 (15.2) | 90.1 (4.2) | 219 (63.8) | 79.6 (2.8) | 72 (21.0) | 53.6 (5.9) | 343 |
| Female | 23 (19.0) | 100.0 | 73 (60.3) | 83.5 (4.3) | 25 (20.7) | 39.0 (9.9) | 121 |
| Age, y | |||||||
| 1-9 | 36 (14.6) | 88.7 (5.3) | 160 (64.8) | 84.2 (2.9) | 51 (20.6) | 48.2 (7.1) | 247 |
| 10-17 | 39 (18.0) | 97.4 (2.5) | 132 (60.8) | 76.4 (3.7) | 46 (21.2) | 51.7 (7.4) | 217 |
| WBC count, /μL | |||||||
| < 100 000 | 58 (21.3) | 94.8 (2.9) | 170 (62.5) | 82.9 (2.9) | 44 (16.2) | 49.5 (7.6) | 272 |
| ≥ 100 000 | 17 (8.9) | 87.8 (8.1) | 122 (63.5) | 77.6 (3.8) | 53 (27.6) | 50.2 (6.9) | 192 |
| Response to prednisone | |||||||
| PGR | 71 (23.8) | 94.1 (2.9) | 194 (65.1) | 83.9 (2.6) | 33 (11.1) | 59.1 (8.8) | 298 |
| PPR | 4 (2.5) | — | 94 (59.1) | 74.0 (4.6) | 61 (38.4) | 44.3 (6.4) | 159 |
| Not known | 0 | — | 4 (57.1) | — | 3 (42.9) | — | 7 |
| Final stratification AIEOP-BFM 2000 | |||||||
| SR | 71 (100.0) | 94.1 (2.9) | 0 | — | 0 | — | 71 |
| IR | 0 | — | 198 (100.0) | 83.8 (2.6) | 0 | — | 198 |
| HR | 4* (2.1) | — | 94† (48.2) | 74.0 (4.6) | 97 (49.7) | 49.8 (5.1) | 195 |
| T-ALL subtype | |||||||
| Early | 6 (5.7) | — | 68 (64.1) | 65.6 (5.9) | 32 (30.2) | 46.9 (8.8) | 106 |
| Cortical | 66 (24.2) | 92.1 (3.4) | 171 (62.6) | 85.9 (2.7) | 36 (13.2) | 50.8 (8.6) | 273 |
| Mature | 2 (2.8) | — | 42 (59.2) | 80.8 (6.1) | 27 (38.0) | 48.2 (9.6) | 71 |
| Not specified | 1 (7.1) | — | 11 (78.6) | — | 2 (14.3) | — | 14 |
MRD affected outcome significantly (P < .001) in every subgroup.
PGR indicates prednisone good-responder; and —, not applicable.
These patients were at high risk for PPR (none relapsed, and one died in first CR).
These patients were at high risk for PPR, and 7 of them also presented no CR day 33.
According to AIEOP-BFM ALL 2000 final stratification, 98 patients were allocated to the HR group because of PPR (7 of them also presented no CR at day 33). Interestingly, their MRD classification was SR in 4 patients (3 are in continuous CR and 1 died during chemotherapy blocks), whereas 94 were MRD-IR and their outcome was favorable, with a 5-year-EFS of 74.0% (4.6), implying that MRD response allows to discriminate prognostic subgroups on top of classic HR criteria (PPR and no CR day 33).
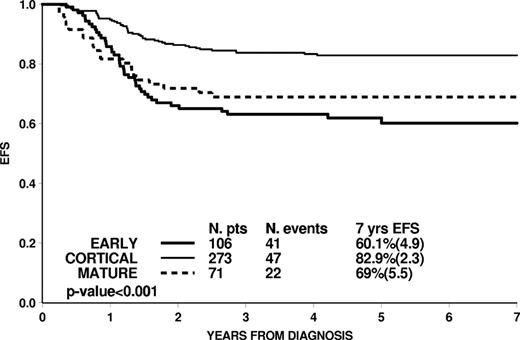
When analyzed by maturational stage, cortical T-ALL was less likely to fall into the MRD-HR group and represented the large majority of MRD-SR patients (66 of 75 patients). Age and leukocyte count were not related to the maturational stage, with age ≥ 10 years in 46.2%, 46.9%, and 49.3% (P = .92) and WBC count ≥ 100 000/mm3 in 40.6%, 41.0%, and 43.7% (P = .91) of early, cortical, and mature T-ALL, respectively. PPR was more frequently observed in mature than in cortical or early T-ALL (59.4% vs 37.1% vs 27.5%, respectively, P < .001).
Overall, the outcome differed by maturational stage, with cortical T-ALL having a better EFS, in keeping with the better profile of early response to initial therapy (Figure 3). MRD levels maintain their prognostic impact within each maturational stage subgroup (Table 2). ETP subtype19 was identified in 19 early T-ALL patients. Of them, 14 were at HR according to AIEOP-BFM final stratification because of MRD-HR (n = 10, with 3 relapses and 2 deaths in continuous CR after transplantation), no CR at day 33 (n = 2, 2 relapses) or PPR (n = 2, 2 relapses); the remaining 5 patients were in the standard (n = 1, in continuous CR) or intermediate risk arm (n = 4, 1 relapse). These data show that most of the ETP patients who fail disease control are identified by poor early response as measured by morphology or MRD.
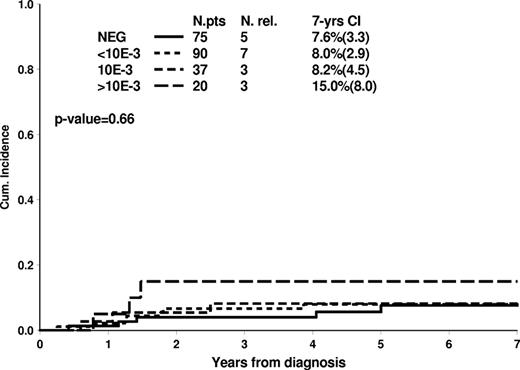
The dynamics of MRD clearance at TP1 and TP2 and its relation with the relapse rates are shown in supplemental Table 2. At TP1, 16% of the T-ALL patients (76 of 462) were MRD negative, and their 7-year cumulative incidence of relapse was 7.5% (3.3). At TP2, the proportion of MRD-negative patients was 48% (222 of 462), and only 18 of them relapsed, this resulting in a 7-year cumulative incidence of 8.5% (1.9). The favorable outcome of the large cohort of patients (48%) MRD-negative at TP2 was relatively independent of MRD levels at TP1 (Figure 4). Indeed, also patients who became MRD-negative only at TP2 (32% of all cases) had an excellent outcome. On the other hand, for patients who were still positive at TP2, the risk of relapse was closely related to MRD levels at TP2: the 7-year cumulative incidence of relapse was 26.3% (3.7), 33.0% (6.2), and 44.7% (8.1) for MRD levels of < 10−3, 10−3, or > 10−3, respectively (Figure 5).
Cumulative incidence of relapse in 222 T-ALL patients with negative MRD at TP2 according to MRD results at TP1.
Cumulative incidence of relapse in 222 T-ALL patients with negative MRD at TP2 according to MRD results at TP1.
Cumulative incidence of relapse in 464 T-ALL patients by MRD levels at TP2.
Results of the Cox regression analysis show that, compared with MRD-IR patients, MRD-HR patients have a significant 2-fold increase of relapse, whereas MRD-SR is related to a nonsignificant decrease in the hazard of relapse (Table 3). Response to prednisone remains borderline significant (hazard ratio = 1.60 for PPR vs prednisone good-response, P = .05), and also T-ALL early subtype is associated with a significantly worse prognosis. If MRD levels are omitted from the model, results remain similar for all factors considered, except that PPR becomes highly significantly related to the hazard of relapse (data not shown).
Cox model stratified on hazard of relapse for T-ALL patients with known prognostic features (n = 443; 89 relapses)
| . | Hazard ratio . | 95% CI . | P . |
|---|---|---|---|
| Age, y | |||
| < 10 | 1 | ||
| 10-17 | 1.15 | 0.75-1.76 | .53 |
| WBC count, /μL | |||
| < 100 000 | 1 | ||
| ≥ 100 000 | 1.31 | 0.84-2.02 | .23 |
| Response to prednisone | |||
| PGR | 1 | ||
| PPR | 1.60 | 1.01-2.53 | .05 |
| PCR-MRD | |||
| SR | 0.49 | 0.19-1.28 | .15 |
| IR | 1 | ||
| HR | 2.02 | 1.27-3.19 | .003 |
| T-ALL subtype | |||
| Early | 2.03 | 1.26-3.27 | .004 |
| Cortical | 1 | ||
| Mature | 1.28 | 0.70-2.34 | .42 |
| . | Hazard ratio . | 95% CI . | P . |
|---|---|---|---|
| Age, y | |||
| < 10 | 1 | ||
| 10-17 | 1.15 | 0.75-1.76 | .53 |
| WBC count, /μL | |||
| < 100 000 | 1 | ||
| ≥ 100 000 | 1.31 | 0.84-2.02 | .23 |
| Response to prednisone | |||
| PGR | 1 | ||
| PPR | 1.60 | 1.01-2.53 | .05 |
| PCR-MRD | |||
| SR | 0.49 | 0.19-1.28 | .15 |
| IR | 1 | ||
| HR | 2.02 | 1.27-3.19 | .003 |
| T-ALL subtype | |||
| Early | 2.03 | 1.26-3.27 | .004 |
| Cortical | 1 | ||
| Mature | 1.28 | 0.70-2.34 | .42 |
PGR indicates prednisone good-responder.
Discussion
The bulk of published data on MRD response in childhood ALL is related to pB-ALL.9,11,22,27,28 Few reports, generally based on relatively small numbers of patients, have specifically addressed this issue in T-ALL.15,21,29-31
AIEOP, BFM, and DCLSG contributed to the study conducted in the early 1990s, which showed that PCR-MRD measured at days 33 and 78 of induction therapy allowed identification of 3 prognostic groups, respectively, with very good prognosis (MRD-SR), intermediate prognosis (MRD-IR), and dismal prognosis (MRD-HR).9 In the context of that study, 43 patients with T-ALL treated according to ALL-BFM 90, or AIEOP-ALL 91, or DCLSG-ALL 8 protocols had full information on MRD: 23% were found to be MRD-SR, 49% MRD-IR, and 28% MRD-HR (compared with the distribution in pB-ALL of 46% in SR, 43% in IR, and 11% in HR) and their 5-year relapse-free survival was 100%, 76%, and 0%, respectively.15 In these patients, according to study design, MRD was not used for tailoring treatment, but approximately two-thirds of MRD-HR T-ALL were treated in the HR arm because of PPR. However, the HR treatment for ALL-BFM 90 or AIEOP-ALL 91, based on block therapy, included neither the induction consolidation phase IB nor protocol II; consequently, the resulting outcome was not satisfactory.32,33 In the subsequent ALL-BFM 95 and AIEOP-ALL 95 studies, treatment for HR patients was modified during consolidation blocks and by introducing one (BFM) or 2 (AIEOP, together with protocol IB) “delayed intensifications” (protocol II), with the result of an improved outcome for these patients.8,34
In the year 2000, AIEOP and BFM started a common prospective study in which children with newly diagnosed ALL were stratified on the basis of PCR-MRD analysis performed at the same 2 time points.16 In this study, patients at HR, also those at HR only for MRD findings, received treatment based on the 1995′ approach. The information gathered from the AIEOP-BFM ALL 2000 study allows for the first time to investigate the actual MRD response pattern in a cohort of patients, which may be considered adequate both for the number of patients and the quality of treatment. Moreover, it provides new information, which may be useful to clarify the conflicting results on the prognostic role of maturational stage in T-ALL.
Indeed, the results of the present study show that MRD risk groups defined by MRD levels at the end of induction (day 33) and after induction-consolidation (day 78) compose the most powerful independent prognostic factor for relapse in T-ALL, as it is for pB-ALL.14 However, in contrast to pB-ALL, MRD in T-ALL has also an impact on the incidence of relapses with extramedullary components. The percentage of this type of relapses in our T-ALL cohort increases from 2.7% in MRD-SR to 9.6% in MRD-IR, and to 18.6% in MRD-HR patients, whereas corresponding values in pB-ALL were 2.1%, 6.0%, and 5.8%, respectively.14 Of note, all patients at MRD-HR received maximal treatment for prevention of central nervous system relapse, including high dose of MTX and of cytosine arabinoside, and cranial radiotherapy.
The pattern of blast clearance in response to chemotherapy is very different in T-ALL compared with pB-ALL in the context of identical therapy, being used for these 2 biologically different diseases. In particular, patients with T-ALL show a slower blast clearance (ie, a higher tumor burden at the selected time points, day 33 and day 78); as a consequence, the proportion of patients falling into the MRD-defined categories is very different, with a higher proportion of patients in the categories with insufficient blast clearance. Thus, only 16.2% show a complete clearance (ie, they belong to the SR) compared with 42.3% in pB-ALL, a comparable proportion remain in the IR group (62.9% vs 51.7%), whereas the proportion of cases with high tumor load is approximately 4-fold (20.9% vs 5.9%).14
Thus, the strategy to analyze MRD at 2 time points revealed distinct response characteristics of pB- and T-ALL to the identical induction-consolidation therapy, whereas the outcome of these 3 MRD-based risk groups is strikingly similar (Figure 2).14 MRD-SR patients defined by being MRD-negative already by day 33 have a very good prognosis, with a 7.6% (3.3) 7-year cumulative incidence of relapse (pB-ALL MRD-SR 7.2%, SE 1.2). Interestingly, in T-ALL patients more than in pB-ALL, incidence of relapse is strongly related to the levels of MRD at day 78 (TP2): patients who are MRD-negative at TP2 (they include also as a subset the MRD-SR cases) are a large fraction (48%) of the T-ALL population, and have again a very good outcome (7-year cumulative incidence of relapse 8.5%; 1.9). This is largely different to pB-ALL in which patients who were MRD-negative at TP2 had a 7-year cumulative incidence of relapse of 16.2% (1.8), and 23.0% (3.6), depending on the level of MRD at TP1.14
On the contrary, patients who remain positive at TP2 have a dismal prognosis, with a risk of relapse escalating from 26.3% for those who are low-positive (< 10−3), up to 33.0% (6.2) and 44.7% (8.1) in those with high burden of residual disease (either 10−3 or > 10−3, respectively). In MRD-HR patients, relapses appeared to be reduced by allogeneic SCT, although the final outcome shows a modest improvement compared with chemotherapy alone. These findings strengthen the value of MRD information at TP2 for T-ALL patients.
Results by maturational stage show that patients with the early T-ALL subtype, as defined by European Group for the Immunologic Characterization of Leukemias, have a worse outcome than those with cortical or mature subtype if they are stratified to the intermediate MRD risk group. By contrast, early T-ALL patients who were stratified into the HR group had the same outcome as those with cortical or mature T-ALL. In the small subgroup identified as very ETP, response by MRD was slow; thus, the large majority (14 of 19 patients) were allocated to the HR treatment. Prognostic impact of this subset of T-ALL is therefore limited. MRD-IR early T-ALL patients who lack HR features (ie, the subset of patients with prednisone good response and CR at day 33, 47 of 68 patients) have a 5-year EFS of 72.2% (6.6), which is reasonably good although inferior to that of other maturational subtypes. The multivariate analysis shows that MRD measurement of early response is the most relevant factor discriminating prognosis, blurring the importance of most of other known prognostic features, such as age and WBC count. However, prednisone response retains some prognostic value in T-ALL, and PPR patients tend to have a worse prognosis, despite the fact that all of them were assigned to the HR treatment regardless of their MRD level (Tables 2 and 3). By contrast, PPR in pB-ALL patients loses its impact after MRD stratification and allocation to HR treatment.14 A higher risk of relapse is also related to early maturational stage.
Comparison of these PCR-MRD findings with MRD data obtained by flow cytometry is very difficult. MRD response was also measured by flow cytometry in a group of patients treated in the context of the study here reported, and day 15 bone marrow evaluation was found very suitable to predict prognosis; the number of T-ALL cases evaluated (n = 87) was, however, too small to draw solid conclusions.13
This study of a large cohort of 464 T-ALL children is able to show some relevant differences with pB-ALL: (1) the clearance of leukemic blast cells is slower in T-ALL; (2) the MRD detection at a late time point (day 78) is more suited to define the risk of relapse in T-ALL than an earlier evaluation at end of induction (day 33); and (3) high levels of MRD at day 78 in T-ALL are not only related to medullar relapses but also to relapses with extramedullary component.
In conclusion, the findings of this study show that measurement of MRD at day 78 allows to identify a large fraction of T-ALL patients (∼ 50%) who become PCR-MRD negative and have an excellent prognosis with current conventional therapy based on the BFM protocol. Conversely, patients (∼ 20%) with high MRD levels at TP2 still have a poor outcome, despite being treated with aggressive HR therapy and SCT.35 Of note, using MRD to assign the risk group and the related treatment did unfortunately not invalidate the prognostic value of MRD, meaning that treatment tailoring still has room for improvement. Treatment response measured by sensitive PCR-MRD at TP2 is, in T-ALL, the most important predictive factor for relapse and allows to identify also patients who may benefit from alternative experimental treatment strategies based on the use of novel antileukemia agents for T-ALL, such as nelarabine or forodesine.36-38 The effort of tailoring front-line therapy can be done either by early interventions in induction-consolidation to impact MRD at TP2, or by applying novel treatments in patients with HR-MRD at TP2. This tailoring is particularly relevant in T-ALL given the very low chances of rescuing these patients once a bone marrow relapse has occurred. By contrast, if MRD testing by sensitive MRD targets is negative at TP2, one is able to save 48% of T-ALL patients, including those with high levels of MRD at TP1, from unnecessary treatment intensification.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the participants of the ESG-MRD-ALL for fruitful discussion on standardization and quality control of the MRD diagnostics, Daniela Silvestri for careful support in data analysis, Marco A. Citterio for help in data revision, AIEOP reference laboratories (G.B., Padova; and A.B., Monza) with their expert technicians, the partners in the reference laboratories and in the central MRD laboratories, all the technicians for their expertise work in cytology and MRD diagnostics, and the data managers for their careful study conduct.
For AIEOP: This work was supported by Comitato M.L. Verga, and Fondazione Tettamanti (Monza), Fondazione Città della Speranza, Fondazione Cariparo (Padova), Associazione Gian Franco Lupo (Pomarico), Associazione Italiana per la Ricerca sul Cancro (ab, gc; mgv IG 5017), Fondazione Cariplo (ab), and Ministero dell'Istruzione, Università e Ricerca (MIUR; ab). For BFM: This work was supported by Deutsche Krebshilfe, Bonn, Germany (grant 50-2698 Schr1 and grant 50-2410 Ba7), the Competence Network Pediatric Hematology and Oncology, which was funded by the Federal Ministry of Research, Oncosuisse/Krebsforschung Schweiz (grant OCS 1230-02-2002), and St Anna Kinderkrebsforschung Austria.
Authorship
Contribution: M.S. planned the study and coordinated the study in Germany; M.G.V. was the study statistician for AIEOP; C.R.B. and A.S. were responsible for diagnosis and PCR MRD analyses for BFM Germany; R.P.-G. was responsible for diagnosis and PCR MRD analyses for BFM Austria; A.M. contributed to study coordination and data revision for BFM Germany; R.P., A.R., T.K., C.M., F.L., B.W.S., M.A., and K.W. contributed to planning the study; M.Z. was the study statistician for BFM; M.D. was responsible for diagnosis for BFM Austria; B.B. contributed to diagnostics; G.B., G.C., and A.B. were responsible for diagnosis and PCR MRD analyses for AIEOP; R.R. was responsible for immunphenotyping in Germany and contributed to planning the study; R.K. was responsible for PCR MRD analyses for BFM Germany; J.J.M.v.D. organized and supervised the standardization and quality control of the PCR-MRD diagnostics, including the development of guidelines for data interpretation; H.G. planned the study and coordinated the study in Austria; V.C. planned the study and contributed to study coordination for AIEOP; V.C., M.G.V., M.S., and A.B. wrote the manuscript; all authors participated in the protocol development, study supervision, and data interpretation stages of this study, and have seen, reviewed, and approved the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A list of the AIEOP-BFM ALL 2000 study group members appears in the supplemental Appendix.
Correspondence: Martin Schrappe, Department of Pediatrics, University Medical Center Schleswig-Holstein, Campus Kiel, Schwanenweg 20, 24105 Kiel, Germany; e-mail: schrappe-office@pediatrics.uni-kiel.de.
References
Author notes
M.S. and M.G.V. contributed equally to this study and share first authorship.
A.B. and V.C. contributed equally to this study and share senior authorship



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal