Abstract
Current guidelines discourage combined oral contraceptive (COC) use in women with hereditary thrombophilic defects. However, qualifying all hereditary thrombophilic defects as similarly strong risk factors might be questioned. Recent studies indicate the risk of venous thromboembolism (VTE) of a factor V Leiden mutation as considerably lower than a deficiency of protein C, protein S, or antithrombin. In a retrospective family cohort, the VTE risk during COC use and pregnancy (including postpartum) was assessed in 798 female relatives with or without a heterozygous, double heterozygous, or homozygous factor V Leiden or prothrombin G20210A mutation. Overall, absolute VTE risk in women with no, single, or combined defects was 0.13 (95% confidence interval 0.08-0.21), 0.35 (0.22-0.53), and 0.94 (0.47-1.67) per 100 person-years, while these were 0.19 (0.07-0.41), 0.49 (0.18-1.07), and 0.86 (0.10-3.11) during COC use, and 0.73 (0.30-1.51), 1.97 (0.94-3.63), and 7.65 (3.08-15.76) during pregnancy. COC use and pregnancy were independent risk factors for VTE, with highest risk during pregnancy postpartum, as demonstrated by adjusted hazard ratios of 16.0 (8.0-32.2) versus 2.2 (1.1-4.0) during COC use. Rather than strictly contraindicating COC use, we advocate that detailed counseling on all contraceptive options, including COCs, addressing the associated risks of both VTE and unintended pregnancy, enabling these women to make an informed choice.
Medscape EDUCATION Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and the American Society of Hematology. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 70% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 2375.
Disclosures
The authors; the Associate Editor David P. Lillicrap; and the CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, declare no competing financial interest.
Learning objectives
Upon completion of this activity, participants will be able to:
Describe findings from previous studies and current guidelines in regard to COC use in women with hereditary thrombophilic defects.
Describe findings from a retrospective family cohort study of the risk for VTE during COC use and pregnancy and the postpartum period among 798 female relatives of symptomatic probands with heterozygous, double heterozygous, or homozygous factor V Leiden or prothombin G20210A mutation.
Describe clinical implications of these findings from the retrospective family cohort study.
Release date: August 25, 2011; Expiration date: August 25, 2012.
Introduction
The risk of venous thromboembolism (VTE) in women using combined oral contraceptives (COCs) is attributed to changes in hemostasis.1-3 These changes may have greater impact in women with thrombophilic defects. Therefore, WHO recommendations state COC use in women with thrombophilic mutations, that is, antithrombin deficiency, protein C deficiency, or protein S deficiency, factor V Leiden, and prothrombin-20210A, as associated with an unacceptable health risk.4 These recommendations are mainly based on case-control studies reporting increased relative risks of VTE during COC use in women with hereditary thrombophilic defects.5-11
However, to qualify all hereditary thrombophilic defects as similarly strong risk factors might be questioned. The absolute risk of VTE in factor V Leiden carriers is estimated being 0.15 per 100 person-years,12 whereas in antithrombin-, protein C–, or protein S–deficient persons these estimates range from 0.7 to 1.7 per 100 person-years, indicating a considerably higher degree of risk.12,13
We previously demonstrated that women with severe hereditary thrombophilic defects, that is, a deficiency of antithrombin, protein C, or protein S, are at very high risk during actual COC use, particularly when concomitant thrombophilic defects are present (4.6 per 100 pill-years), and the use of COCs should be strongly discouraged in these women.14,15
As the absolute risk in women with mild thrombophilic defects is substantially lower than in women with severe thrombophilic defects, withholding COCs in women with mild hereditary thrombophilic defects might be less favorable. When discouraging COC use, an increased risk of unintended pregnancy must be taken into account as alternative nonhormonal contraception is less reliable.16,17
Pregnancy and especially the postpartum period is a strong risk factor for VTE, with an absolute risk of VTE in the general population that is higher than noted for COCs, that is, 0.2 per 100 pregnancy-years18 versus 0.06 per 100 pill-years.19 To balance the risk and benefits of COCs, reliable estimates of the VTE risk associated with both COC use and pregnancy are needed.
To evaluate whether mild thrombophilic defects indeed have lower risk of VTE during COC use, which would be the basis for counseling, the risk of first VTE during COC use and pregnancy postpartum was assessed in a large retrospective family cohort of women with heterozygous, double heterozygous, or homozygous factor V Leiden or prothrombin-G20210A mutation. Women were divided into those with no, single, or combined mild thrombophilic defects, as we recently demonstrated that aggregation of defects is often noted in thrombophilic families, and that especially the presence of multiple defects may significantly increase the absolute risk of VTE.13-15 To put results into perspective, obtained absolute risks are compared with those during use of alternative contraception.
Methods
Subjects
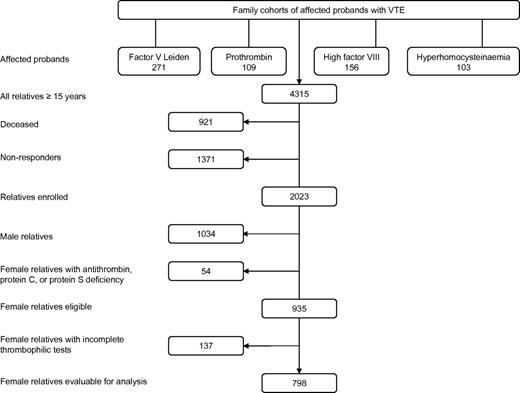
In the present retrospective family cohort study, all female relatives were included from 4 family cohorts, which were described in detail elsewhere.20-23 These were first-degree relatives of consecutive patients (probands) with VTE or premature atherosclerosis (< 50 years) and factor V Leiden, prothrombin-G20210A, high factor VIII levels (> 150 IU/dL), or hyperhomocysteinemia, respectively (Figure 1). Probands and their first-degree relatives were enrolled between 1995 and 1998 (factor V Leiden study) and 1998 and 2004 (prothrombin-G20210A, hyperhomocysteinemia, and factor VIII studies) in 3 university hospitals in The Netherlands (University Medical Center Groningen, University Medical Center Amsterdam, and University Hospital Maastricht). Probands were excluded to avoid bias, as they have experienced VTE by definition. Relatives of probands with premature atherosclerosis were not included in the present study. First-degree relatives aged 15 years or older were identified by pedigree analysis. Relatives were contacted through the probands and were all seen in person at our clinics.
Information on VTE, exposure to exogenous risk factors for VTE, and anticoagulant treatment was collected by physicians and research nurses through medical interviews using a validated questionnaire,24 and by reviewing medical records.
All relatives were tested for the presence of factor V Leiden, prothrombin-G20210A, high factor VIII levels, hyperhomocysteinemia (original index defects), and for a deficiency of antithrombin, protein C, and protein S.
Because of the retrospective nature of the study, collection of medical history took place at the end of the observation period. As thrombophilia testing was performed after collection of these data, the taking of medical histories of relatives was not influenced by the results of thrombophilia testing. For the same reason, any decisions on diagnostic outcome and treatments during the observation periods were made without knowledge on presence of thrombophilia.
Female relatives with a deficiency of antithrombin, protein C, or protein S were excluded, as these deficiencies are strong risk factors for VTE.12-15 As hyperhomocysteinemia is no longer considered an independent thrombophilic risk factor,23,25 this defect was not taken into account, but these women were not excluded from our cohort.
For the purpose of our study, information on contraceptive use and pregnancies (including pregnancy losses) was reconfirmed by a written questionnaire sent by mail. In addition, also general practitioners were contacted for further information. All relatives gave informed consent in accordance with the Declaration of Helsinki and the studies were approved by the institutional review boards of the 3 participating Dutch hospitals.
Diagnosis of VTE
VTE was considered established when diagnosed by compression ultrasound or venography (deep vein thrombosis), by ventilation/perfusion lung-scan, spiral computed tomography scan or pulmonary angiography (pulmonary embolism), or when the patient had received full-dose heparin and vitamin K antagonists for at least 3 months without objective testing at a time when these techniques were not available. VTE was classified as “provoked” when occurring up to 3 months after exposure to exogenous risk factors, which included surgery, trauma, immobilization for at least 7 days, COC use, pregnancy-postpartum up to 3 months, and malignancy. In the absence of these risk factors, VTE was defined as “unprovoked.” Superficial phlebitis was not considered a thrombotic event.
Laboratory studies
Factor V Leiden and prothrombin-G20210A were demonstrated by PCRs.26,27 Factor VIII:C was measured by 1-stage clotting assay and considered increased at levels above 150 IU/dL.28 Protein S– and protein C–Ag levels were measured by ELISA (DAKO); protein C activity and antithrombin levels (Chromogenix) were measured by chromogenic substrate assays. Normal ranges were determined in healthy volunteers without a (family) history of VTE, who were neither pregnant nor used COCs within 3 months before blood sampling. Deficiency of antithrombin, protein S, and protein C was defined by levels below the lower limit of their normal ranges. In probands and symptomatic relatives, blood samples were collected at least 3 months after VTE had occurred. If they were still treated with vitamin K antagonists, samples were taken after temporary change of this therapy to low-molecular-weight heparin for at least 2 weeks.
Statistical analysis
We estimated the overall absolute risk of first VTE in female relatives with no defects, a single defect, or a combination of factor V Leiden and prothrombin-G20210A. Homozygosity for factor V Leiden or prothrombin-G20210A was classified as a combined defect, as their prevalence is too low to calculate reliable estimates separately. Furthermore, the absolute risk during actual COC use and during the pregnancy/postpartum period was estimated.
The absolute risk was expressed as the incidence rate per 100 person-years. Corresponding 95% confidence intervals (CI) were calculated by using the binomial probability model (conditional small-sample approach).29 Person-years were counted from age 15 until age 50 years, first VTE, or end of study. A minimum age of 15 years was chosen because VTE is rare at younger age, and a maximum age of 50 years as end of fertile lifetime. The duration of exposure to COCs (pill-years) included actual use including a 3-month exposure window after COC use was discontinued. For pregnancy, including pregnancy losses, exposure was defined as the gestation time plus 3-month postpartum.
We used a time-varying exposure Cox proportional-hazard model to estimate adjusted hazard ratios (HRs) of actual COC use and the pregnancy-postpartum period next to the presence of single or combined hereditary mild thrombophilic defects. With this model, we specifically took into account that both COC use and the pregnancy-postpartum period are temporary risk periods during fertile lifetime. Effect modification (interactions) of mild thrombophilic defects on both COC use and the pregnancy-postpartum period were also considered. In addition, the influence of an increased factor VIII level (> 150 IU/mL) as an acquired independent risk factor for VTE was estimated.
Furthermore, the absolute risk of VTE during COC use in women with or without mild hereditary thrombophilia was put into the perspective of contraceptive failure of COC and alternative contraceptives. Alternative contraceptives include condom, the copper-intrauterine devices (IUD; 380 mm2), and the levonorgestrel IUD (LNG-IUD). The LNG-IUD is presented as having no increased risk of VTE, as recently reported in a large study.19
Continuous variables were expressed as mean values and SD or median values and range, and categorical data as counts and percentages. A 2-sided P value < .05 indicated statistical significance. Analyses were performed using SAS Version 9.1 software.
Results
Clinical characteristics
The study consisted of 639 unrelated families including 271 probands with factor V Leiden, 109 with prothrombin-G20210A, 156 with high factor VIII levels, and 103 with hyperhomocysteinemia (Figure 1). Of their 4315 relatives aged 15 years or older, 2292 relatives could not be enrolled; 1371 were nonresponders (no consent, geographic distance); and 921 had deceased. Of the 2023 relatives enrolled, 989 were female. Of these women, 54 were not eligible as they had a deficiency of antithrombin, protein C, or protein S, leaving 935 eligible female relatives. A total of 137 eligible women were excluded for incomplete thrombophilic tests. The remaining 798 women were available for analysis (Figure 1).
Recruitment of the study population from families with factor V Leiden, prothrombin G20120A mutation, high factor VIII levels, and hyperhomocysteinemia, respectively.
Recruitment of the study population from families with factor V Leiden, prothrombin G20120A mutation, high factor VIII levels, and hyperhomocysteinemia, respectively.
Table 1 lists the characteristics of the 798 female relatives. Of those, 301 had one or more defects. Among these women, 14 were homozygous for factor V Leiden, of whom 3 were also heterozygous for prothrombin-G20210A mutation, and 4 women were homozygous for the prothrombin-G20210A mutation.
Characteristics of 798 female first-degree relatives with either factor V Leiden, prothrombin G20210A, or a combination of these defects, including homozygosity
| . | All female relatives . | ||
|---|---|---|---|
| No defects . | Single defects . | Combined defects* . | |
| Female relatives, n | 497 | 251 | 50 |
| Follow-up, y† | 32 (0.1-35) | 28 (0.1-35) | 24 (2-35) |
| Factor V Leiden (%) | NA | 160 (64) | 46 (92) |
| Prothrombin G20210A (%) | NA | 91 (36) | 39 (81) |
| Ever COC users, n (%) | 366 (74) | 171 (68) | 34 (68) |
| Age at start of COC, y | 19 (15-47) | 20 (15-49) | 19 (15-49) |
| Duration of COC use, y | 8 (0.1-30) | 6 (0.1-27) | 6 (0.5-16) |
| Ever pregnant, n (%) | 364 (73) | 175 (70) | 36 (72) |
| Age at first pregnancy | 24 (15-41) | 24 (15-36) | 23 (17-38) |
| Number of pregnancies, n | 2 (1-10) | 3 (1-10) | 2 (1-7) |
| Total pregnancy time, y | 2 (0.3-9) | 3 (0.3-9) | 2 (0.8-6) |
| Age at time of VTE, y | 31 (18-46) | 34 (17-48) | 26 (17-39) |
| VTE, n† | 17 | 22 | 11 |
| Unprovoked, n | 1 | 4 | 1 |
| Provoked, n | |||
| COC use | 4 | 1 | 2 |
| Pregnancy/postpartum | 5 | 9 | 7 |
| COC use + postpartum | 2 | 1 | 0 |
| COC use + other risk factor | 0 | 4 | 0 |
| Major trauma, surgery, immobilization | 5 | 3 | 1 |
| . | All female relatives . | ||
|---|---|---|---|
| No defects . | Single defects . | Combined defects* . | |
| Female relatives, n | 497 | 251 | 50 |
| Follow-up, y† | 32 (0.1-35) | 28 (0.1-35) | 24 (2-35) |
| Factor V Leiden (%) | NA | 160 (64) | 46 (92) |
| Prothrombin G20210A (%) | NA | 91 (36) | 39 (81) |
| Ever COC users, n (%) | 366 (74) | 171 (68) | 34 (68) |
| Age at start of COC, y | 19 (15-47) | 20 (15-49) | 19 (15-49) |
| Duration of COC use, y | 8 (0.1-30) | 6 (0.1-27) | 6 (0.5-16) |
| Ever pregnant, n (%) | 364 (73) | 175 (70) | 36 (72) |
| Age at first pregnancy | 24 (15-41) | 24 (15-36) | 23 (17-38) |
| Number of pregnancies, n | 2 (1-10) | 3 (1-10) | 2 (1-7) |
| Total pregnancy time, y | 2 (0.3-9) | 3 (0.3-9) | 2 (0.8-6) |
| Age at time of VTE, y | 31 (18-46) | 34 (17-48) | 26 (17-39) |
| VTE, n† | 17 | 22 | 11 |
| Unprovoked, n | 1 | 4 | 1 |
| Provoked, n | |||
| COC use | 4 | 1 | 2 |
| Pregnancy/postpartum | 5 | 9 | 7 |
| COC use + postpartum | 2 | 1 | 0 |
| COC use + other risk factor | 0 | 4 | 0 |
| Major trauma, surgery, immobilization | 5 | 3 | 1 |
High factor VIII level was present in 8 (22%), 99 (39%), and 197 (40%) women with combined defects, single defects, and no defects, respectively (in 14 women factor VIII levels were not available). Data are given as median (min − max) unless otherwise indicated.
COC indicates combined oral contraceptive; NA, data not applicable; and VTE, venous thromboembolism.
Including 14 homozygote carriers of factor V Leiden, of whom 3 were also heterozygous for prothrombin G20210A, and 4 homozygote carriers of the prothrombin G20210A mutation, respectively.
Restricted to those aged 15-50 years.
A total of 205 (68%) of the 301 women with single or combined defects, and 366 (74%) of the 497 women without a defect reported COC use during their lifetime. The majority of women (76%) had only one period of COC use. Seventy-two percent of women had 1 or more pregnancies, with a median number of 3 (range 1-10) pregnancies. Sixty-nine percent of the women who had used COCs, and 75% of women who never used COCs had 1 or more pregnancies (Table 1).
A total of 50 first episodes of VTE were reported, of which 44 (88%) were provoked by exogenous risk factors. The majority, that is, 35 provoked episodes of VTE, occurred during COC use (11), of which 4 in the presence of another risk factor, or during the pregnancy-postpartum period (21), and 3 VTEs occurred after starting COC use during the 12 weeks postpartum (Table 1).
Absolute risk of first VTE
Table 2 lists the absolute risk of VTE associated with COC use and the pregnancy-postpartum period. The crude overall absolute risk of first VTE in our cohort was 0.25 per 100 person-years (95% CI, 0.18-0.32), based on 50 VTEs in 798 women with 20 317 person-years. In women with no, a single, or combined defects, these were 0.13 (95% CI, 0.08-0.21), 0.35 (95% CI, 0.22-0.53), and 0.94 (95% CI, 0.47-1.67) per 100 person-years, respectively.
Absolute risk of VTE in all 798 female relatives
| Defects . | All female relatives . | ||
|---|---|---|---|
| None . | Single . | Combined* . | |
| All women | |||
| Total no. | 497 | 251 | 50 |
| No. with event | 17 | 22 | 11 |
| Observation period, y | 12 908 | 6234 | 1175 |
| Incidence rate per 100 person-years (95% CI) | 0.13 (0.08-0.21) | 0.35 (0.22-0.53) | 0.94 (0.47-1.67) |
| Actual pill use | |||
| Total no. | 366 | 171 | 34 |
| No. with event | 6 | 6 | 2 |
| Observation period, pill-years | 3211 | 1218 | 232 |
| Incidence rate per 100 pill-years (95% CI) | 0.19 (0.07-0.41) | 0.49 (0.18-1.07) | 0.86 (0.10-3.11) |
| Actual pregnancy | |||
| Total no. | 364 | 175 | 36 |
| No. with event | 7 | 10 | 7 |
| Observation period, pregnancy-years | 955 | 507 | 92 |
| Incidence rate per 100 pregnancy-years (95% CI) | 0.73 (0.30-1.51) | 1.97 (0.94-3.63) | 7.65 (3.08-15.76) |
| Defects . | All female relatives . | ||
|---|---|---|---|
| None . | Single . | Combined* . | |
| All women | |||
| Total no. | 497 | 251 | 50 |
| No. with event | 17 | 22 | 11 |
| Observation period, y | 12 908 | 6234 | 1175 |
| Incidence rate per 100 person-years (95% CI) | 0.13 (0.08-0.21) | 0.35 (0.22-0.53) | 0.94 (0.47-1.67) |
| Actual pill use | |||
| Total no. | 366 | 171 | 34 |
| No. with event | 6 | 6 | 2 |
| Observation period, pill-years | 3211 | 1218 | 232 |
| Incidence rate per 100 pill-years (95% CI) | 0.19 (0.07-0.41) | 0.49 (0.18-1.07) | 0.86 (0.10-3.11) |
| Actual pregnancy | |||
| Total no. | 364 | 175 | 36 |
| No. with event | 7 | 10 | 7 |
| Observation period, pregnancy-years | 955 | 507 | 92 |
| Incidence rate per 100 pregnancy-years (95% CI) | 0.73 (0.30-1.51) | 1.97 (0.94-3.63) | 7.65 (3.08-15.76) |
Absolute risk of VTE in all 798 female relatives with no defects, a single defect (factor V Leiden or prothrombin G20210A), or a combination of these defects (including homozygosity) and during actual use of COCs and during actual pregnancy.
VTE indicates venous thromboembolism; and CI, confidence interval.
Including 14 homozygote carriers of factor V Leiden, of whom 3 were also heterozygous for prothrombin G20210A, and 4 homozygote carriers of the prothrombin G20210A mutation, respectively.
Restricting the observation time to actual COC use, the crude incidence of VTE was 0.30 (95% CI, 0.16-0.50) per 100 pill-years, based on 14 VTEs during 4661 pill-years. In women with no, a single, or combined defects, the incidences were 0.19 (95% CI, 0.07-0.41), 0.49 (95% CI, 0.18-1.07), and 0.86 (95% CI, 0.10-3.11) per 100 pill-years, respectively (Table 2).
When considering only the pregnancy-postpartum periods, the crude incidence of first VTE was 1.55 (95% CI, 0.99-2.30) per 100 pregnancy-years, based on 24 VTEs during 1553 pregnancy-years. In women with no, a single, or combined defects, the incidences rose from 0.73 (95% CI, 0.30-1.51) and 1.97 (95% CI, 0.94-3.63) to 7.65 (95% CI, 3.08-15.76) per 100 pregnancy-years, respectively.
Relative risk of first VTE
The increased risk of VTE in women with single and combined defects in comparison to women without defects was confirmed in our time-dependent multivariable Cox regression analysis (also including pregnancy and COC use). The HRs were 2.7 (95% CI, 1.4-5.1) for a single defect and 8.5 (95% CI, 3.8-19.2) for combined defects. The substantially higher risk of VTE during the pregnancy-partum period was confirmed by a HR of 16.4 (95% CI, 8.2-32.8). For actual COC use, the HR was 2.1 (95% CI, 1.1-4.1).
When adjusted for factor VIII, HRs were 2.7 (95% CI,1.4-5.0) and 10.1 (95% CI, 4.4-23.0) for single and combined defects, 2.2 (95% CI, 1.1-4.0) for COC use and 16.0 (95% CI, 8.0-32.2) for pregnancy.
A high factor VIII level was present in 197 (40%), 99 (39%), and 8 (22%) of women with no, single, and combined defects, respectively. Independent of the presence of factor V Leiden or prothrombin-G20210A mutation, the presence of high factor VIII level was associated with an increased risk of VTE (adjusted HR 2.3; 95% CI, 1.3-4.2).
Absolute risk of VTE in COC users versus users of alternative contraception
The absolute VTE incidence in COC users and in users of alternative contraceptive methods, that is, LNG-IUD users, copper-IUD users, and users of male condom, for a hypothetical group of 100 000 women over 1 year is presented in Table 3. In addition to the absolute risk related to COC use, the hypothetical risks of VTE associated with contraception failure were estimated.
Comparison of thrombosis outcome in women with factor V Leiden or prothrombin G20210A, or a combination of these defects (including homozygosity)
| . | Defects . | No defects . | ||||||
|---|---|---|---|---|---|---|---|---|
| COC . | LNG-IUD . | Copper IUD (380 mm2) . | Condom* . | COC . | LNG-IUD . | Copper IUD (380 mm2) . | Condom* . | |
| Incidence of first VTE per 100 pregnancy-years | 0.55† | 0.25‡ | 0.25‡ | 0.25‡ | 0.19 | 0.09 | 0.09 | 0.09 |
| Cases of VTE per 100 000 pregnancy-years | 550 | 250 | 250 | 250 | 190 | 90 | 90 | 90 |
| Contraceptive failure rate, per 100 women-years§ | 0.2 | 0.7 | 1.4 | 12 | 0.2 | 0.7 | 1.4 | 12 |
| Unintended pregnancies per 100 000 pregnancy-years | 200 | 700 | 1400 | 12 000 | 200 | 700 | 1400 | 12 000 |
| Incidence of VTE per 100 pregnancy-years¶ | 2.8 | 2.8 | 2.8 | 2.8 | 0.7 | 0.7 | 0.7 | 0.7 |
| Additional cases of VTE | 6 | 20 | 40 | 336 | 2 | 5 | 10 | 84 |
| Total number of VTE | 556 | 270 | 290 | 586 | 192 | 95 | 100 | 174 |
| . | Defects . | No defects . | ||||||
|---|---|---|---|---|---|---|---|---|
| COC . | LNG-IUD . | Copper IUD (380 mm2) . | Condom* . | COC . | LNG-IUD . | Copper IUD (380 mm2) . | Condom* . | |
| Incidence of first VTE per 100 pregnancy-years | 0.55† | 0.25‡ | 0.25‡ | 0.25‡ | 0.19 | 0.09 | 0.09 | 0.09 |
| Cases of VTE per 100 000 pregnancy-years | 550 | 250 | 250 | 250 | 190 | 90 | 90 | 90 |
| Contraceptive failure rate, per 100 women-years§ | 0.2 | 0.7 | 1.4 | 12 | 0.2 | 0.7 | 1.4 | 12 |
| Unintended pregnancies per 100 000 pregnancy-years | 200 | 700 | 1400 | 12 000 | 200 | 700 | 1400 | 12 000 |
| Incidence of VTE per 100 pregnancy-years¶ | 2.8 | 2.8 | 2.8 | 2.8 | 0.7 | 0.7 | 0.7 | 0.7 |
| Additional cases of VTE | 6 | 20 | 40 | 336 | 2 | 5 | 10 | 84 |
| Total number of VTE | 556 | 270 | 290 | 586 | 192 | 95 | 100 | 174 |
According to the method described by Koster et al.39
LNG-IUD indicates levonorgestrel intrauterine device; HR, hazard ratio; and VTE, venous thromboembolism.
US data.
(6 + 2) events during (1218 + 232) pill-years (see Table 2).
Based on an adjusted HR of 2.2 for COC use when compared to no COC use.
Pearl Index for correct use (method failure).
(10 + 7) events during (507 + 92) pregnancy-years (see Table 2).
In this analysis, both the direct risk of VTE because of the contraceptive method (only present in COC users) and the additional risk of VTE because of contraceptive failure resulting in unintended pregnancies are taken into account. In Table 3, the absolute risk of VTE of 0.55 per 100 pill-years in COC users with thrombophilic defect(s) is derived from combining both the number of VTEs and observation years in women with single and combined defects as presented in Table 2. The absolute risk of VTE of in users of alternative contraceptives is 2.2-fold lower, 0.25 per 100 years in users of alternative contraceptive methods, that is, based on the adjusted HR of 2.2 for COC use. The absolute risk of VTE of 2.8 per 100 pregnancy years is derived from combining both the number of VTEs and observation years noted in pregnant women with single and combined defects, see Table 2. A similar approach is used in the calculations in women without thrombophilic defects.
In women with a thrombophilic defect, the total number of VTEs is 556 in COC users, 270 in LNG-IUD users, 290 in copper-IUD users, and 586 in condom users. In women without thrombophilic defects, the total number of VTEs is 192, 174, 95, and 100, respectively. In these estimations, the possibility that unintended pregnancies are interrupted is not taken into consideration; in that situation, it is expected that the risk of VTE is lower than presented here.
Discussion
In a cohort of women with a positive family history of VTE, the presence of single or combined factor V Leiden and prothrombin-G20210A mutation, including homozygotes, resulted in a modest increase in absolute risk of VTE. COC use and the pregnancy-postpartum period were confirmed as risk factors for VTE. Although the absolute risk of VTE significantly increased during COC use (up to 0.86 [95% CI, 0.10-3.11] per 100 pill-years) in women with combined defects, the absolute risk during the pregnancy-postpartum period was by far the most important (up to 7.65 [95% CI, 3.08-15.76] per 100 pregnancy years). The substantially higher risk of VTE during the pregnancy-postpartum period was confirmed by an adjusted HR of 16.0 (95% CI, 8.0-32.2), compared with an adjusted HR of 2.2 (95% CI, 1.1-4.0) for COC use.
Our results further show that, in line with recent publications, the a priori absolute risk of VTE during the pregnancy-postpartum period noted in women without any thrombophilic defect is higher than noted in COC users, that is, 0.73 versus 0.19 per 100 person-years. However, as these risks were observed in thrombophilic families, the absolute risk during the pregnancy-postpartum period and during COC use in our study was approximately 3.5 to 5 times higher than reported in the general community, incidences of 0.2 per 100 pregnancy-years18 and 0.06 per 100 pill-years.19
As our cohort also included women from one of the original family studies with familial high factor VIII level, which is an acquired risk factor for VTE, the effect of high factor VIII level was also estimated.21 Independent of the presence or absence of factor V Leiden or prothrombin-G20210A, the presence of high factor VIII level was associated with an increased risk of VTE (adjusted HR 2.3; 95% CI, 1.3-4.2).
Several studies have reported COC use5-11,30-33 and pregnancy-postpartum33-36 as contributing factors to the risk of VTE in women with factor V Leiden and/or prothrombin-G20210A. However, adequate interpretation of risks is hampered as most studies only reported relative risks and these estimates differ considerably. In carriers of factor V Leiden or prothrombin-20210A, odds ratios for COC use ranged from 1.3 to 30, while for the pregnancy-postpartum period these ranged from 2 to 53. In addition, it is often unclear whether the presence of other thrombophilic defects was excluded. Furthermore, although COCs prevent from pregnancy, the clinical consequences of both hormonal risks of VTE are seldom considered simultaneously within 1 study.
The major finding in the present study is the high pregnancy-related risk of VTE, relative to the observed risk during COC use. Therefore, if it is decided against COC use, adequate alternative contraception is needed to keep the risk of unintended pregnancy in these women as low as possible. The actual choice in alternative contraception is very limited; only copper-intrauterine devices (IUDs) and condoms. All hormonal contraceptives are considered to increase the risk of VTE, including progestagen-only contraceptives (desogestrel-only pill: Cerazette; etonogestrel implant: Implanon; medroxyprogesterone-acetate injections: Depoprovera; and LNG-IUD: Mirena), though this is merely on theoretical grounds by extrapolation of data from combined hormonal contraceptives. However, a recent large cohort study reported the LNG-IUD to not increase the risk of VTE, based on > 100 000 women-years of use.19
To put our results into a risk-benefit perspective, we estimated absolute VTE risk of COC use and alternative contraceptive methods, that is, LNG-IUD, copper-IUD, and condoms for a hypothetical group of 100 000 women over 1 year of use, together with VTE risk associated with contraception failure (Table 3). Although COC use results in COC-related VTEs, because of its excellent contraceptive efficacy,37 the number of unintended pregnancies and subsequent number of pregnancy-related VTEs (6) is very low. The pregnancy-related VTE risk associated with the LNG-IUD and Cu-IUD (380 mm2 Cu-IUD) is estimated to be higher because of their slightly lesser contraceptive efficacy.16,38 Condom use has the lowest contraceptive efficacy, with an up to 60-fold increased risk of unintended pregnancy,17 which makes this option the least favorable alternative. Summarizing these extrapolations, the LNG-IUD and copper-IUD both carry a lower overall risk of VTE and are therefore good alternatives to COCs. The reported higher rate of unintended pregnancies versus COC use is expected not clinically relevant in daily practice as these contraceptives are not dependent on compliance. However, the specific side effects of LNG-IUD and copper-IUD related to changes in bleeding pattern, that often lead to discontinuation of use, and women's preferences need be taken into account.
Our study has its limitations. With the retrospective design, not all events were established by objective techniques because these were not yet available at the time. Consequently, the reported absolute risk of VTE may have been overestimated. On the other hand, because of the retrospective design, treating physicians were unaware of the presence of any thrombophilic defects. Furthermore, there was a potential for recall bias on COC exposure. Therefore, extensive efforts were made to minimize the recall bias on COC exposure by verification of patient information through medical files and treating physicians. Not all relatives were tested for all thrombophilic defects. Furthermore, the overall risk of VTE might be overestimated because of the family cohort design by selecting symptomatic affected probands. The risk of VTE associated with these mild hereditary thrombophilic defects will probably be even lower when unselected women are tested for those defects as part of counselling before COC use.
Strong points of our study are its size and the testing for all known thrombophilic defects. Furthermore, we estimated the absolute and relative risk of VTE and took both COC use and pregnancy into account. Moreover, these risks were put into perspective in a modeling exercise, in which both the risks of contraceptive-related VTE and the risk of pregnancy-related VTE (resulting from contraceptive failure) are considered.
In conclusion, factor V Leiden and prothrombin-G20210 are mild risk factors for VTE in fertile women. Although in the women with a factor Leiden and prothrombin-G20210 mutation, the absolute risk of VTE increased during COC use, this risk was importantly lower than the absolute risk observed during the pregnancy-postpartum period. These data provide evidence that the policy to contraindicate COC use in these women needs reconsideration. The results of the study do not allow to “promote” a COC use in asymptomatic family-carriers of FVL or PT G20210A, but indicate that when COC use is discontinued the need for adequate alternative contraception has high priority.
Rather than strictly contraindicating COC use, we advocate that detailed counseling on all contraceptive options, including COCs, should be performed, addressing the associated risks of both VTE and unintended pregnancy, to enable these women to make an informed choice.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This manuscript is dedicated to Jan van der Meer, Professor of Hemostasis and Thrombosis at the University Medical Center Groningen in The Netherlands, who died at the age of only 58 in 2009. He was the inspiration for this study.
This study was supported by grants from The Netherlands Organization for Health Research and Development (ZonMw no. 28-2783) and the Dutch Heart Foundation (no. 99.187).
Authorship
Contribution: J.v.d.M., E.F.W.v.V., and N.J.G.M.V. developed the study concept and design; E.F.W.v.V., S.M., K.H., and H.R.B. acquired data; E.F.W.v.V., N.J.G.M.V., and K.M. analyzed and interpreted data; E.F.W.v.V. drafted the manuscript; N.J.G.M.V., S.M., K.H., M.H.P., H.R.B., and K.M. performed critical revision of the manuscript for important intellectual content; N.J.G.M.V. provided statistical analysis; J.v.d.M. and K.M. supervised the study; and all authors had full access to the database, and have read and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Elizabeth F. W. van Vlijmen, University Medical Center Groningen, Hanzeplein 1, 9713 GZ, Groningen, The Netherlands; e-mail: e.f.w.van.vlijmen@int.umcg.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal