Abstract
Patients with β-thalassemia require lifelong iron chelation therapy from early childhood to prevent complications associated with transfusional iron overload. To evaluate long-term efficacy and safety of once-daily oral iron chelation with deferasirox, patients aged ≥ 2 years who completed a 1-year, phase 3, randomized trial entered a 4-year extension study, either continuing on deferasirox (deferasirox cohort) or switching from deferoxamine to deferasirox (crossover cohort). Of 555 patients who received ≥ 1 deferasirox dose, 66.8% completed the study; 43 patients (7.7%) discontinued because of adverse events. In patients with ≥ 4 years' deferasirox exposure who had liver biopsy, mean liver iron concentration significantly decreased by 7.8 ± 11.2 mg Fe/g dry weight (dw; n = 103; P < .001) and 3.1 ± 7.9 mg Fe/g dw (n = 68; P < .001) in the deferasirox and crossover cohorts, respectively. Median serum ferritin significantly decreased by 706 ng/mL (n = 196; P < .001) and 371 ng/mL (n = 147; P < .001), respectively, after ≥ 4 years' exposure. Investigator-assessed, drug-related adverse events, including increased blood creatinine (11.2%), abdominal pain (9.0%), and nausea (7.4%), were generally mild to moderate, transient, and reduced in frequency over time. No adverse effect was observed on pediatric growth or adolescent sexual development. This first prospective study of long-term deferasirox use in pediatric and adult patients with β-thalassemia suggests treatment for ≤ 5 years is generally well tolerated and effectively reduces iron burden. This trial was registered at www.clinicaltrials.gov as #NCT00171210.
Introduction
Iron overload is a leading cause of morbidity and mortality in transfusion-dependent patients with β-thalassemia major; related complications include liver cirrhosis and cardiac disease.1-3 The introduction of iron chelation therapy has led to a significant improvement in the survival of patients with β-thalassemia,4 but long-term management of iron overload is suboptimal in many patients, in part because of compliance issues associated with the parenteral administration regimen of iron chelation therapy with deferoxamine (DFO).5 Iron overload may also contribute to retardation of growth and sexual development during adolescence in patients with β-thalassemia,6-8 which can be further exacerbated by the toxic effects of intensive iron chelation therapy with DFO on bone structure.9-11 Therefore, in addition to exploring long-term efficacy and safety in patients with β-thalassemia, it is important to address the shortage of data from long-term trials on oral iron chelation and pediatric growth and development.
Deferasirox is a once-daily oral iron chelator that has proven effective in reducing liver iron concentration (LIC) and serum ferritin levels over 1 year in patients with various transfusion-dependent anemias.12-15 Because the requirements for transfusion therapy, and therefore iron chelation therapy, are lifelong in patients with β-thalassemia, there is a need to assess the long-term safety and efficacy of deferasirox in both adult and pediatric patients. This phase 3 study initially randomly assigned patients with β-thalassemia aged ≥ 2 years to receive deferasirox or DFO for 1 year.12 Patients who completed the 1-year core study were eligible to enter a 4-year extension, either continuing to receive deferasirox or switching from DFO to deferasirox. Efficacy and safety data for patients treated with deferasirox for ≤ 5 years are now available. These data represent the first analysis of long-term treatment with deferasirox in pediatric and adult patients with β-thalassemia, including the effects on pediatric growth and development.
Methods
Patients, study design, and dosing
The study was reviewed and approved by the Independent Ethics Committee or Institutional Review Board for all participating centers. Patients with β-thalassemia and transfusional iron overload who completed the 1-year randomized core study (inclusion/exclusion criteria for which have been described previously12 ) were eligible to enter the 4-year extension study. During the extension study, patients either continued to receive deferasirox (deferasirox cohort) or switched from DFO to deferasirox (crossover cohort). Deferasirox dose was initially assigned according to LIC at the start of deferasirox treatment, whereby patients with LIC values of 2-3, > 3-7, > 7-14, and > 14 mg Fe/g dry weight (dw) were assigned deferasirox doses of 5, 10, 20, or 30 mg/kg/day, respectively. One patient in the crossover cohort was assigned a deferasirox starting dose of 15 mg/kg/day (summarized in the ≤ 10 mg/kg/day planned starting dose category in the following analyses) and a further 3 patients in the crossover cohort were assigned a starting dose of 40 mg/kg/day. Subsequent dose increases or decreases of 5-10 mg/kg/day could be made every 3 months according to trends in serum ferritin levels and safety markers. Dose adjustments could also be based on transfusion requirements at the investigator's discretion.
Efficacy assessments
LIC was assessed at the start of deferasirox treatment and at end of study (EOS) by liver biopsy or the superconducting quantum interference device (SQUID) method, as described for the core study.12 Calibration issues noted during the core study meant that LIC values determined by SQUID were approximately one-half those determined by biopsy; because this observation was made after the study was started, a correction factor was not applied to the SQUID data.12 Serum ferritin levels were evaluated every 4 weeks.
Safety assessments
Safety was assessed by monitoring adverse events (AEs) and laboratory parameters, including serum creatinine and liver transaminase levels. In adults, creatinine clearance was estimated on the basis of serum creatinine levels and body mass, according to the Cockcroft-Gault formula.16 In pediatric patients, height was also used to estimate creatinine clearance, using the Schwartz formula.17 Additional specific assessments for pediatric patients included height, body mass, and sexual development. Height was measured every 3 months after the start of deferasirox with an approximation of ± 0.1 cm with the use of a Harpenden stadiometer. Yearly height assessments of individual patients were plotted against the 5%, 50%, and 95% percentiles of the US Clinical Growth Charts for a nonthalassemia population, available at http://www.cdc.gov/growthcharts.18 Body mass was assessed every 3 months, always using the same scale with an approximation of ± 0.1 kg, with patients clothed in a lightweight suit. Sexual development was assessed annually by physical examination with reference to Tanner stages.19,20 Pediatric growth was evaluated by monitoring changes in height and body mass index (BMI), expressed as height- and BMI-standard deviation scores (h-SDSs and BMI-SDSs, respectively). SDSs are standardized normal-distributed scores with a mean of 0 and SD of 1 of an age- and sex-specific normal population.
Statistical methods
Data are reported for all patients who received ≥ 1 dose of deferasirox during the core or extension studies. LIC and serum ferritin levels were further analyzed for patients who were treated with deferasirox for ≥ 4 years. Patients were assigned to planned starting dose categories according to the planned dose at the start of deferasirox treatment. P values for change in LIC in this population were based on 1-sided Student t tests. P values for change in serum ferritin levels were based on a 2-sided sign test.
h-SDS was calculated with the formula h-SDS = ([(X/M)L]−1)/(L × S), where X is the patient's standing height measurement (cm), and L, M, and S are the parameters for the Box-Cox transformation (as provided in the US Clinical Growth Charts18 at http://www.cdc.gov/growthcharts). L is the Box-Cox parameter used to normalize the data (exponent), M is the median height corresponding to age, and S is the measure of the spread of the data (generalized coefficient of variation). BMI-SDS was calculated as for h-SDS, where X is the patient's BMI (kg/m2) and M is the median BMI corresponding to age.
Results
Patient characteristics
In total, 555 patients received ≥ 1 dose of deferasirox in the core or extension studies between February 13, 2003 (first patient, first visit in core study) and November 28, 2008 (last patient, last visit in extension study): 296 patients in the deferasirox cohort and 259 patients in the crossover cohort. Pediatric patients aged 2 to < 16 years accounted for 49.2% of patients (n = 273) overall. The patient demographics at the start of deferasirox treatment, which corresponds to the start of the core study for the deferasirox cohort and the start of the extension study for the crossover cohort, are shown in Table 1. The majority of patients who entered the core study (97.4%) had a history of prior iron chelation therapy.12 However, at the start of deferasirox treatment, serum ferritin levels and LICs were generally lower in the crossover cohort in which patients had received 1 year of DFO treatment in the core study, compared with the deferasirox cohort in which patients had started deferasirox on entry to the study. LIC assessments by SQUID were lower than LIC assessments by biopsy (Table 1). Because assessment by biopsy was optional for pediatric patients, the SQUID method was more likely to be used in this group. Overall, 91 of 555 patients (16.4%) had LIC assessments by SQUID at baseline.
Demographics and patient characteristics at the start of deferasirox treatment
| Characteristic . | Deferasirox cohort (n = 296) . | Crossover cohort (n = 259) . | All patients (n = 555) . |
|---|---|---|---|
| Mean age, y (range) | 17.1 (2-49) | 18.3 (3-54) | 17.7 (2-54) |
| Age group, n (%) | |||
| 2 to < 16 y | 153 (51.7) | 120 (46.3) | 273 (49.2) |
| ≥ 16 y | 143 (48.3) | 139 (53.7) | 282 (50.8) |
| Male:female | 140:156 | 134:125 | 274:281 |
| Race (white:Oriental:other)n | 263:9:24 | 225:10:24 | 488:19:48 |
| Mean LIC ± SD, mg Fe/g dw | |||
| Biopsy | 15.5 ± 9.9 | 11.5 ± 7.8 | 13.3 ± 9.2 |
| SQUID | 6.1 ± 2.8 | 5.2 ± 2.9 | 5.7 ± 2.8 |
| LIC category, n (%)* | |||
| < 7 mg Fe/g dw (biopsy) | 60 (20.3) | 64 (24.7) | 124 (22.3) |
| < 7 mg Fe/g dw (SQUID) | 33 (11.1) | 35 (13.5) | 68 (12.3) |
| 7-14 mg Fe/g dw (biopsy) | 68 (23.0) | 93 (35.9) | 161 (29.0) |
| 7-14 mg Fe/g dw (SQUID) | 15 (5.1) | 6 (2.3) | 21 (3.8) |
| ≥ 14 mg Fe/g dw (biopsy) | 120 (40.5) | 58 (22.4) | 178 (32.1) |
| ≥ 14 mg Fe/g dw (SQUID) | 0 | 2 (0.8) | 2 (0.4) |
| Median serum ferritin (range), ng/mL | 2211 (321-12 646) | 1758 (273-8529) | 2007 (273-12 646) |
| Serum ferritin category, n (%) | |||
| ≤ 1000 ng/mL | 21 (7.1) | 48 (18.5) | 69 (12.4) |
| > 1000-2500 ng/mL | 155 (52.4) | 140 (54.1) | 295 (53.2) |
| > 2500-4000 ng/mL | 64 (21.6) | 50 (19.3) | 114 (20.5) |
| > 4000 ng/mL | 56 (18.9) | 21 (8.1) | 77 (13.9) |
| Characteristic . | Deferasirox cohort (n = 296) . | Crossover cohort (n = 259) . | All patients (n = 555) . |
|---|---|---|---|
| Mean age, y (range) | 17.1 (2-49) | 18.3 (3-54) | 17.7 (2-54) |
| Age group, n (%) | |||
| 2 to < 16 y | 153 (51.7) | 120 (46.3) | 273 (49.2) |
| ≥ 16 y | 143 (48.3) | 139 (53.7) | 282 (50.8) |
| Male:female | 140:156 | 134:125 | 274:281 |
| Race (white:Oriental:other)n | 263:9:24 | 225:10:24 | 488:19:48 |
| Mean LIC ± SD, mg Fe/g dw | |||
| Biopsy | 15.5 ± 9.9 | 11.5 ± 7.8 | 13.3 ± 9.2 |
| SQUID | 6.1 ± 2.8 | 5.2 ± 2.9 | 5.7 ± 2.8 |
| LIC category, n (%)* | |||
| < 7 mg Fe/g dw (biopsy) | 60 (20.3) | 64 (24.7) | 124 (22.3) |
| < 7 mg Fe/g dw (SQUID) | 33 (11.1) | 35 (13.5) | 68 (12.3) |
| 7-14 mg Fe/g dw (biopsy) | 68 (23.0) | 93 (35.9) | 161 (29.0) |
| 7-14 mg Fe/g dw (SQUID) | 15 (5.1) | 6 (2.3) | 21 (3.8) |
| ≥ 14 mg Fe/g dw (biopsy) | 120 (40.5) | 58 (22.4) | 178 (32.1) |
| ≥ 14 mg Fe/g dw (SQUID) | 0 | 2 (0.8) | 2 (0.4) |
| Median serum ferritin (range), ng/mL | 2211 (321-12 646) | 1758 (273-8529) | 2007 (273-12 646) |
| Serum ferritin category, n (%) | |||
| ≤ 1000 ng/mL | 21 (7.1) | 48 (18.5) | 69 (12.4) |
| > 1000-2500 ng/mL | 155 (52.4) | 140 (54.1) | 295 (53.2) |
| > 2500-4000 ng/mL | 64 (21.6) | 50 (19.3) | 114 (20.5) |
| > 4000 ng/mL | 56 (18.9) | 21 (8.1) | 77 (13.9) |
LIC at start of deferasirox was not available for 1 patient in the crossover cohort.
Deferasirox dosing
The mean deferasirox dose was 21.6 ± 6.4 mg/kg/day in the deferasirox cohort and 23.2 ± 5.9 mg/kg/day in the crossover cohort. Over the course of the study, the percentage of patients receiving < 15 mg/kg/day showed a marked decrease; most patients received final doses of ≥ 25 mg/kg/day (Table 2).
Deferasirox dosing
| . | Deferasirox cohort (n = 296) . | Crossover cohort (n = 259) . | All patients (n = 555) . |
|---|---|---|---|
| Patients in planned dose groups at start of deferasirox, n (%) | |||
| < 15 mg/kg/d | 92 (31.1) | 104 (40.2) | 196 (35.3) |
| 15 to < 25 mg/kg/d | 85 (28.7) | 94 (36.3) | 179 (32.3) |
| 25 to < 35 mg/kg/d | 119 (40.2) | 58 (22.4) | 177 (31.9) |
| ≥ 35 mg/kg/d | 0 | 3 (1.16) | 3 (0.54) |
| Patients in final actual dose groups, n (%) | |||
| < 15 mg/kg/d | 43 (14.5) | 13 (5.0) | 56 (10.1) |
| 15 to < 25 mg/kg/d | 113 (38.2) | 100 (38.6) | 213 (38.4) |
| 25 to < 35 mg/kg/d | 108 (36.5) | 101 (39.0) | 209 (37.7) |
| ≥ 35 mg/kg/d | 32 (10.8) | 45 (17.4) | 77 (13.9) |
| . | Deferasirox cohort (n = 296) . | Crossover cohort (n = 259) . | All patients (n = 555) . |
|---|---|---|---|
| Patients in planned dose groups at start of deferasirox, n (%) | |||
| < 15 mg/kg/d | 92 (31.1) | 104 (40.2) | 196 (35.3) |
| 15 to < 25 mg/kg/d | 85 (28.7) | 94 (36.3) | 179 (32.3) |
| 25 to < 35 mg/kg/d | 119 (40.2) | 58 (22.4) | 177 (31.9) |
| ≥ 35 mg/kg/d | 0 | 3 (1.16) | 3 (0.54) |
| Patients in final actual dose groups, n (%) | |||
| < 15 mg/kg/d | 43 (14.5) | 13 (5.0) | 56 (10.1) |
| 15 to < 25 mg/kg/d | 113 (38.2) | 100 (38.6) | 213 (38.4) |
| 25 to < 35 mg/kg/d | 108 (36.5) | 101 (39.0) | 209 (37.7) |
| ≥ 35 mg/kg/d | 32 (10.8) | 45 (17.4) | 77 (13.9) |
Patient discontinuations
Of the 555 patients included, 181 patients (61.1%) in the deferasirox cohort and 190 patients (73.4%) in the crossover cohort completed the 5-year study (Table 3). Thirty-two of 296 patients (10.8%) from the deferasirox cohort chose not to enter the extension phase, and 1 patient left the study at the end of extension year 3, before a protocol amendment increased the extension study length to 4 years. The most common reasons for discontinuation were withdrawal of consent and AEs. The reasons for discontinuation were relatively consistent throughout the study (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), with the exception that discontinuation because of an unsatisfactory therapeutic effect was more likely in the later stages of the trial. Of the AEs that led to discontinuation, the most common (in ≥ 4 patients overall) were increased alanine aminotransferase (ALT; n = 5, 0.9%), increased transaminases (n = 4, 0.7%), and glycosuria (n = 4, 0.7%; supplemental Table 2). Five patients died while receiving deferasirox, 1 during the core study12 and 4 during the extension. One patient died in the crossover cohort after a road traffic accident. The 3 other deaths during the extension (2 in the deferasirox cohort and 1 in the crossover cohort) were because of cardiac disorders (congestive cardiac failure, myocardial dysfunction, and cardiorespiratory arrest, respectively). These 3 patients received their last deferasirox dose 14, 12, and 9 days before death, respectively. Deferasirox was discontinued as a result of atrial flutter in the patient who died of congestive cardiac failure, and DFO was subsequently administered for iron chelation for 10 days before death. In 2 of the patients who died as a result of cardiac disorders, including the patient who switched to DFO, their last available serum ferritin assessments were elevated above levels at the start of deferasirox treatment. No deaths reported during the extension were attributed to deferasirox toxicity.
Adult and pediatric patient disposition
| Disposition, n (%) . | Deferasirox cohort . | Crossover cohort . | All patients (n = 555) . | ||
|---|---|---|---|---|---|
| Adults (n = 143) . | Pediatrics (n = 153) . | Adults (n = 139) . | Pediatrics (n = 120) . | ||
| Completed | 74 (51.7) | 107 (69.9) | 88 (63.3) | 102 (85.0) | 371 (66.8) |
| Discontinued | 69 (48.3) | 46 (30.1) | 51 (36.7) | 18 (15.0) | 184 (33.2) |
| Adverse events | 14 (9.8) | 13 (8.5) | 10 (7.2) | 6 (5.0) | 43 (7.7) |
| Abnormal laboratory value | 1 (0.7) | 2 (1.3) | 4 (2.9) | 2 (1.7) | 9 (1.6) |
| Abnormal test procedure result | 1 (0.7) | — | — | — | 1 (0.2) |
| Unsatisfactory therapeutic effect | 11 (7.7) | 4 (2.6) | 7 (5.0) | 4 (3.3) | 26 (4.7) |
| Protocol violation | 1 (0.7) | 1 (0.7) | — | — | 2 (0.4) |
| Withdrawal of consent* | 24 (16.8) | 6 (3.9) | 26 (18.7) | 6 (5.0) | 62 (11.2) |
| Lost to follow-up | — | — | 1 (0.7) | — | 1 (0.2) |
| Administrative problems | 1 (0.7) | — | 1 (0.7) | — | 2 (0.4) |
| Death | 2 (1.4) | 1 (0.7) | 2 (1.4) | — | 5 (0.9) |
| Stopped at end of core | 13 (9.1) | 19 (12.4) | — | — | 32 (5.8) |
| Stopped at end of extension year 3 | 1 (0.7) | — | — | — | 1 (0.2) |
| Disposition, n (%) . | Deferasirox cohort . | Crossover cohort . | All patients (n = 555) . | ||
|---|---|---|---|---|---|
| Adults (n = 143) . | Pediatrics (n = 153) . | Adults (n = 139) . | Pediatrics (n = 120) . | ||
| Completed | 74 (51.7) | 107 (69.9) | 88 (63.3) | 102 (85.0) | 371 (66.8) |
| Discontinued | 69 (48.3) | 46 (30.1) | 51 (36.7) | 18 (15.0) | 184 (33.2) |
| Adverse events | 14 (9.8) | 13 (8.5) | 10 (7.2) | 6 (5.0) | 43 (7.7) |
| Abnormal laboratory value | 1 (0.7) | 2 (1.3) | 4 (2.9) | 2 (1.7) | 9 (1.6) |
| Abnormal test procedure result | 1 (0.7) | — | — | — | 1 (0.2) |
| Unsatisfactory therapeutic effect | 11 (7.7) | 4 (2.6) | 7 (5.0) | 4 (3.3) | 26 (4.7) |
| Protocol violation | 1 (0.7) | 1 (0.7) | — | — | 2 (0.4) |
| Withdrawal of consent* | 24 (16.8) | 6 (3.9) | 26 (18.7) | 6 (5.0) | 62 (11.2) |
| Lost to follow-up | — | — | 1 (0.7) | — | 1 (0.2) |
| Administrative problems | 1 (0.7) | — | 1 (0.7) | — | 2 (0.4) |
| Death | 2 (1.4) | 1 (0.7) | 2 (1.4) | — | 5 (0.9) |
| Stopped at end of core | 13 (9.1) | 19 (12.4) | — | — | 32 (5.8) |
| Stopped at end of extension year 3 | 1 (0.7) | — | — | — | 1 (0.2) |
May include patients who left the study when deferasirox became commercially available.
Efficacy
No clinically relevant differences were observed in the transfusion requirements of the deferasirox and crossover cohorts. Overall blood intake after the start of deferasirox was < 7 mL/kg/mo in 69 patients (12.4%), 7-14 mL/kg/mo in 454 patients (81.8%), and > 14 mL/kg/mo in 32 patients (5.8%). Mean iron intake was 0.37 ± 0.1 and 0.38 ± 0.1 mg/kg/day in the deferasirox and crossover cohort, respectively, and was similar irrespective of planned starting dose.
In 103 patients in the deferasirox cohort who had liver biopsies at the start of deferasirox and after ≥ 4 years of deferasirox exposure, mean LIC significantly decreased from 17.4 ± 10.5 to 9.6 ± 8.0 mg Fe/g dw (1-sided P < .001). Deferasirox treatment for ≥ 4 years had a dose-dependent effect on LIC (Figure 1A), with the largest reduction in LIC observed in patients assigned a planned starting dose of 30 mg/kg/day (supplemental Table 3). Reductions in LIC were similarly dose dependent in the crossover cohort (Figure 1B; supplemental Table 3). Overall, the mean LIC of 68 patients in the crossover cohort who had liver biopsies at the start of deferasirox and after ≥ 4 years of deferasirox exposure significantly decreased from 12.5 ± 6.8 to 9.3 ± 6.4 mg Fe/g dw (1-sided P < .001). An additional 28 patients in the deferasirox cohort and 19 patients in the crossover cohort had LIC measurements by SQUID assessment at the start of deferasirox and after ≥ 4 years of deferasirox treatment. In the deferasirox cohort, SQUID-assessed mean LIC significantly reduced from 5.6 ± 2.7 to 4.0 ± 4.0 mg Fe/g dw (1-sided P = .024). In the crossover cohort, the mean SQUID-assessed LIC was similar at the start of deferasirox and after ≥ 4 years' deferasirox (5.2 ± 2.8 vs 5.1 ± 4.5 mg Fe/g dw). Patient numbers in the crossover cohort were too low to determine statistical significance.
Serum ferritin, LIC, and actual dose in cohorts for patients with ≥ 4 years of deferasirox exposure. Median serum ferritin ± range, mean LIC ± SD (biopsy), and mean actual dose ± SD in (A) the deferasirox cohort and (B) the crossover cohort for patients with ≥ 4 years of deferasirox exposure by planned starting dose category ≤ 10, 20, or 30 mg/kg/day. Only patients with ≥ 4 years of exposure to deferasirox are included. LIC values are for patients assessed by biopsy at start of deferasirox and at EOS, whereby EOS represents the last available biopsy assessment occurring after ≥ 4 years of exposure to deferasirox. Patients with LIC assessments by SQUID are not included in this figure. One crossover cohort patient included in the ≤ 10 mg/kg/day category had a planned starting dose of 15 mg/kg/day. Error bars indicate the minimum and maximum values for the serum ferritin plot and the SD for both the LIC and dose plots.
Serum ferritin, LIC, and actual dose in cohorts for patients with ≥ 4 years of deferasirox exposure. Median serum ferritin ± range, mean LIC ± SD (biopsy), and mean actual dose ± SD in (A) the deferasirox cohort and (B) the crossover cohort for patients with ≥ 4 years of deferasirox exposure by planned starting dose category ≤ 10, 20, or 30 mg/kg/day. Only patients with ≥ 4 years of exposure to deferasirox are included. LIC values are for patients assessed by biopsy at start of deferasirox and at EOS, whereby EOS represents the last available biopsy assessment occurring after ≥ 4 years of exposure to deferasirox. Patients with LIC assessments by SQUID are not included in this figure. One crossover cohort patient included in the ≤ 10 mg/kg/day category had a planned starting dose of 15 mg/kg/day. Error bars indicate the minimum and maximum values for the serum ferritin plot and the SD for both the LIC and dose plots.
In 393 patients with LIC assessed with the same method at both start of deferasirox and EOS (last observation carried forward), > 40% had LIC < 7 mg Fe/g dw at EOS, irrespective of iron intake. The mean deferasirox dose during the study generally reflected iron intake; in the < 0.3 mg/kg/day iron intake category, 15 patients (18.1%) received mean doses of 5 to < 15 mg/kg/day, and 23 patients (27.7%) received mean doses of 25 to < 35 mg/kg/day, whereas in the > 0.5 mg/kg/day iron intake category,2 patients (4.5%) received mean doses of 5 to < 15 mg/kg/day and 19 patients (43.2%) received mean doses of 25 to < 35 mg/kg/day. This trend was further reinforced in the final dose received; most patients in the > 0.5 mg/kg/day iron intake category received final doses ≥ 25 mg/kg/day with a corresponding decrease in the proportion receiving 15 to < 25 mg/kg/day (Table 4).
Proportion of patients with LIC < 7, 7-14, or > 14 mg Fe/g dw at EOS (last observation carried forward) and mean actual dose categories by iron intake
| . | Iron intake during study, mg/kg/d . | ||
|---|---|---|---|
| < 0.3 (n = 83) . | 0.3-0.5 (n = 266) . | > 0.5 (n = 44) . | |
| LIC category at EOS, n (%) | |||
| < 7 mg Fe/g dw | 36 (43.4) | 120 (45.1) | 20 (45.5) |
| 7-14 mg Fe/g dw | 30 (36.1) | 77 (28.9) | 13 (29.5) |
| > 14 mg Fe/g dw | 17 (20.5) | 69 (25.9) | 11 (25.0) |
| Mean actual dose during study, n (%) | |||
| 5 to < 15 mg/kg/d | 15 (18.1) | 29 (10.9) | 2 (4.5) |
| 15 to < 25 mg/kg/d | 45 (54.2) | 145 (54.5) | 23 (52.3) |
| 25 to < 35 mg/kg/d | 23 (27.7) | 89 (33.5) | 19 (43.2) |
| ≥ 35 mg/kg/d | 0 | 3 (1.1) | 0 |
| Final actual dose during study, n (%) | |||
| 5 to < 15 mg/kg/d | 16 (19.3) | 20 (7.5) | 2 (4.5) |
| 15 to < 25 mg/kg/d | 32 (38.6) | 115 (43.2) | 11 (25.0) |
| 25 to < 35 mg/kg/d | 28 (33.7) | 93 (35.0) | 26 (59.1) |
| ≥ 35 mg/kg/d | 7 (8.4) | 38 (14.3) | 5 (11.4) |
| . | Iron intake during study, mg/kg/d . | ||
|---|---|---|---|
| < 0.3 (n = 83) . | 0.3-0.5 (n = 266) . | > 0.5 (n = 44) . | |
| LIC category at EOS, n (%) | |||
| < 7 mg Fe/g dw | 36 (43.4) | 120 (45.1) | 20 (45.5) |
| 7-14 mg Fe/g dw | 30 (36.1) | 77 (28.9) | 13 (29.5) |
| > 14 mg Fe/g dw | 17 (20.5) | 69 (25.9) | 11 (25.0) |
| Mean actual dose during study, n (%) | |||
| 5 to < 15 mg/kg/d | 15 (18.1) | 29 (10.9) | 2 (4.5) |
| 15 to < 25 mg/kg/d | 45 (54.2) | 145 (54.5) | 23 (52.3) |
| 25 to < 35 mg/kg/d | 23 (27.7) | 89 (33.5) | 19 (43.2) |
| ≥ 35 mg/kg/d | 0 | 3 (1.1) | 0 |
| Final actual dose during study, n (%) | |||
| 5 to < 15 mg/kg/d | 16 (19.3) | 20 (7.5) | 2 (4.5) |
| 15 to < 25 mg/kg/d | 32 (38.6) | 115 (43.2) | 11 (25.0) |
| 25 to < 35 mg/kg/d | 28 (33.7) | 93 (35.0) | 26 (59.1) |
| ≥ 35 mg/kg/d | 7 (8.4) | 38 (14.3) | 5 (11.4) |
Only patients with a LIC value at both start of deferasirox and EOS evaluated with the same method (either SQUID or biopsy) are included. EOS values represent the last observation carried forward after starting deferasirox, irrespective of length of deferasirox exposure.
Median serum ferritin levels generally decreased over the study duration in both the deferasirox (Figure 1A) and crossover (Figure 1B) cohorts, with decreases in serum ferritin levels becoming more pronounced when the average actual dose increased to > 20 mg/kg/day. In the deferasirox cohort, for 196 patients who had serum ferritin levels assessed at start of deferasirox and after ≥ 4 years of deferasirox exposure, median serum ferritin levels decreased significantly from 2117 to 1124 ng/mL (P < .001). A total of 100 patients (51.0%) achieved serum ferritin levels of ≤ 1000 ng/mL (considered a target of iron chelation therapy21 ) after ≥ 4 years of deferasirox, of whom 13, 58, 16, and 13 had serum ferritin levels of ≤ 1000, > 1000-2500, > 2500-4000, and ≥ 4000 ng/mL, respectively, at the start of treatment. A total of 167 patients (85.2%) attained serum ferritin levels ≤ 2500 ng/mL (a threshold associated with a decreased risk of cardiac failure and death22 ) after ≥ 4 years of deferasirox treatment. Median serum ferritin levels also significantly decreased in 147 patients in the crossover cohort with assessments at start of deferasirox and after ≥ 4 years of deferasirox treatment, from 1731 to 1047 ng/mL (P < .001). Sixty-four patients (42.4%) achieved serum ferritin levels ≤ 1000 ng/mL after ≥ 4 years' exposure, of whom 17, 37, 8, and 2 had serum ferritin levels of ≤ 1000, > 1000-2500, > 2500-4000, and ≥ 4000 ng/mL, respectively, at the start of deferasirox treatment. A total of 121 patients (80.1%) in the crossover cohort had serum ferritin levels of ≤ 2500 ng/mL after ≥ 4 years of deferasirox exposure. As observed for LIC, deferasirox had a dose-dependent effect on serum ferritin levels in both the deferasirox and crossover cohorts (Figure 1); the median absolute change in serum ferritin levels was greatest in patients assigned a planned starting dose of 30 mg/kg/day (supplemental Table 4).
The median serum ferritin levels of pediatric patients in the deferasirox cohort who received 5 years of exposure to deferasirox decreased significantly from 2409 ng/mL at the start of deferasirox treatment to 1208 ng/mL (n = 107; P < .001). After 4 years of exposure to deferasirox, the serum ferritin levels of pediatric patients in the crossover cohort also significantly decreased from 1922 ng/mL at the start of deferasirox to 1047 ng/mL (n = 83; P < .001).
Safety and tolerability
Adverse events.
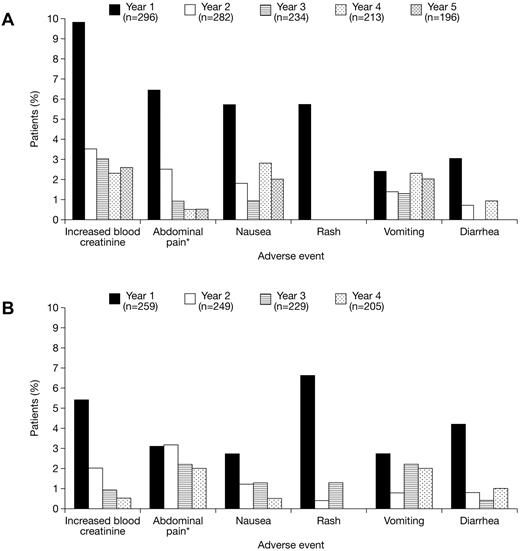
The most common (≥ 5% overall) investigator-assessed deferasirox-related AEs were increased blood creatinine levels (n = 62; 11.2%), abdominal pain (including upper abdominal pain; n = 50; 9.0%), nausea (n = 41; 7.4%), rash (n = 36; 6.5%), vomiting (n = 35; 6.3%), and diarrhea (n = 28; 5.0%). Gastrointestinal disorders with a suspected relationship to deferasirox treatment were observed more frequently in patients aged ≥ 16 years (n = 82; 29.1%) than < 16 years (n = 43; 15.8%). AEs were predominantly transient and mild to moderate in nature. Their incidence generally decreased after the first year of deferasirox treatment in both cohorts (Figure 2). Overall, the frequency of AEs with a suspected relationship to deferasirox was higher in patients receiving doses of 25 to < 35 mg/kg/day (n = 136; 39.4%) than for patients receiving 15 to < 25 mg/kg/day (n = 118; 31.1%) or < 15 mg/kg/day (n = 101; 29.4%), but there was no marked increase in AE frequency in 66 patients treated with doses ≥ 35 mg/kg/day (n = 13; 19.7%). Serious AEs, irrespective of relationship to deferasirox treatment, were reported in 163 patients (29.4%) overall; 102 patients (34.5%) in the deferasirox cohort and 61 patients (23.6%) in the crossover cohort. The most common (≥ 2% overall) were pyrexia (n = 16; 2.9%), hypersplenism (n = 14; 2.5%), cholelithiasis (n = 13; 2.3%), and abdominal pain (n = 13; 2.3%). Serious AEs considered by investigators to be related to deferasirox treatment were reported in 26 patients (4.7%) overall. Full details are provided in supplemental Table 5.
Drug-related AEs during deferasirox treatment. Annual frequency of the most common (≥ 5% overall) investigator-assessed drug-related AEs during deferasirox treatment in the (A) deferasirox and (B) crossover cohorts. *Reports of abdominal pain and abdominal pain (top) are combined and presented as abdominal pain
Drug-related AEs during deferasirox treatment. Annual frequency of the most common (≥ 5% overall) investigator-assessed drug-related AEs during deferasirox treatment in the (A) deferasirox and (B) crossover cohorts. *Reports of abdominal pain and abdominal pain (top) are combined and presented as abdominal pain
Laboratory parameters.
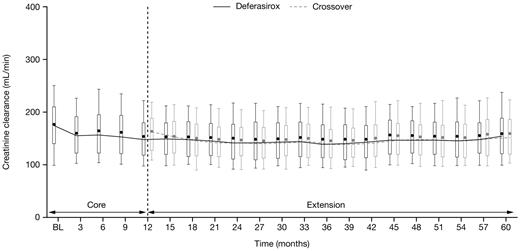
Median serum creatinine levels remained in the normal range during the study in both the deferasirox and crossover cohorts; observed increases in serum creatinine were generally mild and nonprogressive. Two consecutive serum creatinine level increases > 33% above the value at the start of deferasirox and greater than the upper limit of normal (ULN) were reported in 26 patients (8.8%) in the deferasirox cohort and 11 patients (4.2%) in the crossover cohort. All patients had serum creatinine levels ≤ ULN at the start of deferasirox treatment. Such increases in serum creatinine were observed throughout the study, most frequently in patients receiving 25 to < 35 mg/kg/day (supplemental Table 6). After 2 consecutive increases in serum creatinine levels > 33% above the value at the start of deferasirox and > ULN, 3 patients in the deferasirox cohort and 1 patient in the crossover cohort had dose adjustments, whereas 4 patients in the deferasirox cohort had temporary dose interruptions. No patients discontinued the study as a result of such increases in serum creatinine levels. Median creatinine clearance decreased slightly during the first 6 months of deferasirox treatment in both cohorts then remained stable for the remainder of the study (Figure 3).
Creatinine clearance over time after start of deferasirox. Creatinine clearance in patients aged 2 to < 16 years was adjusted to a typical adult size of 1.7 m2. Length of the box represents the interquartile range; whiskers extend to 10th and 90th percentiles. The median values are connected.
Creatinine clearance over time after start of deferasirox. Creatinine clearance in patients aged 2 to < 16 years was adjusted to a typical adult size of 1.7 m2. Length of the box represents the interquartile range; whiskers extend to 10th and 90th percentiles. The median values are connected.
Mean ALT levels were slightly increased during the first 2 years of deferasirox treatment before showing a downward trend for the remainder of the study in both cohorts. Two consecutive increases in ALT levels > 10 × ULN were reported in 3 patients (1.0%) in the deferasirox cohort and 2 patients (0.8%) in the crossover cohort. ALT levels were < ULN in 1 patient in the deferasirox cohort at the start of deferasirox treatment. The other 4 patients had elevated ALT levels > ULN to ≤ 5 × ULN. In the deferasirox cohort, 1 patient discontinued deferasirox and another had a temporary dose interruption after 2 consecutive increases in ALT levels ≥ 10 × ULN. No apparent relationship was observed between these ALT increases and the deferasirox dose at the time of onset. Progressive increases in ALT were reported in 1 patient in the crossover cohort; ALT levels increased to 265 U/L, which was considered by investigators to be a serious AE related to deferasirox, and treatment was permanently discontinued. No patients had increases in aspartate aminotransferase levels > 1 × ULN at 2 consecutive visits.
Pediatric growth and development
For the 273 pediatric patients included in this study, individual growth curves showed continuous growth in male and female pediatric patients from both cohorts during deferasirox treatment, although growth trends tended to lie within the lower percentiles of US Clinical Growth Charts (supplemental Figure 1). Absolute change for h-SDS from start of deferasirox to EOS for patients aged 2 to < 6 and 6 to < 12 years also suggested slightly reduced growth compared with a nonthalassemic North American control population, with evidence of growth normalization in parallel with pubertal development in patients aged 12 to < 16 years (Figure 4).
Change in h-SDS from start of deferasirox treatment. Change in h-SDS from start of deferasirox treatment to EOS in (A) male and (B) female pediatric patients receiving deferasirox for ≤ 5 years. Age groups refer to age at start of deferasirox treatment. EOS value corresponds to last available value after start of deferasirox for patients aged 2 to < 16 years at the start of deferasirox treatment. Height data for patients aged 2 to < 16 years at the start of deferasirox are included for as long as they remain in the study. Length of the box represents the interquartile range; whiskers extend to minimum and maximum values.
Change in h-SDS from start of deferasirox treatment. Change in h-SDS from start of deferasirox treatment to EOS in (A) male and (B) female pediatric patients receiving deferasirox for ≤ 5 years. Age groups refer to age at start of deferasirox treatment. EOS value corresponds to last available value after start of deferasirox for patients aged 2 to < 16 years at the start of deferasirox treatment. Height data for patients aged 2 to < 16 years at the start of deferasirox are included for as long as they remain in the study. Length of the box represents the interquartile range; whiskers extend to minimum and maximum values.
BMI-SDS was stable during deferasirox treatment; the mean change from the start of deferasirox treatment to EOS in pediatric patients aged 2 to < 6, 6 to < 12, and 12 to < 16 years, respectively, was −0.42 ± 0.9, −0.13 ± 0.8, and −0.05 ± 0.7 in the deferasirox cohort, and 0.28 ± 1.1, 0.2 ± 0.7, and −0.07 ± 0.6 in the crossover cohort. Values were similar for male and female patients.
During the study, the observed transition through Tanner stages for female breast development, male testes volume, and both male and female pubic hair was as expected for patients aged 12 to < 16 at the start of deferasirox treatment (supplemental Figure 2). The proportion of patients at Tanner stage 5 for female breast development increased from 8.8% and 18.1% at the start of deferasirox to 51.5% and 72.7% at EOS in the deferasirox and crossover cohorts, respectively, and the proportion of female patients at Tanner stage 5 for pubic hair increased from 8.8% and 27.2% to 51.5% and 77.2% in the deferasirox and crossover cohorts, respectively. No male patients aged 12 to < 16 were at Tanner stage 5 for testicular volume at the start of deferasirox treatment; at EOS, 27.2% and 28.5% were at stage 5 in the deferasirox and crossover cohorts, respectively. Similarly, none of the male patients aged 12 to < 16 were at Tanner stage 5 for pubic hair at the start of deferasirox treatment. At EOS, 18.1% in the deferasirox cohort and 28.5% in the crossover cohort were at stage 5 for pubic hair.
Discussion
Iron chelation therapy is a lifelong requirement for transfusion-dependent patients with β-thalassemia, but to date, long-term efficacy and safety data from prospective clinical trials in pediatric and adult patients are limited. This is the first prospective study to report long-term monitoring of the efficacy and safety of iron chelation with deferasirox in both pediatric and adult patients with β-thalassemia. It also represents the first report of observed long-term effects on pediatric growth and adolescent sexual development for any oral iron chelation therapy.
Overall, two-thirds of patients completed the 5-year study. Deferasirox became commercially available during the trial; therefore, the patients who discontinued as a result of consent withdrawal or loss to follow-up may include subjects who left the study but continued to take deferasirox. The completion rate is similar to that observed in a 5-year prospective study of deferiprone–DFO combination therapy compared with deferiprone monotherapy23 and higher than a 4-year prospective analysis of long-term deferiprone (in which dose-adjustments were not permitted by the study protocol), in which fewer than one-half of the enrolled patients completed the study.24
Long-term deferasirox treatment led to a sustained reduction in the iron burden of the patients enrolled in the study. The limited efficacy of the 10-mg/kg/day dose was noted during the core phase,12 and patients with relatively low LIC who were planned doses of 10 mg/kg/day were dose escalated during the extension; the final actual doses of more than one-half the patients studied were ≥ 25 mg/kg/day. Significant decreases in both LIC and serum ferritin levels were observed in patients who received ≥ 4 years of exposure to deferasirox, irrespective of whether patients had switched from DFO. Changes in LIC generally reflected changes in serum ferritin levels, despite fewer patients having EOS liver biopsy compared with serum ferritin assessments. The effects of deferasirox on cardiac iron were not examined in this study, but other long-term trials are ongoing to investigate this.25,26 Overall, 83.0% of patients attained serum ferritin levels of ≤ 2500 ng/mL. Serum ferritin levels above this threshold are associated with decreased survival and increased risks of cardiac disease and impaired puberty in patients with β-thalassemia.22,27 Of 347 patients who received deferasirox for ≥ 4 years, 30 patients (8.6%) had serum ferritin levels of ≤ 1000 ng/mL at the start of deferasirox treatment compared with 164 patients (47.3%) after 4 years. Of these 164 patients, almost a quarter (23.8%) had serum ferritin levels of > 2500 ng/mL at the start of deferasirox treatment. The largest decreases in LIC and serum ferritin were reported in patients assigned planned deferasirox doses of 30 mg/kg/day. Because the initial deferasirox dose was assigned on the basis of the LIC at the start of deferasirox treatment, this observation agrees with reports that deferasirox doses of ≥ 30 mg/kg/day may be required in some patients to reduce iron burden to clinically acceptable levels.28 Furthermore, although > 40% of patients had an LIC < 7 mg Fe/g dw at EOS, patients with higher mean iron intake appeared more likely to require doses in the range of 25 to < 35 mg/kg/day to reach this threshold. In clinical practice, the need for doses ≥ 30 mg/kg/day may be particularly associated with higher transfusion requirements. It should be noted, however, that changes in iron burden were subject to interpatient variability even at higher doses. There is some evidence that variable gastrointestinal absorption may contribute to a decreased response in some patients, which should be considered when evaluating dose adjustments and timing.29
The invasive nature of performing a liver biopsy resulted in many patients or investigators electing not to repeat the procedure at EOS, meaning the number of patients included in the EOS LIC analysis was markedly reduced compared with the actual number of patients who completed the study. This issue was confounded by the identification during the core trial and other studies12,15 of differences in the calibration of SQUID and biopsy LIC values, whereby SQUID values were approximately one-half of those obtained by biopsy and subsequently reported separately from LIC values. Since the initiation of this study, magnetic resonance imaging technology has now emerged as a robust, noninvasive technique for determining LIC,30,31 meaning that such calibration and patient uptake issues will probably be less problematic in future iron chelation studies.
Deferasirox was generally well tolerated over the long term in both pediatric and adult patients. Overall, 72 patients (14.2%) discontinued the long-term study as a result of AEs, abnormal laboratory values, abnormal test procedure results, or unsatisfactory therapeutic effects. The most common AEs with a suspected relationship to deferasirox during the extension study were similar to those reported for the core study,12 being predominantly gastrointestinal, transient, and mild to moderate in nature. Of note, gastrointestinal AEs with a suspected relationship to deferasirox treatment were more likely to be reported by adult than pediatric patients. Deferasirox tolerance appeared to improve over the long term, because the proportions of patients presenting with the most common drug-related AEs decreased considerably after the first year of deferasirox treatment.
Renal and liver functions were closely monitored. No progressive increases in serum creatinine or liver transaminase levels were observed during long-term deferasirox treatment, and creatinine clearance remained stable. Fanconi-like syndrome in the kidney during deferasirox treatment has been reported rarely32-34 but was not observed in this study. Patients with 2 consecutive increases in serum creatinine > 33% above the start of deferasirox and > ULN were most frequently in higher average actual dose categories, but such increases were manageable and did not lead to permanent discontinuation of deferasirox. No apparent relationship was observed between deferasirox dose and liver transaminase increases. A relatively high cutoff for elevated liver transaminases of 10 × ULN was used in this study because iron overload itself is known to increase liver enzyme levels, in particular in patients with high LIC.35 Although changes in renal and liver parameters were manageable in this trial, note that the study enrollment criteria excluded patients with clinically significant kidney and liver dysfunctions and the tolerability of deferasirox in such patients cannot therefore be deduced from these observations. Ongoing monitoring of renal and liver functions during deferasirox treatment in clinical practice remains a requirement.
Deferasirox-dosing strategy was the same for adult and pediatric patients. Because transfusion therapy to manage β-thalassemia is started in early childhood and continues for life, it is important to evaluate the long-term effects of iron chelation therapy in pediatric patients. Growth of pediatric patients and sexual development of adolescent patients with β-thalassemia is of particular relevance, because multiple factors, including iron toxicity, can lead to reduced stature and delayed puberty in this population.6-8 Although the prevalence has decreased since iron chelation therapy has become available,4 many patients continue to experience complications of growth and sexual development, which are often associated with poor compliance to DFO.4,6,36 In this 5-year study, pediatric patients showed continued growth and development during deferasirox treatment. The observed growth assessments suggested that patients aged < 12 years had slightly reduced growth compared with a control population, although it should be noted that the control data have some limitations for use in an international thalassemia study, being derived from a healthy population in the United States. h-SDS appeared to improve during adolescence, most probably with the onset of puberty. Sexual development was observed to progress through the Tanner stages as expected in patients aged 12 to < 16 years. It is therefore unlikely that growth problems such as those encountered in early DFO studies9-11 are an issue with deferasirox at the doses used in this study. It is possible that deferasirox activity in removing iron from the endocrine organs contributes to growth progression observed during the study; however, in the absence of a satisfactory control cohort (ie, untreated patients with β-thalassemia major), it is difficult to draw more definitive conclusions.
This is the first study to show significant reduction of liver and total body iron overload with long-term deferasirox treatment in both adult and pediatric patients with β-thalassemia, and the data presented are supportive of published shorter-term clinical trials of 1-year duration.13,37 Many patients achieved maintenance serum ferritin levels of ∼ 1000 ng/mL, and treatment for ≤ 5 years was well tolerated. Deferasirox did not show an adverse effect on pediatric growth or adolescent sexual development in pediatric patients who are prone to growth retardation as a result of iron overload. Appropriate dose adjustments led to clinically relevant decreases in LIC and serum ferritin, highlighting the necessity for dose titration to achieve negative iron balance. Deferasirox, with dosing tailored to individual patient requirements, is therefore an effective long-term treatment for transfusional iron overload in adult and pediatric patients with β-thalassemia.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
The authors thank Helen Little for medical editorial assistance with this manuscript.
This work was supported by Novartis Pharma AG. Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals.
Authorship
Contribution: M.D.C., M.B., L.A., D.C., M.C., A.C., G.D., M.E., S.F., A.K., Y.K., S.P., A.P., J.B.P., and Y.A. served as investigators on this trial, enrolling patients. The manuscript was drafted by M.D.C. with contribution from Y.A. on the pediatrics section. All other authors reviewed and contributed their comments on each draft of this manuscript and approved the final version. M.D.C., A.C., A.P., and A.K. also served as members of the Study Monitoring Committee, overseeing the conduct of the trial. L.G. and V.D. provided clinical insight, assisted in the interpretation of the trial data, and contributed their comments on this manuscript. J.C. served as the trial statistician and contributed comments on the manuscript.
Conflict-of-interest disclosure: M.D.C. is a member of the Novartis Speakers Bureau. A.C. has received research funding from Novartis Pharmaceuticals and reimbursement from ApoPharma for travel expenses for an annual meeting of the Safety Committee. A.K. has received honoraria and research funding from Novartis Pharmaceuticals and reports membership of the Novartis Speakers Bureau. S.P. has received research funding from Novartis Pharmaceuticals. A.P. has received honoraria and research funding from Novartis Pharmaceuticals. J.B.P. has received research funding from Novartis Pharmaceuticals and reports membership of Novartis advisory boards and Speakers Bureau. L.G., V.D., and J.C. are employed by a company (Novartis Pharmaceuticals) whose product was studied in the present work. Y.A. has received honoraria and research funding from Novartis Pharmaceuticals and reports membership of Novartis advisory boards. The remaining authors declare no competing financial interests.
Correspondence: M. Domenica Cappellini, Universitá di Milano, Ca Granda Foundation IRCCS, Via F Sforza 35, 20122 Milan, Italy; e-mail: maria.cappellini@unimi.it.