Abstract
In phagocytes, GTPases of the Rac family control crucial antimicrobial functions. The RacGAP ArhGAP15 negatively modulates Rac activity in leukocytes, but its in vivo role in innate immunity remains largely unknown. Here we show that neutrophils and macrophages derived from mice lacking ArhGAP15 presented higher Rac activity but distinct phenotypes. In macrophages, the loss of ArhGAP15 induced increased cellular elongation and membrane protrusions but did not modify chemotactic responses. Conversely, the lack of ArhGAP15 in neutrophils affected critical Rac-dependent antimicrobial functions, specifically causing enhanced chemotactic responses, straighter directional migration, amplified reactive oxygen species production, increased phagocytosis, and improved bacterial killing. In vivo, in a model of severe abdominal sepsis, these effects contributed to increase neutrophil recruitment to the site of infection, thereby limiting bacterial growth, controlling infection spread, reducing systemic inflammation, and ultimately improving survival in ArhGAP15-null mice. Altogether, these results demonstrate the relevance of ArhGAP15 in the selective regulation of multiple neutrophil functions, suggesting that ArhGAP15 targeting might be beneficial in specific pathologic settings like severe sepsis.
Introduction
Rac proteins are members of the Rho family of GTPases and are key mediators of phagocyte functions, through their involvement in the control of migration to the site of infection, phagocytosis, and reactive oxygen species (ROS) production.1 In neutrophils, Rac is needed for proper gradient sensing2-4 and filamentous actin polymerization2 during migration. On the other hand, in macrophages, Rac can alter cell morphology and mode of migration but is not essential for chemotaxis.5 In phagocytosis, Rac plays an important role in neutrophils6 and macrophages by localizing to the phagosomal membrane and controlling the closure of the phagocytic cup.7 Rac also mediates phagocyte-mediated bacterial killing, through its ability to stimulate ROS production,3,6,8 as Rac can directly bind to p67phox, thereby inducing the activation of the nicotinamide adenine dinucleotide phosphate oxidase complex (NADPH).9 Consistent with these roles, human patients carrying a dominant negative Rac mutation show defective neutrophil chemotaxis, reduced superoxide production, and increased susceptibility to infections.10,11
Like all GTPases, Rac proteins can oscillate between two states: the guanosine diphosphate-bound inactive and guanosine triphosphate-bound active state. The switch between these two conditions is regulated by guanine nucleotide exchange factors and GTPase-activating proteins (GAPs): whereas guanine nucleotide exchange factors promote the exchange of guanosine diphosphate for guanosine triphosphate thus causing Rac activation, GAPs turn off Rac activity by accelerating the hydrolysis of guanosine triphosphate. It is estimated that 0.5% of all predicted human genes encode putative GAPs, thereby suggesting that these proteins have widespread and important roles in GTPase regulation12 ; however, how RacGAPs regulate phagocyte functions remains largely unknown.
In phagocytes, the best characterized RacGAPs are Bcr and Abr, which have been found to have overlapping roles. In particular, these proteins negatively control most of the Rac-dependent functions in macrophages,13 whereas, conversely, they only regulate specific activities in neutrophils.14,15 Nonetheless, although the presence of other RacGAPs in neutrophils has been reported,16,17 their activity has not been thoroughly investigated.
ArhGAP15 is a RacGAP activated by membrane recruitment triggered by docking, through its PH domain, to the phosphatidylinositol 3-kinase product phosphatidylinositol 3,4,5-trisphosphate.18,19 Our previous studies identified ArhGAP15 as protein expressed in macrophages19 but did not define a specific role of this protein in phagocyte functions. To explore the role of this protein in vivo, we generated ArhGAP15-deficient mice and found them to be viable and fertile. Analysis of phagocyte functions showed that macrophages lacking ArhGAP15 exhibited an atypical morphology but normal chemotaxis. Conversely, knockout neutrophils displayed multiple alterations, including increased directional migration, phagocytosis, ROS production, and bacterial killing. Consistently, the improved neutrophil migration to the site of infection and the subsequent control of bacterial colonization and dissemination protected ArhGAP15-null mice from severe polymicrobial abdominal sepsis.
Methods
Details regarding the generation of the ArhGAP15 knockout mice, RT-PCR, Western blot analysis, immunohistochemistry, and fluorescence-activated cell sorter analysis are available in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Cell purification
Bone marrow–derived macrophages (BMDMs) were derived and used as previously described.19 To isolate neutrophils, murine bone marrows were resuspended in saline plus 0.1% FCS and then centrifuged (1100g, 30 minutes, 4°C) through discontinuous Percoll gradients (72%, 64%, and 52%) in Hanks buffered salt solution. Mature neutrophils were derived from the 64%-72% interface, and cell purity was determined by flow cytometric analysis (fluorescence-activated cell sorter).
Rac glutathione S-transferase-PAK pull-down assay
A glutathione-S-transferase-PAK-CD (PAK-CRIB domain) fusion protein, containing the Rac binding region from human PAK1, was used to determine Rac activity. Briefly, BMDMs and neutrophils were stimulated with 20nM or 100nM C5a, respectively. Cell lysates were centrifuged at 4°C for 10 minutes at 13 000 revolutions per minute, and the supernatant was incubated with glutathione S-transferase-PAK glutathione-coupled Sepharose 4B beads (GE Healthcare) for 30 minutes at 4°C. Proteins bound to the beads were washed 3 times in lysis buffer, and the amount of bound proteins was quantified by Western blot analysis.
Immunofluorescence of actin
BMDMs were plated on glass coverslips for 24 hours and then starved for 12 hours before stimulation with 20nM C5a. Cells were incubated with phalloidin tetramethylrhodamine isothiocyanate (Sigma-Aldrich), followed by Hoechst (Sigma-Aldrich) to stain nuclei. Images were acquired with an Axio Observer Zeiss microscope (Carl Zeiss) equipped with a MRm Axiocam at a 63× magnification. MetaMorph software (Meta Imaging Series Version 6.1; Universal Imaging Corporation) was used to examine differences in macrophage morphology, including the elongation ratio, automatically calculated by the software program as a ratio of the longest/shortest cellular cross section of each cell, and cellular perimeter, automatically calculated by the software program as the distance around the outer edge of each cell. More than 60 macrophages per genotype were analyzed.
Migration assays
Chemotactic migration of BMDMs and neutrophils toward 100nM CXCL2 (Sigma-Aldrich), 10μM formyl-methionyl-leucyl-phenylalanine (fMLF; Sigma-Aldrich), 50nM C5a (Sigma-Aldrich), or culture medium (negative control) was tested using Boyden chambers as previously described.19 For chemokinesis experiments, macrophages were seeded in the presence of 50nM C5a in both chambers. After incubation at 37°C for 1 hour for BMDMs or 2 hours for neutrophils, migration was quantified by counting cells that migrated to the lower surface of the filter, on which 5 random fields were examined.
In vivo neutrophil migration toward the chemoattractants IL-8 and Escherichia coli were tested as previously described.20 Directional migration was determined by the under-agarose method.21 A total of 1 × 106 neutrophils were loaded to the right, and 10 pmol of C5a or 5 pmol of CXCL2 was loaded to the left in 2 opposing points in the agarose along the x-axis and incubated for 1 hour at 37°C. Gels were then transferred to a Zeiss Axiovert 200M (Carl Zeiss) inverted microscope equipped with a CCD cooled camera (Princeton Instrument), a heated enclosure (Incubator PM S1; Carl Zeiss), and imaged with a phase-contrast 32× objective. Cells were filmed for 1 hour with one frame every 4 seconds. Analysis was performed using a purpose-made software (available at www.cbu.mbcunito.it/ctrack), which generated x-y coordinates for each cell at each time point, to calculate the chemotactic index defined by the displacement along the x-axis (Δx; migration distance toward the chemoattractant) divided by the total migrated distance (Σdist). Analysis was determined on low cell density fields to avoid pathway interference between cells; experiments were excluded from the analysis if manual inspection showed incorrectly reconstructed cellular paths. The elongation ratio of migrating cells was analyzed during the first 5 minutes, examining one frame every 12 seconds.
To assess the cellular ability to turn, similar under-agarose experiments were performed except for a third smaller well that was cut below the x-axis at an equal distance between the two original wells.22 Along the x-axis, 1 × 106 neutrophils were loaded to the right and 5 pmol of CXCL2 was loaded to the left. Gels were incubated for 1.5 to 2 hours, allowing neutrophils to migrate into a target area, located between the two wells in the x-axis. Subsequently, cells were filmed for 1 hour with one frame every 4 seconds. A baseline recording was taken for 10 minutes, 5 pmol of C5a was then added to the lower middle well, and the cellular migration was recorded for an additional 50 minutes. The resulting time lapse image series were then analyzed calculating the displacement along the y-axis (Δy; migration toward C5a) divided by the total migrated distance (Σdist).
ROS production, MPO release, phagocytosis, and in vitro killing
Production of ROS was determined by priming isolated neutrophils (5 × 106/mL) with lipopolysaccharide (1μg/mL) for 1 hour and then preincubating them with luminol (20 μg/mL) and horseradish peroxidase (35 μg/mL) in RPMI medium plus 10% FCS. Cells were subsequently stimulated with 2.5μM fMLF, 50nM C5a, or IgG-opsonized zymogen particles (ratio of 10:1 zymogen/:neutrophil; Invitrogen), and light emission was recorded by a Berthold MicroLumat Plus luminometer (Berthold Technologies).
Myeloperoxidase (MPO) release was assessed using tetramethylbenzidine based on a previously established technique23 after stimulation with 2.5 μmol cytochalasin B (Sigma-Aldrich) and 100 nmol C5a, in the presence of 50 U/mL of superoxide dismutase (Sigma-Aldrich).
To assess phagocytosis, E coli bacteria conjugated to Texas Red (Invitrogen) were serum-opsonized or IgG opsonized and mixed with neutrophils at a ratio of 10:1 (bacteria/neutrophil). Phagocytosis was initiated by incubating the cells at 37°C for 1 hour and was stopped by placing the mixture on ice immediately after incubation. Cells were washed twice with cold PBS, fixed, and stained for the neutrophil marker Gr-1 (BD Immunocytometry). Similarly, phagocytosis was assessed in macrophages using serum-opsonized bacteria. Images were acquired with an Axio Observer Zeiss microscope (Carl Zeiss) equipped with an MRm Axiocam at a 63× or 40× magnification for neutrophils and macrophages, respectively; mean fluorescence intensities for Gr-1–positive populations were compared.
The ability of neutrophils to kill bacteria in vitro was assayed by incubating E coli bacteria with neutrophils (ratio of 100:1 bacteria/neutrophil) in Hanks balanced salt solution plus 10% FCS plus 1mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid for 1 hour at 37°C. Serial dilutions of the incubated mixture were plated on Luria-Bertani plates and incubated overnight at 37°C. Colony-forming units were then counted.
Cecal ligation and perforation-induced sepsis
Mice were anesthetized with 100 mg/kg ketamine (Pfizer) and 5 mg/kg xylazine (Bayer) and randomized to either cecal ligation and perforation-induced sepsis (CLP) or laparotomy (sham) surgery. To induce severe sepsis, the cecum was isolated and punctured twice with an 18-gauge needle, after which a small amount of fecal matter was extruded from each puncture site. Sham animals received a laparotomy with no manipulation of the cecum. Animals were killed 6 hours after surgery, at which time the peritoneal cavity was washed with 5 mL of sterile phosphate-buffered saline plus 1mM ethylenediaminetetraacetic acid, the spleen was isolated and homogenized in sterile phosphate-buffered saline plus 1mM ethylenediaminetetraacetic acid, and blood serum was collected and snap frozen for later analysis. Aliquots of the peritoneal lavage and spleen homogenate were then serially diluted, plated on Muller-Hinton agar dishes, and incubated at 37°C. Colony-forming units were counted after 24 hours, and results were expressed as the total colony-forming unit per peritoneal cavity or spleen. Separate aliquots of the peritoneal lavage fluid were assessed for cellular influx by automated cell counter Countess (Invitrogen). In addition, the percentage of neutrophil influx was determined by fluorescence-activated cell sorter using the targeting antibody Gr-1. To assess systemic inflammation, serum concentration of the cytokines IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, GM-CSF, CXCL2, and IFN-γ were measured using a commercially available BioPlex kit (Bio-Rad). In a separate cohort of animals, the lung, liver, kidney, ileum, and cecum were collected after 18 hours of CLP, fixed in 4% paraformaldehyde overnight, embedded in paraffin, sectioned and stained by hematoxylin and eosin, and assessed at 20× and 40× magnification by light microscopy on an Olympus BH2-RFCA microscope equipped with a Leica DFC320 camera for image acquisition. The overall pathophysiologic effect was assessed by Kaplain-Meier analysis of survival over 7 days, during which mice were supplemented with fluids subcutaneously at 6 hours and every subsequent 12 hours after surgery.
Statistical analysis
Values were presented as mean ± SEM. P values were calculated using the nonparametric 2-tailed Mann-Whitney U test, Student t test, and 1- and 2-way ANOVA, Wilcoxon test, and Kaplain-Meier when appropriate (GraphPad Software Version 4). The number of experiments is indicated by the n values, and a P value of < .05 was considered statistically significant. Each experiment was independently performed at least 3 times.
Results
Generation of ArhGAP15 knockout mice
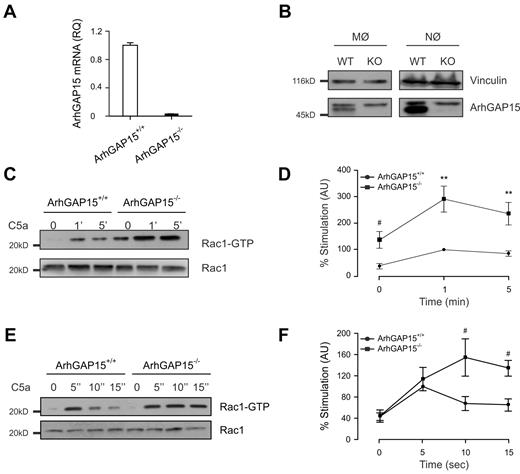
To investigate the functional role of ArhGAP15 in vivo, a knockout mouse line was generated (supplemental Figure 1A-B). The mutation disrupted the ArhGAP15 gene by insertion of a LacZ and neomycin resistance cassette in the first coding exon (supplemental Figure 1A). Fertile homozygous mice (ArhGAP15−/−) were obtained with the expected Mendelian ratios (supplemental Figure 1C) and did not show any overt phenotype. Although ArhGAP15 expression was confirmed in wild-type macrophages and neutrophils, no ArhGAP15 mRNA and protein could be detected in either ArhGAP15−/− spleen or phagocyte lineages, respectively (Figure 1A-B).
ArhGAP15 regulates Rac activity in phagocytes. (A) Quantitative RT-PCR analysis of ArhGAP15 expression in spleens derived from ArhGAP15+/+ and ArhGAP15−/− mice; n = 3/genotype. (B) Representative detection of ArhGAP15 Western blot from macrophage (MØ) and neutrophils (NØ) of ArhGAP15+/+ (WT) and ArhGAP15−/− (KO) mice. Only the lower band, exclusively present in WT lanes, corresponds to the correct size of ArhGAP15. Equal loading was monitored by vinculin expression; n = 3. (C-F) Determination of Rac1 activation after C5a stimulation in macrophages (C-D) and neutrophils (E-F). Representative detection of active (Rac1-guanosine triphosphate) and total Rac1 is shown. Normalization was obtained by setting the percentage stimulation of ArhGAP15+/+ cells after 1 minute for macrophages and after 5 seconds for neutrophils to 100%; at each point, n = 8/genotype. #P < .05. **P < .001.
ArhGAP15 regulates Rac activity in phagocytes. (A) Quantitative RT-PCR analysis of ArhGAP15 expression in spleens derived from ArhGAP15+/+ and ArhGAP15−/− mice; n = 3/genotype. (B) Representative detection of ArhGAP15 Western blot from macrophage (MØ) and neutrophils (NØ) of ArhGAP15+/+ (WT) and ArhGAP15−/− (KO) mice. Only the lower band, exclusively present in WT lanes, corresponds to the correct size of ArhGAP15. Equal loading was monitored by vinculin expression; n = 3. (C-F) Determination of Rac1 activation after C5a stimulation in macrophages (C-D) and neutrophils (E-F). Representative detection of active (Rac1-guanosine triphosphate) and total Rac1 is shown. Normalization was obtained by setting the percentage stimulation of ArhGAP15+/+ cells after 1 minute for macrophages and after 5 seconds for neutrophils to 100%; at each point, n = 8/genotype. #P < .05. **P < .001.
In ArhGAP15−/− mice, circulating neutrophils and monocyte/macrophages were decreased by 36% ± 6% and 43% ± 9%, respectively, whereas lymphocytes were decreased by 28% ± 4% (Table 1). Despite this effect, the number of CD18-positive leukocytes as well as F4/80-positive macrophages in various organs, including the liver, kidney, and heart, was similar between wild-type and ArhGAP15-deficient mice (supplemental Figure 2A-B; and data not shown). In addition, analysis of surface markers, such as CD88, CD11b (MAC-1), and F4/80 in BMDMs and Gr-1 in bone marrow-purified neutrophils, were equally expressed in both mutant and control cells (supplemental Figure 3A-D), suggesting that there was no effect of the loss of ArhGAP15 on phagocyte differentiation.
Blood parameters in ArhGAP15+/+ and ArhGAP15−/− mice
| Parameter . | ArhGAP15+/+ . | ArhGAP15−/− . |
|---|---|---|
| Hematocrit, % | 42.88 ± 0.81 | 40.21 ± 1.19 |
| WBCs, 103/μL | 5.63 ± 0.49 | 3.81 ± 0.34* |
| Neutrophils, 103/μL | 1.07 ± 0.11 | 0.68 ± 0.09* |
| Lymphocytes, 103/μL | 4.0 ± 0.37 | 2.89 ± 0.31* |
| Monocytes, 103/μL | 0.17 ± 0.03 | 0.097 ± 0.01* |
| Eosinophils, 103/μL | 0.14 ± 0.02 | 0.10 ± 0.01 |
| Basophils, 103/μL | 0.014 ± 0.003 | 0.009 ± 0.002 |
| Platelets, 103/μL | 1145 ± 49 | 998 ± 28* |
| Parameter . | ArhGAP15+/+ . | ArhGAP15−/− . |
|---|---|---|
| Hematocrit, % | 42.88 ± 0.81 | 40.21 ± 1.19 |
| WBCs, 103/μL | 5.63 ± 0.49 | 3.81 ± 0.34* |
| Neutrophils, 103/μL | 1.07 ± 0.11 | 0.68 ± 0.09* |
| Lymphocytes, 103/μL | 4.0 ± 0.37 | 2.89 ± 0.31* |
| Monocytes, 103/μL | 0.17 ± 0.03 | 0.097 ± 0.01* |
| Eosinophils, 103/μL | 0.14 ± 0.02 | 0.10 ± 0.01 |
| Basophils, 103/μL | 0.014 ± 0.003 | 0.009 ± 0.002 |
| Platelets, 103/μL | 1145 ± 49 | 998 ± 28* |
Data are mean ± SEM; n = 15 in each group.
WBCs indicates white blood cells.
P < .05
Nonetheless, Rac1 activation after C5a stimulation in ArhGAP15−/− BMDMs and mutant neutrophils was significantly higher than wild-types (Figure 1C-F), thus indicating that ArhGAP15 is a crucial negative regulator of phagocyte Rac1 in response to G-protein-coupled receptor (GPCR) agonists, such as C5a.
Elongated morphology but normal migration in ArhGAP15−/− macrophages
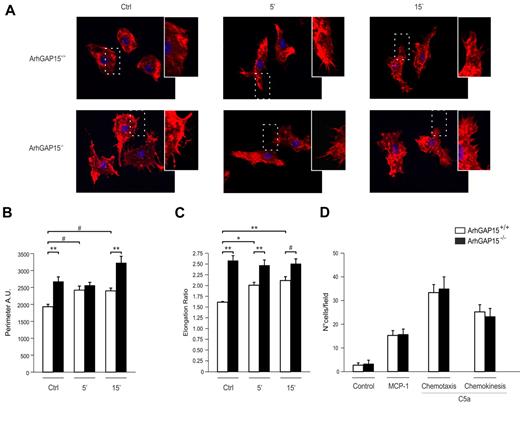
In macrophages, Rac1 is thought to control membrane protrusions and cell shape.5 Consistently, in the absence of ArhGAP15, BMDMs developed a significantly higher number of membrane protrusions than wild-type controls and showed a more elongated shape, either in the absence or presence of C5a stimulation (Figure 2A). In agreement with these findings, mutant BMDMs displayed an increased perimeter (Figure 2B) and a greater elongation ratio (Figure 2C). Conversely, these cells showed no difference with wild-type controls in chemotactic responses induced by either monocyte chemoattractant protein-1 or C5a (Figure 2D). To test whether this lack of difference was the result of adaptation to chronic loss of ArhGAP15, similar experiments were performed in RAW 264.7 cells where ArhGAP15 was acutely down-regulated by a lentiviral-transduced shRNA expression. In agreement with genetically modified BMDMs, RAW 264.7 expressing one of two different shRNAmir sequences (shRNAmir GAP15-1 and shRNAmir GAP15-2) showed a similar atypically elongated shape but normal migration compared with controls (supplemental Figure 4A-B). These results thus indicate that, in macrophages, ArhGAP15 mediates cellular morphology but not chemotactic responses.
Loss of ArhGAP15 affects macrophage morphology but not migration. (A) Representative images of ArhGAP15+/+ and ArhGAP15−/− BMDMs were stimulated with C5a and stained for filamentous actin (red) and nuclei (blue). (B-C) Perimeter analysis and elongation ratio of ArhGAP15+/+ and ArhGAP15−/− BMDMs before and after C5a stimulation (n ≥ 60 cells/genotype). (D) Boyden chamber chemotaxis and chemokinesis analysis of ArhGAP15+/+ and ArhGAP15−/− BMDMs; n = 3/genotype. #P < .05. *P < .01. **P < .001.
Loss of ArhGAP15 affects macrophage morphology but not migration. (A) Representative images of ArhGAP15+/+ and ArhGAP15−/− BMDMs were stimulated with C5a and stained for filamentous actin (red) and nuclei (blue). (B-C) Perimeter analysis and elongation ratio of ArhGAP15+/+ and ArhGAP15−/− BMDMs before and after C5a stimulation (n ≥ 60 cells/genotype). (D) Boyden chamber chemotaxis and chemokinesis analysis of ArhGAP15+/+ and ArhGAP15−/− BMDMs; n = 3/genotype. #P < .05. *P < .01. **P < .001.
Increased migration of ArhGAP15−/− neutrophils
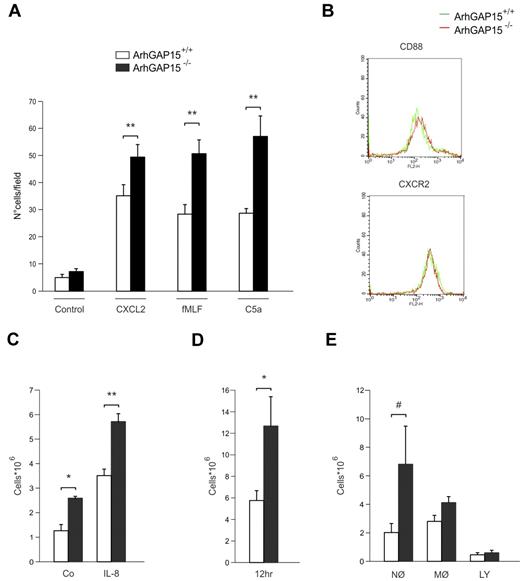
In neutrophils, analysis of chemotactic responses in Boyden chamber assays revealed that migration toward different chemoattractants, such as C5a and fMLF, as well as chemokines, such as CXCL2, was significantly higher in ArhGAP15−/− than wild-type controls (Figure 3A). Analysis of CD88 and CXCR2 expression revealed equal expression in both mutant and control neutrophils (Figure 3B), thus excluding their involvement in the observed differences of cell migration.
Loss of ArhGAP15 causes increased in vitro and in vivo neutrophil migration. (A) Boyden chamber chemotaxis analysis of ArhGAP15+/+ and ArhGAP15−/− neutrophils; n = 5/genotype. (B) Representative fluorescence-activated cell sorter analysis of neutrophil CD88 and CXCR2 expression; n = 3/genotype. (C) Neutrophil recruitment to air pouches after saline (Co) or IL-8 injection; n = 6/genotype. (D-E) Peritoneal total cell recruitment and cellular subpopulation (MØ macrophages, NØ neutrophils, and LY lymphocytes) analysis after intraperitoneal administration of 1 × 107 colony-forming unit of E. coli; n = 14/genotype. #P < .05. *P < .01. **P < .001.
Loss of ArhGAP15 causes increased in vitro and in vivo neutrophil migration. (A) Boyden chamber chemotaxis analysis of ArhGAP15+/+ and ArhGAP15−/− neutrophils; n = 5/genotype. (B) Representative fluorescence-activated cell sorter analysis of neutrophil CD88 and CXCR2 expression; n = 3/genotype. (C) Neutrophil recruitment to air pouches after saline (Co) or IL-8 injection; n = 6/genotype. (D-E) Peritoneal total cell recruitment and cellular subpopulation (MØ macrophages, NØ neutrophils, and LY lymphocytes) analysis after intraperitoneal administration of 1 × 107 colony-forming unit of E. coli; n = 14/genotype. #P < .05. *P < .01. **P < .001.
To further test the role of ArhGAP15 as a negative regulator of neutrophil chemotaxis, migration assays were performed in vivo after the administration of IL-8 in preformed subcutaneous air pouches. After 4 hours, neutrophil accumulation was significantly higher in ArhGAP15−/− mice than in wild-type controls (Figure 3C). In addition, ArhGAP15−/− mice also displayed increased neutrophil influx in vehicle-treated controls (Figure 3C), as well as in nontreated air pouches (data not shown), thus suggesting a stronger local response to air-pouch induction.
Neutrophil recruitment was subsequently assessed 12 hours after the peritoneal administration of E coli, demonstrating that the amount of recruited leukocytes was 2-fold higher in mutant than in wild-type peritoneal lavages (Figure 3D). Analysis of cellular subpopulations showed that, whereas macrophages and lymphocytes responded equally in both genotypes, neutrophils appeared 3-fold more abundant in mutant than in wild-type mice, thus accounting for differences in total cell numbers (Figure 3E).
Enhanced directional migration in ArhGAP15−/− neutrophils
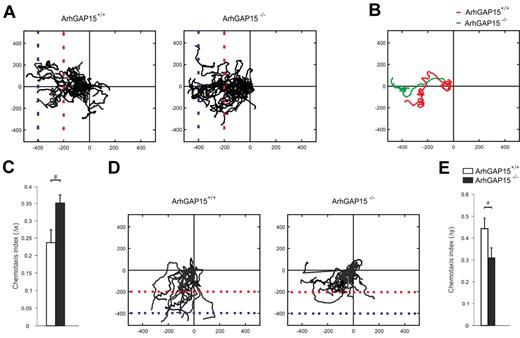
To better address the specific aspects of neutrophil migration affected by the loss of ArhGAP15, neutrophil chemotaxis was studied by time-lapse microscopy in under-agarose chemotactic assays. Results from these experiments showed that, 1 hour after exposure to C5a, 24% ± 3% of ArhGAP15-null neutrophils reached the last horizon (blue dotted line) whereas only 13% ± 2% of wild-type controls traveled the same space (Figure 4A), thus indicating that mutant neutrophils migrated to a greater distance than wild-type controls. As migratory speed did not reveal differences between the genotypes (ArhGAP15+/+: 11.45 ± 3.44 μm/min, ArhGAP15−/−: 10.23 ± 4.32 μm/min), tracks of migrating cells were further analyzed to test potential differences in path convolution. This revealed that ArhGAP15−/− neutrophils migrated in straighter paths than wild-type controls (Figure 4A-B). In agreement, quantification of the ratio between cell displacement in the correct direction (Δx) and the total length of the migration path indicated that the loss of ArhGAP15 reduces path tortuosity (Figure 4C). Similar results were obtained with CXCL2 (supplemental Figure 5A-B). Of note, these experiments did not show any difference between genotypes in the displacement in the y-axis, thus indicating that loss of ArhGAP15 does not affect gradient sensing during migration.
ArhGAP15-null neutrophils showed increased directional migration but decreased turning ability. (A-E) Under-agarose migration experiments. (A) Center-zeroed tracks of ArhGAP15+/+ and ArhGAP15−/− neutrophils migrating toward C5a (left). (B) Single-cell tracks of a typical ArhGAP15+/+ and ArhGAP15−/− neutrophil. (C) Measurement of chemotactic index toward C5a (Δx). (D) Center-zeroed tracks of ArhGAP15+/+ and ArhGAP15−/− neutrophils migrating from a primary CXCL2 gradient in the x-axis (left) toward a secondary C5a gradient in the y-axis (bottom). (E) Measurement of chemotactic index toward C5a (Δy). For each experiment, n = 4 movies/genotype. #P < .05.
ArhGAP15-null neutrophils showed increased directional migration but decreased turning ability. (A-E) Under-agarose migration experiments. (A) Center-zeroed tracks of ArhGAP15+/+ and ArhGAP15−/− neutrophils migrating toward C5a (left). (B) Single-cell tracks of a typical ArhGAP15+/+ and ArhGAP15−/− neutrophil. (C) Measurement of chemotactic index toward C5a (Δx). (D) Center-zeroed tracks of ArhGAP15+/+ and ArhGAP15−/− neutrophils migrating from a primary CXCL2 gradient in the x-axis (left) toward a secondary C5a gradient in the y-axis (bottom). (E) Measurement of chemotactic index toward C5a (Δy). For each experiment, n = 4 movies/genotype. #P < .05.
To test whether this increased directional migration was caused by differences in cell polarization, the elongation ratio of these migrating cells was measured. Interestingly, ArhGAP15-null neutrophils showed a limited but significant increase (3.3% ± 0.1%) in cell polarization compared with wild-type controls (ArhGAP15+/+: 1.519% ± 0.014%, ArhGAP15−/−: 1.571% ± 0.019%; P < .05).
Next, cells moving toward a specific chemotactic cue were challenged by the addition of a second perpendicular chemotactic gradient causing the bending of migratory paths. ArhGAP15-deficient neutrophils, stimulated first with CXCL2 and subsequently with C5a, changed direction significantly less than wild-type cells (Figure 4D, ArhGAP15+/+ video 1 and ArhGAP15−/− video 2). Consistently, analysis of individual migratory paths showed that the ratio between the displacement of a cell toward the second chemoattractant (Δy) and the total length of its migration path was significantly lower in ArhGAP15−/− neutrophils (Figure 4E), thus indicating that the loss of ArhGAP15 leads to more persistent directional migration.
Increased phagocytosis, oxidase activation, and bacterial killing in ArhGAP15−/− neutrophils
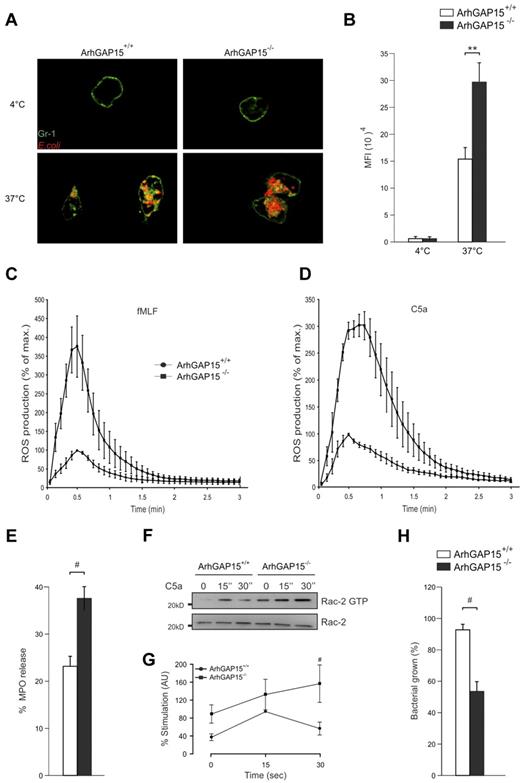
The finding that ArhGAP15 altered migration suggested that other Rac-dependent neutrophil functions, such as phagocytosis and ROS generation, could be affected by the loss of this protein. Measurement of in vitro phagocytosis revealed that ArhGAP15−/− neutrophils engulfed 2-fold more serum-opsonized E coli cells than wild-type controls (Figure 5A-B). Conversely, no difference was detected when bacteria were coated with IgG alone (supplemental Figure 6A) or when phagocytosis of serum-opsonized particles was assessed in macrophages (supplemental Figure 6B).
ArhGAP15 regulates neutrophil-mediated phagocytosis, ROS generation, and in vitro bacterial killing. (A) Representative images of E. coli (red) phagocytosed by neutrophils (Gr-1 staining in green). (B) Quantification of Texas Red fluorescence (E. coli) in ArhGAP15+/+ and ArhGAP15−/− neutrophils; n ≥ 100 cells/genotype. (C-D) ROS generation by neutrophils induced by (C) 2.5μM fMLF or (D) 100nM C5a; n = 5/genotype. (E) Quantification of MPO release by ArhGAP15+/+ and ArhGAP15−/− neutrophils; n = 4/genotype. (F-G) Determination of Rac2 activation after C5a stimulation in neutrophils. Representative detection of active (Rac2-guanosine triphosphate) and total Rac2 is shown. Normalization was obtained by setting the percentage stimulation of ArhGAP15+/+ neutrophils after 15 seconds for 100%; n = 5/genotype. (H) In vitro killing of E. coli by isolated ArhGAP15+/+ and ArhGAP15−/− neutrophils; n = 4/genotype. #P < .05. **P < .001.
ArhGAP15 regulates neutrophil-mediated phagocytosis, ROS generation, and in vitro bacterial killing. (A) Representative images of E. coli (red) phagocytosed by neutrophils (Gr-1 staining in green). (B) Quantification of Texas Red fluorescence (E. coli) in ArhGAP15+/+ and ArhGAP15−/− neutrophils; n ≥ 100 cells/genotype. (C-D) ROS generation by neutrophils induced by (C) 2.5μM fMLF or (D) 100nM C5a; n = 5/genotype. (E) Quantification of MPO release by ArhGAP15+/+ and ArhGAP15−/− neutrophils; n = 4/genotype. (F-G) Determination of Rac2 activation after C5a stimulation in neutrophils. Representative detection of active (Rac2-guanosine triphosphate) and total Rac2 is shown. Normalization was obtained by setting the percentage stimulation of ArhGAP15+/+ neutrophils after 15 seconds for 100%; n = 5/genotype. (H) In vitro killing of E. coli by isolated ArhGAP15+/+ and ArhGAP15−/− neutrophils; n = 4/genotype. #P < .05. **P < .001.
On the other hand, superoxide production analysis revealed that, although the kinetics of ROS production was similar in both genotypes, the generation of oxygen species in response to either fMLF or C5a showed a peak 3-fold higher in ArhGAP15-null neutrophils than in wild-type controls (Figure 5C-D). In contrast, ROS production in response to IgG-zymosan was equal in ArhGAP15-deficient and wild-type neutrophils (supplemental Figure 6C), thus further supporting a more substantial involvement of ArhGAP15 in GPCR than in FcγR-mediated signaling. In agreement with increased ROS production after GPCR stimulation, C5a triggered in ArhGAP15-null neutrophils significantly higher MPO release than in wild-type controls (Figure 5E).
Because previous studies have shown that phagocytosis, ROS production, and azurophil granule release are more dependent on Rac2 than on Rac1, the C5a-mediated activation of Rac2 was determined in ArhGAP15-null neutrophils. Rac2 activity reached at 30 seconds higher levels in mutant cells than in controls (Figure 5F-G) and presented a kinetics similar to that observed in ROS production (Figure 5D). Enhanced Rac2 activity could thus explain the increased response detected in mutant phagocytes and imply that ArhGAP15-deficient neutrophils may have stronger bactericidal properties. In agreement with this hypothesis, in vitro killing of live E coli cells was found to be 42% ± 7% higher in ArhGAP15-deficient neutrophils than in wild-type controls (Figure 5H).
Protection of ArhGAP15−/− mice from severe, polymicrobial, abdominal sepsis
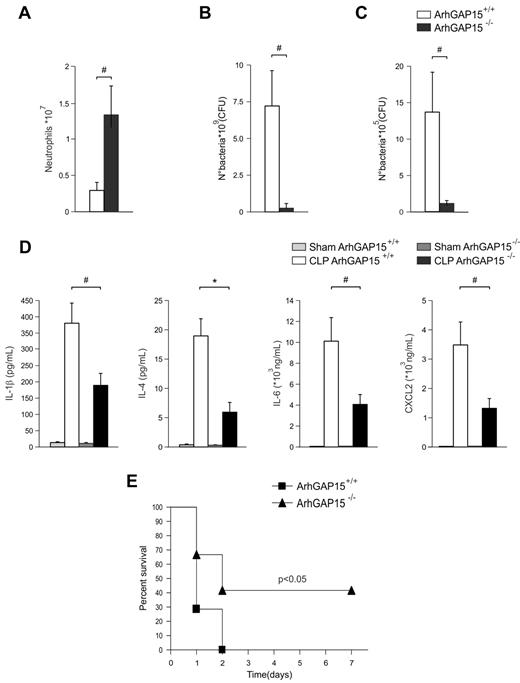
The enhancement of neutrophil functions caused by ArhGAP15 deficiency suggested that blockade of this RacGAP may be beneficial in pathologic conditions that critically depend on rapid neutrophil recruitment, such as severe abdominal polymicrobial sepsis. To test this hypothesis, mutant and wild-type mice were exposed to a model of CLP-inducing severe sepsis. In this pathologic setting, ArhGAP15-null mice showed in sections of the cecum an increased neutrophil load (supplemental Figure 7A) and in the peritoneum a 3-fold higher neutrophil recruitment than wild-type controls (Figure 6A), as well as a dramatic reduction in peritoneal bacterial load (Figure 6B). Furthermore, the spleens of ArhGAP15−/− septic mice contained significantly less bacteria than wild-type controls (Figure 6C), thus indicating decreased bacterial decompartmentalization in the absence of ArhGAP15. After CLP, no differences were observed between genotypes in neutrophil recruitment to the lung, liver, kidney, or ileum (data not shown).
ArhGAP15-null mice are protected against CLP-induced severe sepsis. (A) Number of neutrophils recruited to the peritoneal cavity after CLP; n = 7/genotype. (B) Bacterial load of peritoneal lavage; n = 7/genotype. (C) Bacterial load of isolated spleens; n = 7/genotype. (D) Serum cytokine concentrations 6 hours after CLP; n = 7/genotype. (E) Kaplan-Meier survival analysis of ArhGAP15+/+ and ArhGAP15−/− mice after CLP-induced sepsis; n = 7/genotype. #P < .05. *P < .01.
ArhGAP15-null mice are protected against CLP-induced severe sepsis. (A) Number of neutrophils recruited to the peritoneal cavity after CLP; n = 7/genotype. (B) Bacterial load of peritoneal lavage; n = 7/genotype. (C) Bacterial load of isolated spleens; n = 7/genotype. (D) Serum cytokine concentrations 6 hours after CLP; n = 7/genotype. (E) Kaplan-Meier survival analysis of ArhGAP15+/+ and ArhGAP15−/− mice after CLP-induced sepsis; n = 7/genotype. #P < .05. *P < .01.
Septic injury and mortality are strongly associated with exaggerated systemic inflammation and involve the increased production of multiple cytokines and chemokines.24 Interestingly, serum concentrations of cytokines known to be involved in the septic response, such as IL-1β, IL-2, IL-4, IL-5, IL-6, CXCL2, IL-10, GM-SF, and IFN-γ, were significantly lower in ArhGAP15-null than wild-type mice after CLP (Figure 6D; supplemental Figure 7B). Consistently, although all wild-type controls died within 48 hours after CLP, 40% of ArhGAP15-deficient mice survived > 7 days (Figure 6E). Overall, these results demonstrate that, in severe sepsis, the loss of ArhGAP15 significantly reduces local infection as well as systemic inflammation, ultimately improving survival.
Discussion
The human genome is predicted to encode 60 to 70 RhoGAPs,25 far exceeding the 22 genes encoding Rho family GTPases.26 The excess of RhoGAPs over their target Rho GTPases suggests that each RhoGAP may selectively regulate a specific Rho GTPase signaling pathway activated during defined cellular events.12 ArhGAP15 is a RhoGAP with in vitro specificity toward Rac18 that is regulated by membrane recruitment through its PH domain.18,19 Although the expression of ArhGAP15 has been reported in inflammatory cells,19 its in vivo function has so far remained unclear. In this study, the effects of the lack of ArhGAP15 were analyzed in macrophages and neutrophils derived from ArhGAP15-deficient mice.
Despite the potential presence of numerous other RacGAPs in phagocytes, ArhGAP15 was found to play a nonredundant role in controlling Rac deactivation in both macrophages and neutrophils. In resting conditions, Rac1 activity was elevated in macrophages but not in neutrophils, suggesting a different regulation of Rac1 in the 2 cells types. In response to GPCR agonists, such as C5a, both mutant macrophages and neutrophils showed a higher peak of Rac1 activity. These observations thus indicate that, in neutrophils but not in macrophages, ArhGAP15 down-regulates Rac1-guanosine triphosphate only in response to specific stimuli.
Alteration of Rac activity is thought to differently affect functions of distinct phagocyte populations.27 In agreement, our findings showed that the loss of ArhGAP15 causes less severe phenotypes in macrophages than in neutrophils. For example, in BMDMs, ArhGAP15 deficiency only triggered abnormal elongated morphology. This is consistent with the previously established role of Rac in controlling macrophage cell shape5 and similar to what was detected in macrophages concomitantly lacking the 2 RacGAPs Abr and Bcr.13 As the single loss of either of these 2 molecules fails to induce an effect, our results point to a more dominant role of ArhGAP15 in this process. Consistently, ArhGAP15 and these other 2 RacGAPs may modulate parallel pathways eventually converging in controlling macrophage morphology. Nonetheless, the effect of the loss of ArhGAP15 on cell shape did not significantly alter the ability of macrophages to migrate toward a chemotactic gradient. Consistent with this observation, neither the knockdown of ArhGAP1519 nor the loss of Rac proteins5 affects macrophage chemotaxis. Although the absence of the RacGAPs Abr and Bcr in double-knockout macrophages is reported to cause increased cell migration,13 our results reinforce the view that Rac proteins are not crucial for macrophage chemotaxis and raise the possibility that the effects seen in Abr/Bcr-deficient cells are the result of mechanisms potentially independent from the modulation of Rac activity.
In further agreement with the notion of different Rac functions in specific phagocyte populations, the absence of ArhGAP15 in neutrophils severely affected their responses to inflammatory mediators and infectious agents. Our results showed that ArhGAP15 plays a crucial negative role in the control of chemotaxis in vitro and in vivo. Previous observations support the model that, in neutrophils, active Rac accumulates at the front of the cell through a positive feedback involving the phosphatidylinositol 3-kinase-product phosphatidylinositol 3,4,5-trisphosphate, thus locally driving leading edge formation, cell polarization, and subsequent directional migration.28-30 By down-modulating Rac activity, ArhGAP15 might function as a negative regulator of this feedback. Through phosphatidylinositol 3,4,5-trisphosphate-mediated membrane recruitment,18,19 ArhGAP15 could locally block Rac activity, destabilize the leading edge, and negatively influence directional migration. In support of this hypothesis, our results showed that the increased chemotaxis of ArhGAP15-null neutrophils results from the combined effects of a series of different events, including increased polarization and improved straightness in the migratory path. However, it is possible to envisage that, although the absence of ArhGAP15 improves the efficacy of chemotactic migration, its loss might also come at a cost to other neutrophil responses. Supporting this hypothesis, the lack of ArhGAP15 reduced the ability of cells to modify their trajectory. Indeed, although ArhGAP15-null neutrophils showed more efficient migration toward a single chemoattractant source, their ability to change direction in response to multiple chemotactic agonists appeared significantly impaired. Therefore, the inhibitory function of ArhGAP15 on Rac may be essential to provide neutrophils with the plasticity required to finely regulate and/or alter their directional movements as well as prioritize various chemotactic cues.
Of note, the absence of ArhGAP15 not only affected neutrophil migration but also other critical antimicrobial activities of these cells, such as phagocytosis and respiratory burst, thus suggesting that this RacGAP is a master regulator of neutrophil function. ArhGAP15-null neutrophils displayed increased phagocytosis of serum-opsonized bacteria, indicating that a single RacGAP can affect neutrophil-mediated phagocytosis. Indeed, the lack of Bcr is not sufficient to alter neutrophil-mediated bacterial ingestion.15 Nonetheless, when Ig opsonization was used to trigger phagocytosis, the absence of ArhGAP15 did not cause an abnormal response. Although our results cannot exclude the possibility that, besides immunoglobulins, other tyrosine kinase signaling agonists present in the serum might activate ArhGAP15, this result suggests that ArhGAP15 is more substantially activated downstream of GPCRs. Nonetheless, the finding that ArhGAP15 can block phagocytosis in a more physiologic response to serum-opsonized bacteria is in keeping with the observation that bacterial toxins can mimic RhoGAP activity to reduce their internalization and subsequent killing by neutrophils. For example, ExoS and ExoT, 2 toxins from Pseudomonas aeruginosa, possess RacGAP activity that blocks phagocytosis by disrupting RhoGTPase-mediated actin rearrangement.31
In neutrophils, the main Rac isoform involved in phagocytosis is Rac26 and, in agreement with increased bacterial ingestion, ArhGAP15-deficient neutrophils showed enhanced GPCR-induced Rac2 activity. Rac2 is also critically required for nicotinamide adenine dinucleotide phosphate oxidase complex assembly and consequent ROS production.3,9 Consistent with ArhGAP15 being a negative regulator of Rac2, the lack of this protein caused a significant increase in ROS production after stimulation of different GPCRs but interestingly not of FcγR. This reinforces the view that, within the RacGAP family, ArhGAP15 possesses unique signaling properties mainly involving Rac2 modulation after GPCR signaling, a concept further supported by the observation that C5a stimulation led to the enhancement of azurophile granule release, a process known to depend on Rac2.23 Overall, as GPCR engagement is typically occurring in vivo in response to invading pathogens, our results explain the increased ability of mutant neutrophils to kill bacteria in vitro.
Bactericidal activity of neutrophils after recruitment to the site of infection is critical in conditions, such as severe polymicrobial sepsis, where systemic spread of a localized bacterial contamination becomes life-threatening. In this setting, insufficient neutrophil recruitment and bacterial elimination have been shown to increase mortality.32 Therefore, it could be envisaged that enhancing polymorphonuclear leukocyte functions, as observed in the absence of ArhGAP15, may be beneficial. In line with this hypothesis, in a model of polymicrobial peritoneal sepsis, induced by severe cecal ligation and perforation, the absence of ArhGAP15 increased neutrophil migration to the site of infection, decreased bacterial growth and decompartmentalization, lowered systemic inflammation, and ultimately improved survival at one week. Although this is in apparent contrast to the increased mortality observed in Abr/Bcr double knockout mice at 48 hours after mild sepsis,14 several reasons could account for this discrepancy. First, the functions of ArhGAP15 and Abr/Bcr probably do not completely overlap because of structural differences, such as the additional presence, in both Abr and Bcr, of a DH motif and a PDZ-binding domain. Second, there are significant differences reported between mild CLP, used in the Abr/Bcr study, and severe CLP, used in this study, with survival and the proinflammatory response varying depending on the point of ligation, size of puncture, number of punctures, fluid resuscitation, and antibiotic treatment.33 Third, although both studies show heightened neutrophil activity, the loss of ArhGAP15 in sepsis improved recruitment to the site of infection (cecum and peritoneal cavity), thereby decreasing bacterial decompartmentalization and systemic inflammation, but had no effect on recruitment to distal organs (lung, liver, kidney, and ileum), suggesting that decreased systemic inflammation is the dominant cause of improved survival. In contrast, the loss of Abr/Bcr increases unwanted migration to distal organs resulting in unwarranted inflammation, organ damage, and death.
In conclusion, this study shows that ArhGAP15 plays a nonredundant role as a regulator of multiple neutrophil functions, providing a balance between antimicrobial activities and allowing the versatility to respond to distinct chemotactic signals. Therefore, it is tempting to speculate that, in pathologic conditions with a severe focalized infection where neutrophil-mediated antibacterial activity is imperative, ArhGAP15 may be beneficial as a novel therapeutic target.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by Associazione italiana ricerca sul cancro, Piedmont Region, Fondation Leducq, the European Union Sixth Framework Program EUGeneHeart, and Fondazione Cariplo 2009-2455.
Authorship
Contribution: C.C., G.G., and E.L.M.-C. designed and performed research, analyzed data, and wrote the manuscript; I.M., E.B., S.M., and O.A. performed research and analyzed data; V.M.R. and F.A. analyzed data; and E.H. designed research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Carlotta Costa, Department of Genetic, Biology and Biochemistry and Molecular Biotechnology Center, University of Torino, Via Nizza 52, 10126 Torino, Italy; e-mail: carlotta.costa@unito.it; and Emilio Hirsch, Department of Genetic, Biology and Biochemistry and Molecular Biotechnology Center, University of Torino, Via Nizza 52, 10126 Torino, Italy; e-mail: emilio.hirsch@unito.it.
References
Author notes
C.C. and G.G. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal