Abstract
Platelet activation via Fcγ receptor IIA (FcγRIIA) is a critical event in immune-mediated thrombocytopenia and thrombosis syndromes (ITT). We recently identified signaling by the guanine nucleotide exchange factor CalDAG-GEFI and the adenosine diphosphate receptor P2Y12 as independent pathways leading to Rap1 small GTPase activation and platelet aggregation. Here, we evaluated the contribution of CalDAG-GEFI and P2Y12 signaling to platelet activation in ITT. Mice transgenic for the human FcγRIIA (hFcR) and deficient in CalDAG-GEFI−/− (hFcR/CDGI−/−) were generated. Compared with controls, aggregation of hFcR/CDGI−/− platelets or P2Y12 inhibitor-treated hFcR platelets required more than 5-fold and approximately 2-fold higher concentrations of a FcγRIIA stimulating antibody against CD9, respectively. Aggregation and Rap1 activation were abolished in P2Y12 inhibitor-treated hFcR/CDGI−/− platelets. For in vivo studies, a novel model for antibody-induced thrombocytopenia and thrombosis was established. FcγRIIA-dependent platelet thrombosis was induced by infusion of Alexa750-labeled antibodies to glycoprotein IX (CD42a), and pulmonary thrombi were detected by near-infrared imaging technology. Anti-GPIX antibodies dose-dependently caused thrombocytopenia and pulmonary thrombosis in hFcR-transgenic but not wild-type mice. CalDAG-GEFI-deficient but not clopidogrel-treated hFcR-transgenic mice were completely protected from ITT. In summary, we established a novel mouse model for ITT, which was used to identify CalDAG-GEFI as a potential new target in the treatment of ITT.
Introduction
Platelets are essential components of the hemostatic response to vascular injury. However, platelets also play a role in pathologic conditions, such as atherothrombosis, and in immune-mediated thrombocytopenia and thrombosis (ITT). Several ITT syndromes, including heparin-induced thrombocytopenia and thrombosis (HIT),1-3 bacterial sepsis-associated thrombocytopenia and disseminated intravascular coagulation,4,5 and the thrombotic manifestations of antiphospholipid syndromes6 are characterized by immune-mediated platelet activation through the platelet Fcγ receptor, FcγRIIA. In addition, thrombotic complications have been observed with the expanded use of therapeutic IgG antibodies, such as bevacizumab.7-9 One of the barriers to successful treatment of these thrombotic syndromes is that therapeutic targeting of platelet activation pathways to prevent thrombosis is either not effective or comes with an inherent risk of bleeding complications.
In humans, FcγRIIA is expressed on platelets, neutrophils, monocytes, and macrophages and activates these cells following the binding of the Fc region of IgG-coated cells or IgG-containing immune complexes.10 Mice lack the genetic equivalent of human FcγRIIA and, indeed, do not express a platelet Fc receptor. Consequently, most of the studies on the role of FcγRIIA in platelet activation were entirely dependent on the use of inhibitors, and they did not provide information on whether or not these inhibitors would reduce the risk of excessive platelet activation as observed in the clinical settings of ITT or HIT. To circumvent this limitation, we generated and characterized human FcγRIIA-transgenic (hFcR) mice in which FcγRIIA is expressed on mouse platelets and macrophages at levels equivalent to that in human cells.11 For studies on HIT, we further crossed hFcR mice with mice deficient for mouse PF4 but transgenic for human PF4.12 Using these mouse models, we demonstrated a critical role for FcγRIIA expression in antiplatelet antibody-induced and heparin-induced thrombocytopenia and thrombosis in vivo.11-14
FcγRIIA is unique among the activating Fcγ receptors in that its cytoplasmic tail contains an immunoreceptor tyrosine-based activation motif.15 Residues in the immunoreceptor tyrosine-based activation motif domain become rapidly phosphorylated on receptor engagement and induce cell activation after binding by nonreceptor protein tyrosine kinases, such as spleen tyrosine kinase.16,17 It is widely accepted that stimulation of FcγRIIA on platelets stimulates spleen tyrosine kinase, leading to PLCγ activation and the generation of the second messengers Ca2+ and DAG. In our recent work, we have described a central role for Ca2+ and diacylglycerol regulated guanine nucleotide exchange factor I (CalDAG-GEFI) in Ca2+-dependent platelet activation.18-21 CalDAG-GEFI catalyzes the activation of the small GTPases Ras-proximate (Rap)1 and Rap2. In platelets, Rap1B accounts for 90% of the total Rap protein,22 and its importance in αIIbβ3 activation was demonstrated in Rap1B-deficient mice.23 Our studies with CalDAG-GEFI−/− mice in combination with inhibitors to protein kinase C or the Gi-coupled adenosine diphosphate receptor, P2Y12, identified a 2-pathway model for integrin activation downstream of PLC activation. CalDAG-GEFI is a high-affinity sensor for Ca2+, which mediates the rapid but reversible activation of αIIbβ3. In the absence of CalDAG-GEFI, Rap1/integrin activation is delayed but sustained and depends on signaling by protein kinase C and P2Y12. Notably, our studies further suggested that CalDAG-GEFI is particularly important for platelet activation through GPVI, the immunoreceptor tyrosine-based activation motif-coupled platelet collagen receptor.21
In this study, we evaluated the contribution of 2 critical platelet signaling pathways, Ca2+/CalDAG-GEFI/Rap1 and P2Y12/Rap1, to platelet activation in ITT. Deficiency in CalDAG-GEFI, and to a lesser extent inhibition of P2Y12, provided protection from FcγRIIA-mediated platelet aggregation both in vitro and in vivo.
Methods
Reagents and antibodies
Lovenox (enoxaparin sodium; Sanofi-Aventis), heparin-coated capillaries (VWR), BSA (fraction V), prostacyclin, human fibrinogen (type I), acetylsalicylic acid (aspirin; all from Sigma-Aldrich), 2-methylthio-AMP triethylammonium salt hydrate (2-MeSAMP, P2Y12 inhibitor, BioLog), U46619 (Cayman Chemical), PAR4-activating peptide (Advanced Chemtech), TxB2 immunoassay (Assay Design), and RalGDS-RBD coupled to agarose beads and polyvinylidene fluoride membranes (Millipore) were purchased. Monoclonal antibodies against murine CD9 (rat IgG2a, BD Biosciences Pharmingen) and murine GPIX, GPIbα, αIIbβ3, GPVI, β1 integrin, control IgG (Emfret Analytics), murine Rap1 (Santa Cruz Biotechnology), human FcγRIIA (IV.3, StemCell Technologies), and goat anti–mouse IgG (Fab′2; Santa Cruz Biotechnology) were purchased.
Mice
Mice transgenic for hFcγRIIA,11 CalDAG-GEFI−/−,18 and littermate control wild-type (WT) mice were bred on a C57Bl6/J strain background and housed in the mouse facility of Thomas Jefferson University. Experimental procedures were approved by the Animal Care and Use Committee of Thomas Jefferson University. Where indicated, mice were treated with clopidogrel 24 and 3 hours before the experiment at a dosage of 75 mg/kg body weight orally by gavage feeding.
Platelet preparation
Blood was drawn from the retroorbital plexus into heparinized tubes. Platelet-rich plasma (PRP) was obtained by centrifugation at 100g for 5 minutes at room temperature. PRP was centrifuged at 700g in the presence of prostacyclin (2 μg/mL) for 5 minutes at room temperature. After 2 washing steps, pelleted platelets were resuspended at the concentration of 4 × 108 platelets/mL in modified Tyrode buffer (137mM NaCl, 0.3mM Na2HPO4, 2mM KCl, 12mM NaHCO3, 5mM HEPES, 5mM glucose, pH 7.3) containing 0.35% BSA and 1mM CaCl2.
Flow cytometry
Surface expression of FcγRIIA.
Blood was obtained from the retro-orbital plexus of anesthetized mice. Blood samples were stained with a phycoerythrin-labeled antibody against GPIbα and an Alexa488-labeled antibody against FcγRIIA (IV.3). Surface expression of FcγRIIA was determined as the mean fluorescence intensity in channel 1 (FL-1) for all events that stained positive for FL-2.
Platelet count.
Blood samples were stained with a phycoerythrin-labeled antibody against αIIbβ3, and platelets were counted by flow cytometry gating for FL-2 (phycoerythrin) positive events. Platelet counts at t = 0 were defined as 100%.
Aggregometry
Light transmission was measured in PRP or washed platelets activated under stirring conditions at 37°C. Inhibitors and agonists were added at the indicated concentrations, and light transmission was recorded for 10 to 15 minutes on a Chrono-log 4-channel optical aggregation system (Chronolog).
Rap1 pull-down assay
Platelets were stimulated in standard aggregometry for 1 or 10 minutes. Reactions were stopped with ice-cold 2× lysis buffer (100mM Tris/HCl, pH 7.4, 400mM NaCl, 5mM MgCl2, 2% Nonidet P-40, 20% glycerol, and complete protease inhibitor cocktail lacking ethylenediaminetetraacetic acid). Cell lysis was completed on ice for 15 minutes. Rap1-GTP was precipitated from lysates using RalGDS-RBD beads as described previously.18 Pellets were solubilized in sample buffer (75mM Tris/HCl pH 6.8, 10% SDS, 10% glycerol, 5% 2-mercaptoethanol, 0.004% bromophenol blue) for the detection of Rap1 by immunoblot. Small aliquots were saved to control that each sample contained equal amounts of proteins.
Western blotting
Proteins were separated by SDS-PAGE on 10%-20% gradient gels and transferred to polyvinylidene fluoride membranes. Standard Western blotting procedures were used. Rap1 was detected, and band intensity was quantified with the Odyssey Infrared Imaging System (LICOR Biosystems).
Thromboxane generation assay
Platelets were stimulated in standard aggregometry. At different time points, 50 μL of sample was withdrawn and 5mM EDTA /1mM acetylsalicylic acid was added. The cells were removed by centrifugation, and TxB2 levels were determined in the supernatant by enzyme immunoassay. TxB2 is the stable product of the nonenzymatic hydration of thromboxane (TxA2), which itself has a half-life of only 37 seconds under physiologic conditions.
In vivo immunoglobulin-mediated thrombosis model
Animals were matched according to their platelet Fc-receptor expression before the experiment. Mice were anesthetized with isoflurane, and anti–GPIX-750 was administered by retro-orbital injection at a dose of 0.5 μg/g or 1 μg/g body weight. After 4 hours, the mice were anesthetized and killed by cervical dislocation. Laporotomy and thoracotomy were performed to gain access to the internal organs. The organs were carefully flushed with 2 mL PBS by left ventricular and right ventricular puncture to perfuse the lung and systemic vessels. After flushing, the organs were harvested, cleared from fatty and connective tissue, and incubated in 4% paraformaldehyde for 24 hours before they were read on a conventional LICOR Odyssey scanner (LICOR).
Pearl imaging method.
WT animals and hFcR animals were anesthetized with 100 mg/kg ketamine (Fort Dodge Animal Health) and 10 mg/kg xylazine (Lloyd Lab), the abdominal and thoracic region was shaved with an electric clipper, and the remaining hair was removed with a depilatory cream. Subsequently, the mice were injected with an anti–GPIX-750 antibody. After 4 hours, bio imaging was performed using a Pearl Impulse in vivo imaging chamber (LICOR Biosystems) at 800 nm.
H&E staining.
Extracted lungs were cryosectioned (8-μm sections) and stained with H&E at the histology core within the Pathology Department at Thomas Jefferson University.
Statistics
Results are reported as mean ± SEM. Statistical significance was assessed by unpaired 2-tailed Student t test. A P value < .05 was considered significant.
Results
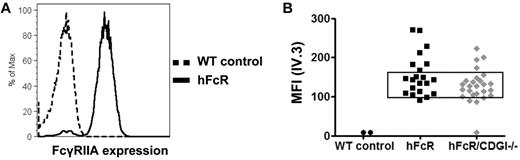
To evaluate the role of CalDAG-GEFI in FcγRIIA-mediated platelet activation, we generated mice transgenic for hFcR and deficient in CalDAG-GEFI (hFcR/CDGI−/−). Staining of platelets for surface-expressed FcγRIIA demonstrated variation in its expression level from mouse to mouse. Thus, for further experiments, we selected mice with similar platelet FcγRIIA expression (Figure 1). Importantly, the surface expression of GPIbα, GPIX, GPVI, αIIbβ3 integrin, and β1 integrins on platelets from hFcR/CDGI−/− mice did not differ significantly compared with platelets from WT or hFcR mice (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Surface expression of human FcγRIIA. (A) Representative histograms for platelets from a WT mouse (dashed black trace) and a mouse transgenic for the hFcR (black trace) stained with IV.3-Alexa488. (B) FcγRIIA expression levels on platelets from hFcR mice and mice transgenic for hFcR and deficient in CalDAG-GEFI (hFcR/CDGI−/−). Only mice with a platelet mean fluorescence signal (IV.3-Alexa488) between 100 and 150 were used for further experiments.
Surface expression of human FcγRIIA. (A) Representative histograms for platelets from a WT mouse (dashed black trace) and a mouse transgenic for the hFcR (black trace) stained with IV.3-Alexa488. (B) FcγRIIA expression levels on platelets from hFcR mice and mice transgenic for hFcR and deficient in CalDAG-GEFI (hFcR/CDGI−/−). Only mice with a platelet mean fluorescence signal (IV.3-Alexa488) between 100 and 150 were used for further experiments.
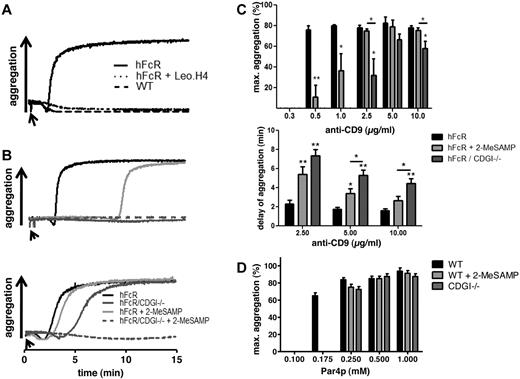
In a first step, we evaluated the contribution of CalDAG-GEFI and P2Y12 to FcγRIIA-mediated platelet aggregation. Confirming previous results,14 addition of antibodies to the cell surface protein CD9 led to rapid, integrin αIIbβ3-dependent aggregation of hFcR-transgenic but not WT platelets (Figure 2A). hFcR/CDGI−/− platelets did not aggregate in response to low-dose anti-CD9 (1μg/mL, Figure 2B top panel), and aggregation induced by high-dose anti-CD9 (10μg/mL, Figure 2B bottom panel) was markedly delayed compared with hFcR platelets. Compared with hFcR/CDGI−/− platelets, hFcR platelets treated with the P2Y12 inhibitor 2-MeSAMP showed a more robust aggregation response both at the low and the high dose of the agonist. Aggregation was abolished in hFcR/CDGI−/− platelets pretreated with 2-MeSAMP, confirming that signaling by both CalDAG-GEFI and P2Y12 is required for FcγRIIA-mediated platelet activation (Figure 2B bottom panel). Importantly, dose-response studies with anti-CD9 antibodies revealed that maximum aggregation in hFcR/CDGI−/− platelets required more than 5-fold higher concentrations of anti-CD9 compared with hFcR platelets. Addition of 2-MeSAMP caused only an approximately 2-fold shift in the dose-response to anti-CD9 in hFcR platelets (Figure 2C top panel). Furthermore, the delay in the aggregation response observed in hFcR/CDGI−/− platelets was significantly greater than that in 2-MeSAMP–treated and untreated hFcR platelets (Figure 2C bottom panel). In contrast, aggregation of both CDGI−/− and 2-MeSAMP–treated WT platelets required only 2-fold higher concentrations of PAR4 receptor-activating peptide (Par4p) compared with untreated WT platelets (Figure 2D). Thus, CalDAG-GEFI is particularly important for FcγRIIA-dependent platelet activation. To confirm this result, we directly cross-linked FcγRIIA with the monoclonal antibody IV.3 and anti-IgG (Fab′2) fragments (supplemental Figure 1). Aggregation was observed with hFcR platelets but not hFcR/CDGI−/− platelets stimulated with 2 μg/mL IV.3 and 30 μg/mL anti-IgG (Fab′2). Inhibition of P2Y12 in hFcR platelets partially inhibited aggregation at this dose (top panel). At 8 μg/mL IV.3 and 60 μg/mL anti-IgG (Fab′2), aggregation was still reduced in hFcR/CDGI−/− platelets, whereas normal aggregation was observed in 2-MeSAMP–treated hFcR platelets (bottom panel). At both agonist concentrations, aggregation was completely abolished in 2-MeSAMP–treated hFcR /CDGI−/− platelets.
CalDAG-GEFI deficiency protects from FcγRIIA-dependent platelet aggregation. (A-B) Representative aggregation traces in response to anti-CD9. (A) WT (black dashed line) and transgenic hFcR platelets were stimulated with 5 μg/mL anti-CD9 in the presence (dotted black line) or absence (black line) of 75 μg/mL of the αIIbβ3 blocking antibody Leo.H4. (B) Black line represents hFcR; light gray line, hFcR treated with the P2Y12 inhibitor 2-MeSAMP; dark gray line, hFcR/CDGI−/−; and dashed dark gray trace, hFcR/CDGI−/− treated with 2-MeSAMP. Platelets were stimulated with low-dose (top panel, 1 μg/mL) or high-dose (bottom panel, 10 μg/mL) anti-CD9. Traces are representative of 3 independent experiments. (C) Dose-response of anti–CD9-induced platelet aggregation. Maximum aggregation (%, top panel) and delay of aggregation (minutes, bottom panel) measured in PRP from hFcR (black bar), hFcR preincubated with 100μM 2-MeSAMP (light gray bar), and hFcR/CDGI−/− platelets (dark gray bar) stimulated with increasing doses of anti-CD9 antibodies. Data are mean ± SEM (n = 5 or 6). *P < .05. **P < .01. (D) Maximum aggregation measured in PRP from WT (black bar), WT preincubated with 100μM 2-MeSAMP (light gray bar), and CDGI−/− platelets (dark gray bar) stimulated with increasing doses of PAR4 receptor-activating peptide. Data are mean ± SEM (n = 5).
CalDAG-GEFI deficiency protects from FcγRIIA-dependent platelet aggregation. (A-B) Representative aggregation traces in response to anti-CD9. (A) WT (black dashed line) and transgenic hFcR platelets were stimulated with 5 μg/mL anti-CD9 in the presence (dotted black line) or absence (black line) of 75 μg/mL of the αIIbβ3 blocking antibody Leo.H4. (B) Black line represents hFcR; light gray line, hFcR treated with the P2Y12 inhibitor 2-MeSAMP; dark gray line, hFcR/CDGI−/−; and dashed dark gray trace, hFcR/CDGI−/− treated with 2-MeSAMP. Platelets were stimulated with low-dose (top panel, 1 μg/mL) or high-dose (bottom panel, 10 μg/mL) anti-CD9. Traces are representative of 3 independent experiments. (C) Dose-response of anti–CD9-induced platelet aggregation. Maximum aggregation (%, top panel) and delay of aggregation (minutes, bottom panel) measured in PRP from hFcR (black bar), hFcR preincubated with 100μM 2-MeSAMP (light gray bar), and hFcR/CDGI−/− platelets (dark gray bar) stimulated with increasing doses of anti-CD9 antibodies. Data are mean ± SEM (n = 5 or 6). *P < .05. **P < .01. (D) Maximum aggregation measured in PRP from WT (black bar), WT preincubated with 100μM 2-MeSAMP (light gray bar), and CDGI−/− platelets (dark gray bar) stimulated with increasing doses of PAR4 receptor-activating peptide. Data are mean ± SEM (n = 5).
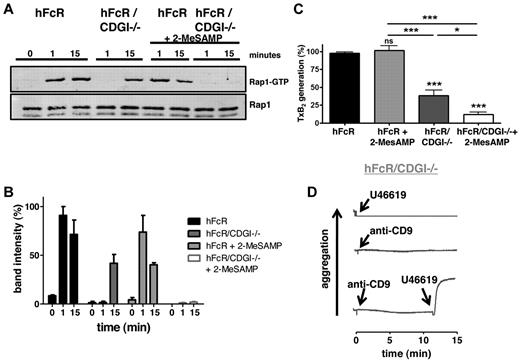
In agreement with our recent studies on the role of CalDAG-GEFI and P2Y12 in Rap1 activation in platelets stimulated via the receptors for thrombin, PAR4, or collagen, GPVI,20,21 we observed impaired Rap1 activation downstream of FcγRIIA in hFcR/CDGI−/− or 2-MeSAMP–treated hFcR platelets (Figure 3A-B). More specifically, hFcR/CDGI−/− platelets lacked the early phase of Rap1 activation, whereas 2-MeSAMP–treated hFcR platelets were defective in sustained activation of the small GTPase. Rap1 activation was completely abolished in 2-MeSAMP–treated hFcR/CDGI−/− platelets. An important platelet response controlled by Rap1 is the generation of the second wave mediator thromboxane (TxA2).21 As expected, the generation TxB2, a stable analog of TxA2, was almost completely abolished in 2-MeSAMP–treated hFcR/CDGI−/− platelets (Figure 3C). Deficiency in CalDAG-GEFI led to a significant reduction in TxB2 formation, whereas inhibition of P2Y12 alone did not affect TxB2 release under these experimental conditions. Importantly, addition of an exogenous thromboxane analog (U46619) restored aggregation in hFcR/CDGI−/− platelets activated with a threshold dose of anti-CD9 antibodies, whereas either agonist alone failed to induce aggregation (Figure 3D). Inhibition of TxA2 generation by the COX-1 inhibitor acetylsalicylic acid (aspirin), however, had only a mild inhibitory effect on the aggregation of both hFcR and hFcR/CDGI−/− platelets (supplemental Figure 2). This impairment was much weaker than that observed in hFcR/CDGI−/− platelets in response to anti-CD9 (Figure 2), indicating that (1) CalDAG-GEFI predominantly affects FcγRIIA-dependent platelet aggregation because of its critical role in integrin αIIbβ3 activation, and (2) the defect in TxA2 feedback is critical only in the absence of CalDAG-GEFI-mediated integrin activation.
CalDAG-GEFI synergizes with P2Y12 signaling in Rap1 activation downstream of FcγRIIA. (A) Time course of Rap1 activation on stimulation of hFcR and hFcR/CDGI−/− platelets with 10 μg/mL anti-CD9 in the absence or presence of 100μM 2-MeSAMP. (Bottom panel) Total Rap1 as loading control. (B) Densitometric analysis of Rap1-GTP shown as percentage of maximal activation (mean ± SEM, n = 3). (C) TxB2 levels in the supernatant of hFcR and hFcR/CDGI−/− platelets 4 minutes after stimulation with 5 μg/mL anti-CD9 in the absence or presence of 100μM 2-MeSAMP. Data are mean ± SEM (n = 6). *P < .05. ***P < .001. ns indicates not significant. (D) Aggregation response of hFcR/CDGI−/− platelets activated with 1 μg/mL anti-CD9, 5μM U46619 (TxA2 analog), or the combination of both agonists. Traces are representative of 4 independent experiments.
CalDAG-GEFI synergizes with P2Y12 signaling in Rap1 activation downstream of FcγRIIA. (A) Time course of Rap1 activation on stimulation of hFcR and hFcR/CDGI−/− platelets with 10 μg/mL anti-CD9 in the absence or presence of 100μM 2-MeSAMP. (Bottom panel) Total Rap1 as loading control. (B) Densitometric analysis of Rap1-GTP shown as percentage of maximal activation (mean ± SEM, n = 3). (C) TxB2 levels in the supernatant of hFcR and hFcR/CDGI−/− platelets 4 minutes after stimulation with 5 μg/mL anti-CD9 in the absence or presence of 100μM 2-MeSAMP. Data are mean ± SEM (n = 6). *P < .05. ***P < .001. ns indicates not significant. (D) Aggregation response of hFcR/CDGI−/− platelets activated with 1 μg/mL anti-CD9, 5μM U46619 (TxA2 analog), or the combination of both agonists. Traces are representative of 4 independent experiments.
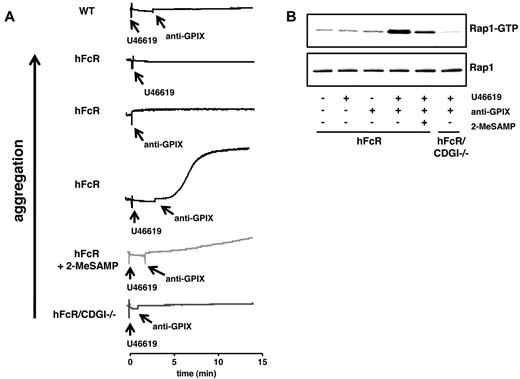
Next, we sought to determine the contribution of CalDAG-GEFI and P2Y12 signaling to FcγRIIA-mediated platelet activation in ITT. In our previous work, we showed that infusion of antibodies against CD9 (rat IgG2a isotype) causes thrombocytopenia in mice independent of FcγRIIA and that FcγRIIA-independent platelet consumption protects mice from FcγRIIA-mediated thrombotic complications.14 We also showed that antibodies to membrane-proximal domains within the GPIb-IX subunit of the von Willebrand factor receptor complex have minimal effects on the survival of circulating platelets in regular WT mice, irrespective of their isotype.24,25 Thus, we speculated that antibodies to GPIX could be well suited to induce FcγRIIA-dependent thrombocytopenia and thrombosis in mice. In vitro, antibodies to GPIX caused aggregation of U46619-primed hFcR but not hFcR/CDGI−/− platelets (Figure 4A). Inhibition of P2Y12 signaling partially inhibited anti-GPIX–induced aggregation of hFcR platelets. Consistent with these findings, we observed that anti-GPIX antibodies induced Rap1 activation in hFcR platelets only in the presence of U46619. Whereas Rap1 activation induced by anti-GPIX/U46619 was abolished in hFcR/CDGI−/− platelets, it was partially reduced in 2-MeSAMP–treated hFcR platelets (Figure 4B).
CalDAG-GEFI deficiency protects from platelet aggregation induced by antibodies to GPIX. (A) Aggregation of hFcR (black line), hFcR pretreated with 100μM 2-MeSAMP (light gray line), and hFcR/CDGI−/− (dark gray line) platelets in response to 0.5 μg/mL anti-GPIX in combination with a threshold dose of U46619 (0.25μM). Aggregation responses of hFcR platelets to either 0.5 μg/mL anti-GPIX or 0.25μM U46619 alone are shown for comparison. (B) Rap1 activation in hFcR and hFcR/CDGI−/− platelets at t = 15 minutes of stimulation with anti-GPIX (0.5 μg/mL) and/or U46619 (0.25μM) in the absence or presence of 100μM 2-MeSAMP. (Bottom panel) Total Rap1 as loading control. Representative of 3 independent experiments.
CalDAG-GEFI deficiency protects from platelet aggregation induced by antibodies to GPIX. (A) Aggregation of hFcR (black line), hFcR pretreated with 100μM 2-MeSAMP (light gray line), and hFcR/CDGI−/− (dark gray line) platelets in response to 0.5 μg/mL anti-GPIX in combination with a threshold dose of U46619 (0.25μM). Aggregation responses of hFcR platelets to either 0.5 μg/mL anti-GPIX or 0.25μM U46619 alone are shown for comparison. (B) Rap1 activation in hFcR and hFcR/CDGI−/− platelets at t = 15 minutes of stimulation with anti-GPIX (0.5 μg/mL) and/or U46619 (0.25μM) in the absence or presence of 100μM 2-MeSAMP. (Bottom panel) Total Rap1 as loading control. Representative of 3 independent experiments.
Infusion of antibodies to GPIX dose-dependently caused severe thrombocytopenia in hFcR but not regular WT mice (Figure 5A), and thrombocytopenia was associated with the formation of pulmonary microthrombi (Figure 5B). To quantify thrombus formation in the lungs, we injected mice with Alexa750 fluorophore-coupled antibodies to GPIX followed by near-infrared scanning of the whole animal (Figure 5B) or isolated lungs (Figure 6).
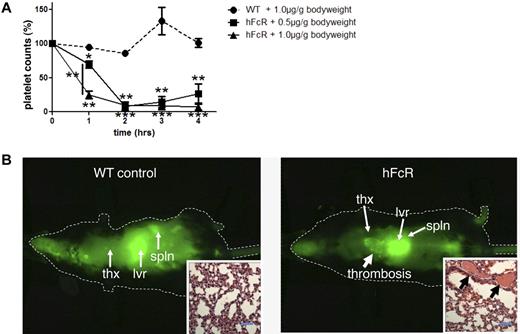
Antiplatelet antibody-induced thrombocytopenia and thrombosis in vivo. (A) Platelet count of hFcR transgenic mice (solid line) injected with 0.5 μg/g (■) or 1.0 μg/g (▴) body weight of anti-GPIX antibody. Platelet count of WT mice injected with anti-GPIX (1 μg/g body weight) is shown as control (dashed line, ●). The platelet counts in whole blood were determined at baseline (t = 0) and every hour up to 4 hours. Results shown are percentage platelet count compared with baseline. *P < .05. **P < .01. ***P < .001. (B) Whole-body scan with a Pearl in vivo Imager of hFcR mice and WT mice 4 hours after the injection of Alexa750-labeled anti-GPIX antibody (1 μg/g body weight) LICOR, Pearl infrared chamber, software Pearl cam Version 2.0. thx indicates thorax; lvr, liver; and spln, spleen. (Insets) Representative H&E-stained histology sections of lungs isolated from WT or hFcR mice. Arrows point at fibrin and platelet-rich thrombi in the lung vasculature. Images are representative of 3 independent experiments. Scale bar represents 50 μm. The images were obtained with a Nikon Eclipse 80i microscope (Nikon) and a 40×/0.8 numeric aperture oil-immersion objective lens, imaging medium: oil, H&E staining, Spot Flex Color camera (Diagnostic Instruments), Spot Advanced Version 4.5 software (Diagnostic Instruments).
Antiplatelet antibody-induced thrombocytopenia and thrombosis in vivo. (A) Platelet count of hFcR transgenic mice (solid line) injected with 0.5 μg/g (■) or 1.0 μg/g (▴) body weight of anti-GPIX antibody. Platelet count of WT mice injected with anti-GPIX (1 μg/g body weight) is shown as control (dashed line, ●). The platelet counts in whole blood were determined at baseline (t = 0) and every hour up to 4 hours. Results shown are percentage platelet count compared with baseline. *P < .05. **P < .01. ***P < .001. (B) Whole-body scan with a Pearl in vivo Imager of hFcR mice and WT mice 4 hours after the injection of Alexa750-labeled anti-GPIX antibody (1 μg/g body weight) LICOR, Pearl infrared chamber, software Pearl cam Version 2.0. thx indicates thorax; lvr, liver; and spln, spleen. (Insets) Representative H&E-stained histology sections of lungs isolated from WT or hFcR mice. Arrows point at fibrin and platelet-rich thrombi in the lung vasculature. Images are representative of 3 independent experiments. Scale bar represents 50 μm. The images were obtained with a Nikon Eclipse 80i microscope (Nikon) and a 40×/0.8 numeric aperture oil-immersion objective lens, imaging medium: oil, H&E staining, Spot Flex Color camera (Diagnostic Instruments), Spot Advanced Version 4.5 software (Diagnostic Instruments).
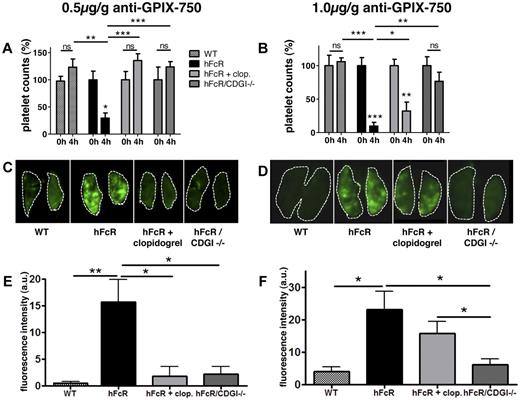
CalDAG-GEFI deficiency protects from immunoglobulin-induced thrombosis in vivo. (A-B) Platelet counts in whole blood at baseline and 4 hours after infusion of anti-GPIX antibody. (C-D) Representative near-infrared images of lungs extracted from the respective mice 4 hours after anti-GPIX-Alexa750 injection obtained with a LICOR Odyssey scanner, software; LICOR Odyssey, Version 3.0. (E-F) Quantitative analysis of the integrated fluorescence density (Adobe Photoshop CS3 extended, Version 10.0.1 Adobe). Results shown are mean fluorescence intensity (a.u.) ± SEM; n = 3 to 5. WT (checkered bar), transgenic hFcR (black bar), hFcR pretreated with 75 mg/kg body weight clopidogrel (light gray bar), and hFcR/CDGI−/− (dark gray bar) mice were injected with an Alexa750-labeled antibody against GPIX at a dosage of 0.5 μg/g (A,C,E) or 1 μg/g (B,D,F) body weight. ***P < .001. **P < .01. *P < .05. ns indicates not significant.
CalDAG-GEFI deficiency protects from immunoglobulin-induced thrombosis in vivo. (A-B) Platelet counts in whole blood at baseline and 4 hours after infusion of anti-GPIX antibody. (C-D) Representative near-infrared images of lungs extracted from the respective mice 4 hours after anti-GPIX-Alexa750 injection obtained with a LICOR Odyssey scanner, software; LICOR Odyssey, Version 3.0. (E-F) Quantitative analysis of the integrated fluorescence density (Adobe Photoshop CS3 extended, Version 10.0.1 Adobe). Results shown are mean fluorescence intensity (a.u.) ± SEM; n = 3 to 5. WT (checkered bar), transgenic hFcR (black bar), hFcR pretreated with 75 mg/kg body weight clopidogrel (light gray bar), and hFcR/CDGI−/− (dark gray bar) mice were injected with an Alexa750-labeled antibody against GPIX at a dosage of 0.5 μg/g (A,C,E) or 1 μg/g (B,D,F) body weight. ***P < .001. **P < .01. *P < .05. ns indicates not significant.
When scanned on a Pearl in vivo Imaging Chamber (LICOR) 4 hours after infusion of the antibody, WT mice did show accumulation of the fluorescence dye in the liver and spleen, but not in the thorax. However, when injecting hFcR mice with the labeled GPIX antibody, there was a distinct accumulation of fluorescence in the thorax (Figure 5B), suggesting the formation of platelet-rich thrombi in the lungs. H&E-stained histology sections of extracted lungs confirmed the formation of microthrombi rich in platelets and fibrin (Figure 5B inset). For studies on the role of CalDAG-GEFI and P2Y12 in experimental ITT, we decided to quantify ITT in lungs extracted 4 hours after infusion of the antibody. At a dosage of 0.5 μg/g body weight of the antibody, both hFcR/CDGI−/− mice and clopidogrel-treated hFcR mice were completely protected from thrombocytopenia (Figure 6A) and pulmonary thrombosis (Figure 6C,E) compared with hFcR mice. At 1 μg/g body weight, however, hFcR/CDGI−/− mice were still protected from ITT, whereas clopidogrel treatment did not significantly affect pulmonary thrombosis in hFcR mice. hFcR mice injected with 1 μg/g anti-GPIX antibodies also displayed a shock phenotype with marked tactile hypothermia (not shown), severely reduced motor activity, and shallow, rapid, and irregular breathing (supplemental Video). Clopidogrel treatment did not prevent these anti-GPIX–induced shock-like symptoms in hFcR mice, whereas no symptoms were observed in hFcR/CDGI−/− mice.
Discussion
We here describe the generation of a novel mouse model for ITT, in which Alexa750 fluorophore-labeled antibodies against the platelet surface receptor GPIX are used (1) to selectively induce thrombocytopenia and thrombosis in FcγRIIA-positive but not WT FcγRIIA-negative mice and (2) to facilitate the rapid and quantitative visualization of pulmonary thrombi by near-infrared imaging technology. Using this cutting-edge technology, we were able to demonstrate that FcγRIIA-dependent platelet aggregation occurs in the systemic venous system and/or the lungs of mice and that pulmonary platelet accumulation was completely abolished in mice transgenic for hFcγRIIA but deficient in CalDAG-GEFI. In contrast, clopidogrel bisulfate (Plavix) treatment only partially inhibited ITT in mice.
Studies in mice on the mechanisms regulating immune-mediated thrombosis have been difficult for several reasons. First, mice do not express the activating Fc receptor, FcγRIIA, on circulating platelets and thus do not develop HIT/ITT. To circumvent this limitation, we previously generated and characterized mice transgenic for the human FcγRIIA receptor.11 Another important limitation for studies on ITT in mice is based on the ability of many antibodies directed against mouse platelet surface receptors to induce thrombocytopenia independent of FcγRIIA expression. Platelet elimination by the reticuloendothelial phagocytic system, however, complicates studies on ITT, as FcγRIIA-independent platelet removal protects mice from FcγRIIA-dependent thrombosis.14 Clinically relevant antibodies/immune complexes, such as the heparin/PF4/anti-PF4 complex12 or the heparin/vascular endothelial growth factor (VEGF)/anti-VEGF complex,7 however, do not cause thrombocytopenia in the absence of platelet FcγRIIA. Finally, there is a lack of technologies that allow a simple but quantitative visualization of microvascular thrombi in the vital organs of ITT mice. So far, most studies relied on the peripheral platelet count in combination with hematoxylin and eosin tissue sections as a read-out for thrombotic complications. We here describe a novel mouse model for ITT in FcγRIIA-transgenic mice, which showed good correlation to HIT and antibody-induced thrombotic complications, both in vitro and in vivo. Our model also markedly simplifies the detection of tissue microthrombi, as it facilitates the quantitative visualization of thrombosis in antibody-treated mice. We selected antibodies to the GPIX subunit of the von Willebrand factor receptor complex to induce thrombocytopenia and thrombosis in an FcγRIIA-dependent manner, as such antibodies show marginal effects on circulating platelet counts in control mice compared with antibodies against other platelet surface receptors, such as αIIbβ3, GPIbα, or CD9.14,24,25 In mice transgenic for hFcR, however, anti-GPIX antibodies caused severe thrombocytopenia within hours (Figure 5). Thrombocytopenia was accompanied by the formation of fibrin-rich thrombi, particularly in the lungs (Figures 5–6), marked tactile hypothermia, severely reduced motor activity, and shallow, rapid, and irregular breathing. Very similar findings have been made for hFcR/hPF4 double-transgenic mice after injection of heparin and anti-PF4 antibodies (S.E.M., unpublished observation) and for FcγRIIA-transgenic mice treated with the antiangiogenic drug bevacizumab in combination with VEGF and heparin.7 Importantly, patients with acute HIT can develop a plethora of complications of arterial and venous thrombosis, including pulmonary thromboembolism, stroke, and myocardial infarction. Consistently, they can present with a variety of symptoms, including dyspnea, neurologic deficiencies, chest pain, and shock.26,27
Interestingly, antibodies to GPIX are weak agonists for FcγRIIA-dependent mouse platelet activation/aggregation in vitro (Figure 4), similar to what has been described for immune complex-mediated activation of these cells.7 Consistent with this observation, it is also widely known that preactivation of low responding human platelets can produce positive FcγRIIA-dependent aggregation responses to HIT complexes.28,29 Thus, our findings that FcγRIIA-dependent mouse platelet activation by anti-GPIX antibodies benefits from priming of these cells are consistent with observations on immune complex-mediated activation in human and murine platelets.
We have previously demonstrated that CalDAG-GEFI is critical for Ca2+-dependent activation of Rap1 and αIIbβ3 integrin in platelets activated by thrombin or collagen, especially under physiologic flow conditions.18,20,21 We here demonstrate that CalDAG-GEFI is critical for FcγRIIA-mediated activation of Rap1 and αIIbβ3 in platelets stimulated by antibodies directed against the surface receptors CD9, GPIX, or FcγRIIA itself (Figures 2 and 4; supplemental Figure 1). Compared with hFcR control platelets, aggregation of hFcR/CDGI−/− platelets required only a 2-fold increase in the concentration of PAR4 receptor-activating peptide, whereas more than 5-fold higher concentrations of stimulating antibodies (anti-CD9) were needed to aggregate hFcR/CDGI−/− cells. Feedback activation via P2Y12, however, was equally important for PAR4- and FcγRIIA-dependent platelet activation, as preincubation of hFcR platelets with 2-MeSAMP caused an approximately 2-fold shift in the dose-response to PAR4p or anti-CD9 antibodies. Consistent with previous findings on the role of Ca2+/CalDAG-GEFI signaling for platelet activation via the collagen receptor, GPVI,21 TxA2 release from antibody-stimulated hFcR/CDGI−/− platelets was markedly reduced and addition of exogenous TxA2 restored aggregation in hFcR/CDGI−/− platelets stimulated via FcγRIIA. Importantly, inhibition of TxA2 synthesis had only a minor effect on the aggregation response of hFcR platelets, demonstrating that CalDAG-GEFI is critical for FcγRIIA-dependent platelet activation because of its critical role in both integrin αIIbβ3 activation and TxA2 biosynthesis, a finding that is well in line with the documented role of TxA2 in FcγRIIA-mediated platelet activation.30,31
Consistent with these in vitro findings, we observed complete protection from ITT in hFcR/CDGI−/− mice, whereas clopidogrel treatment was not effective when mice were challenged with a higher dose of anti-GPIX antibodies. This limited protective effect of clopidogrel in the setting of experimental ITT is in accordance with clinical studies demonstrating incomplete protection from HIT in patients receiving both aspirin and clopidogrel.32 Our studies may explain the limited effect of P2Y12 inhibitors in the setting of HIT/ITT. P2Y12 inhibitors affect the sustained activation of Rap1, important for stable platelet adhesion at sites of vascular injury. However, they do not affect the rapid, CalDAG-GEFI–mediated activation of Rap1, which is sufficient for the formation of reversible platelet aggregates under low and high shear stress conditions.33 In HIT/ITT, these aggregates occur systemically and lead to a permanent obstruction of the pulmonary microcirculation. In contrast, signaling via the Ca2+/CalDAG-GEFI/Rap1 signaling module is critical in the initiation of FcγRIIA-dependent platelet activation. Deficiency in CalDAG-GEFI results in (1) a marked right shift in the dose-response to FcγRIIA agonists and (2) a significant delay in FcγRIIA-mediated platelet activation/aggregation. Consequently, hFcR mice deficient for CalDAG-GEFI were completely protected from ITT.
In conclusion, our novel mouse model for ITT closely mimics experimental HIT and experimental thrombocytopenia and thrombosis induced by heparin/VEGF/anti-VEGF complexes. Our model represents an important step toward more standardized studies on the mechanisms underlying platelet activation in ITT, as it (1) eliminates the need for complicated mouse crosses, (2) depends on a single antibody instead of preformed immune complexes, and (3) provides a simple readout for the quantitative visualization of FcγRIIA-dependent thrombosis in vivo. Our studies also provide a rationale for the documented limited protective effect of P2Y12 inhibitors in HIT patients, and it establishes the Ca2+-sensor CalDAG-GEFI as a promising new target for the intervention with HIT/ITT.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Jill Crittenden and Ann Graybiel for providing CalDAG-GEFI knockout mice, Joseph Rabinowitz for help with the Pearl in vivo imaging system, and the members of the Platelet Interest Group at TJU for helpful discussions and support.
This work was supported by the American Heart Association (SDG0630044N; W.B.), the American Society of Hematology (W.B.), and National Heart, Lung, and Blood Institute, National Institutes of Health (grant R01 HL094594; W.B.).
National Institutes of Health
Authorship
Contribution: M.S. designed the study, performed most of the experiments, and wrote the paper; L.S. performed experiments and helped write the paper; W.B. designed the study and wrote the paper; P.A. performed experiments; T.D.O. maintained the mouse colony and helped with experiments; and M.P.R. and S.E.M. provided the transgenic mice and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Wolfgang Bergmeier, PhD, Dept of Biochemistry and Biophysics, 306A Mary Ellen Jones Bldg, 98 Manning Dr, Campus Box 7035, Chapel Hill, NC 27599-7035; e-mail: bergmeie@email.unc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal