Abstract
The oxygen-sensing transcription factor hypoxia-inducible factor-1α (HIF-1α) plays a critical role in the regulation of myeloid cell function. The mechanisms of regulation are not well understood, nor are the phenotypic consequences of HIF modulation in the context of neutrophilic inflammation. Species conservation across higher metazoans enables the use of the genetically tractable and transparent zebrafish (Danio rerio) embryo to study in vivo resolution of the inflammatory response. Using both a pharmacologic approach known to lead to stabilization of HIF-1α, and selective genetic manipulation of zebrafish HIF-1α homologs, we sought to determine the roles of HIF-1α in inflammation resolution. Both approaches reveal that activated Hif-1α delays resolution of inflammation after tail transection in zebrafish larvae. This delay can be replicated by neutrophil-specific Hif activation and is a consequence of both reduced neutrophil apoptosis and increased retention of neutrophils at the site of tissue injury. Hif-activated neutrophils continue to patrol the injury site during the resolution phase, when neutrophils would normally migrate away. Site-directed mutagenesis of Hif in vivo reveals that hydroxylation of Hif-1α by prolyl hydroxylases critically regulates the Hif pathway in zebrafish neutrophils. Our data demonstrate that Hif-1α regulates neutrophil function in complex ways during inflammation resolution in vivo.
Introduction
Neutrophilic inflammation is of fundamental importance in the innate immune response to bacterial and fungal infection in vertebrates, and it can be initiated by sterile tissue injury. Irrespective of its etiology, inflammation must resolve in a timely manner to avoid damage to surrounding tissues.1 Persisting, noninfectious inflammation is the hallmark of inflammatory diseases, a major cause of morbidity and mortality in the developed world. The resolution phase of inflammation is critical to the restoration of normal tissue function after an inflammatory response, and thus has a central role in determining the outcome of inflammation.2 Despite the central place of failed resolution in the pathogenesis of inflammatory disease, much remains to be known about the cellular and molecular events involved.
Although neutrophil apoptosis, and subsequent uptake and removal by macrophages (efferocytosis), is well documented as a disposal route for inflammatory neutrophils,3-5 there is emerging evidence that other mechanisms also may contribute to certain types of inflammation resolution. In the lung, some neutrophils are lost into the airways and expectorated,6 and in rheumatoid arthritis, neutrophils may leave the inflammatory site while still alive and re-enter the circulation.7 Neutrophils also can be removed by migration through tissues away from the infection site; a process termed retrograde chemotaxis, or reverse migration.8-10 This process is most widely characterized in the zebrafish (Danio rerio) embryo and is less well studied in mammalian systems.
Each of these aspects of inflammation resolution may contribute to the removal of neutrophils and be influenced by a range of extracellular signals encountered by neutrophils at the site of inflammation, including cytokines,11,12 bacterial products,12,13 and low oxygen tensions.14-16 Physiologic hypoxia is important because it induces both extended survival and preserved activity in neutrophils. Hypoxia exerts its effects in part through stabilization of hypoxia-inducible factor (HIF), with activation of the HIF pathway in murine myeloid cells by myeloid-specific Vhl knockout leading to increased myeloid cell recruitment.17 HIF signaling can be activated in normoxia by exposure of myeloid cells to bacterial products, demonstrating the fundamental importance of this pathway to immune cell function.18 The effects of HIF-1α activation and silencing on resolution of neutrophilic inflammation have yet to be fully characterized, especially in an in vivo setting.
Three HIF-α isoforms have been identified in humans, of which HIF-1α is a key regulator of neutrophil function and life span.17-19 HIF-1α stability is regulated by a group of oxygen-sensitive enzymes: prolyl hydroxylases (PHDs) and factor-inhibiting HIF (FIH). Oxygen-dependent PHD activity leads to degradation of HIF-1α via a ubiquitin ligase complex coordinated by the von Hippel Lindau (VHL) protein (pVHL).20-22 In contrast, hypoxia reduces PHD activity, stabilizing HIF-1α, which joins a nuclear complex with the constitutively expressed HIF-β (or aryl hydrocarbon nuclear translocator [ARNT]) and transduces the cellular response.22-24 The HIF pathway can be manipulated pharmacologically using nonspecific inhibitors of PHD enzymes, for example, dimethyloxaloylglycine (DMOG), leading to stabilization of HIF-1α in vitro.25 Such pan-hydroxylase inhibitors lack specificity, however, with potential widespread effects on cellular physiology.
Here, using a transgenic zebrafish line expressing a neutrophil-specific reporter gene,26 we investigate the effects of pharmaceutical and genetic manipulation of Hif-1α activity on neutrophil behavior in vivo. We show for the first time that the resolution of neutrophilic inflammation in vivo is delayed after DMOG treatment in a whole organism model. We show this delay in resolution is a consequence both of a decrease in neutrophil apoptosis and of increased retention of neutrophils at the site of inflammation. Furthermore, using dominant-active and dominant-negative variants of the 2 zebrafish homologs of HIF-1α (Hif-1αa and Hif-1αb), we show timely resolution of neutrophilic inflammation is dependent on prolyl hydroxylation of Hif-1αb, demonstrating the importance of the HIF pathway in determining the outcome of inflammation in vivo.
Methods
Fish husbandry
Two neutrophil-specific fluorescent zebrafish lines were used: Tg(mpx:GFP)i11426 and Tg(lyz:GAL4.VP16)i252;Tg(UAS-E1b:Kaede)s1999t, subsequently termed mpx:GFP and lyz:Kaede, respectively. Zebrafish strains were maintained according to standard protocols.27 Adult fish were maintained on a 14:10-hour light/dark cycle at 28°C in UK Home Office approved facilities in the MRC Centre for Developmental and Biomedical Genetics aquaria at the University of Sheffield.
Inflammation assay
Inflammatory responses were elicited in zebrafish embryos by tail transection as described previously.26 Embryos were anesthetized at 2 or 3 days postfertilization (dpf) by immersion in 0.168 mg/mL tricaine (Sigma-Aldrich), and transection of the tail was performed with a scalpel blade briefly immersed in the chemoattractant f-Met-Leu-Phe (100nM; Sigma-Aldrich). Treatment with 100μM DMOG (or DMSO vehicle control) was performed by immersion at 4 hours postinjury (hpi) or for recruitment assays for 2 hours before injury. Neutrophils were counted at the site of transection at 6, 24, and 48 hpi using a fluorescent dissecting stereomicroscope (Leica) as described previously.26 Where possible, counting was performed blind to experimental conditions.
Apoptosis assays
Rates of apoptosis were assessed blindly by TUNEL/Tyramide Signal Amplification (TSA) or by anti–active caspase-3/TSA staining, as described previously.28,29
Mpx:GFP embryos were injured at 3 dpf (2 dpf for RNA–injected embryos) and 10 dpf. Embryos were treated with DMSO/DMOG at 4 hpi and fixed at 12 hpi in 4% paraformaldehyde. TUNEL (ApopTag Red; Millipore Corporation) staining labeled apoptotic cells with red fluorescence, and TSA (TSAplus kit; Fluorescence Systems, PerkinElmer Life and Analytical Sciences) staining labeled neutrophils with fluorescein green fluorescence. Anti–active caspase-3 (R&D Systems) staining with a fluorescein secondary antibody (Alexa Fluor 488, goat anti–rabbit IgG; Invitrogen) gave green fluorescence. Neutrophils were labeled with red fluorescence using cyanine-3 TSA (TSAplus kit; Fluorescence Systems, PerkinElmer Life and Analytical Sciences).
Neutrophils in the tail transection region were imaged on an UltraVIEWVoX spinning-disk confocal microscope (PerkinElmer Life and Analytical Sciences), and apoptosis was assessed by the percentage of TSA-positive neutrophils labeled with TUNEL or active caspase-3.
Development of a stable Tg(lyz:GAL4.VP16)i252 transgenic line
The Tol2kit multisite Gateway-based transposon system was used to make a transgenic construct from which a stable line was raised.30 Eleven kilobases of the lysosyme C promoter (kindly provided by Phil Crosier, School of Medical Sciences, The University of Aukland, New Zealand),10 was cloned into the p5E-MCS entry vector (Xho-I and Sma-I sites). An LR Clonase II Plus enzyme (Invitrogen) Gateway reaction was performed with the resulting p5E-lyz along with pME-Gal4VP16, p3E-polyA inserted into pDestTol2pA2 to produce lyz:Gal4VP16 construct. This construct was co-injected with tol2-transposase RNA into zebrafish 1-cell-stage embryos to create the Tg(lyz:GAL4.VP16)i252 transgenic line.
Photoconversion of neutrophil-specific Kaede protein
Tail transection of lyz:Kaede larvae was performed at 2 or 3 dpf as described previously.26 Embryos were raised to 6 hpi and mounted in 1% low-melting-point agarose (Sigma-Aldrich). An UltraVIEW PhotoKinesis device on an UltraVIEWVoX spinning disk confocal microscope (PerkinElmer Life and Analytical Sciences) was used to photoconvert the Kaede–labeled cells using 120 pulses of the 405 nm laser at 40% laser power (optimized in previous experiments; data not shown). Embryos were transferred to an Eclipse TE2000-U inverted compound fluorescence microscope (Nikon), where a 1394 ORCA-ERA camera (Hamamatsu Photonics Inc) was used to capture a time-lapse series. Cells fluorescing in the red channel were then tracked over a 3.5-hour period.
Neutrophil tracking
Tracking analysis was performed in Volocity 5 (Improvision; PerkinElmer Life and Analytical Sciences), using the intensity of fluorescence to identify individual labeled neutrophils over time.
Morpholino knockdown of arnt-1
The arnt-1 morpholino (Genetools) was used as reported previously.31 A standard control morpholino (Genetools) was used as a negative control.
hif-1α cloning
Zebrafish 2 dpf RNA purified using TRIzol (Invitrogen) was used for RT-PCR reaction cloning of zebrafish hif-1αa and hif-1αb (using primers in supplemental Table 1; available on the Blood Web site; see the Supplemental Materials link at the top of the online article) using Pfusion polymerase (Finnzymes). These were cloned into TOPOBlunt (Invitrogen) and were subsequently subcloned into pCS2+ (Invitrogen) for RNA synthesis and in situ hybridization expression studies.
Dominant-negative forms of hif-1αa and hif-1αb were generated using primers amplifying DNA corresponding to amino acids 1-330 of human HIF-1α.32 Dominant-active forms of hif-1αa and hif-1αb were generated by successive rounds of site-directed mutagenesis.20,33,34 In each round, one of the hydroxylation sites was mutated into nonhydroxylatable amino acids as described previously in human HIF-1α.20,33,34
Dominant-negative and dominant-active RNAs were transcribed (mMessageMachine; Ambion, Invitrogen) and microinjected into zebrafish embryos at the 1-cell stage as described previously.27,35 A phd-3 in situ hybridization was performed to assess the function of dominant hif-1α variants, using previously described methods.35,36
Development of a stable Tg(UAS:da-hif-1αb-IRES-GFP)i218 transgenic line
Dominant-active hif-1αb was cloned into the middle entry pME-MCS. An LR Clonase (Invitrogen) Gateway reaction was performed with p5E-UAS, pME-da-hif-1αb and p3E-IRES-EGFPpA inserted into pDestTol2pA2. The resulting plasmid was microinjected with tol2-transposase RNA into 1-cell-stage embryos. Internal ribosome entry site–green fluorescent protein (IRES-GFP) expression was weak, so founders were screened by heart marker GFP expression. The resulting Tg(UAS:da-hif-1αb-IRES-GFP)i218 is subsequently referred to as UAS:da-hif-1αb.
Functional Hif expression was tested in UAS:da-hif-1αb, by injection with a cmv:Gal4VP16-IRES-nlsEGFP construct, made from the tol2kit as described previously.30 Injected larvae were screened for expression of nlsEGFP at 3 dpf and a phd-3 in situ hybridization was performed to assess levels of Hif-1α signaling. To investigate the effects of da-hif-1αb expression on the resolution of neutrophilic inflammation, UAS:da-hif-1αb were crossed to Tg(lyz:Gal4)i252 fish to generate double transgenic embryos identified by the presence of GFP heart marker and mCherry-labeled leukocytes.
Statistical analysis
Data were analyzed (Prism 5.0; GraphPad Software) using unpaired, 2-tailed t tests for comparisons between 2 groups and 1-way ANOVA (with Bonferroni posttest adjustment) for other data.
Results
DMOG treatment delays resolution of inflammation
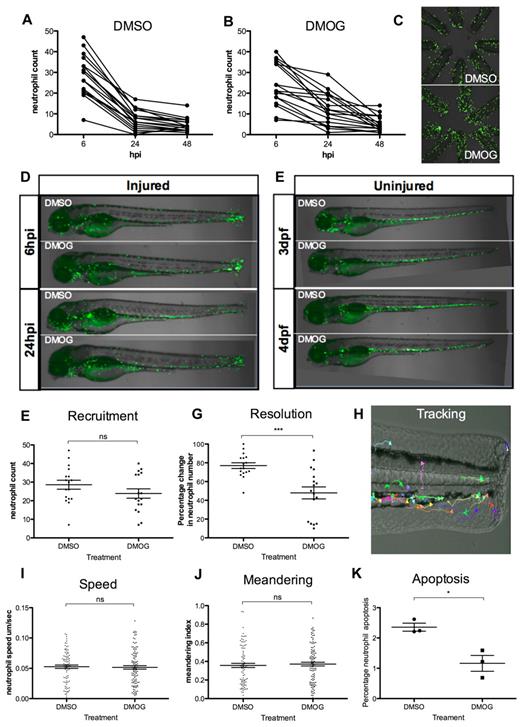
After tail transection in mpx:GFP embryos at 3 dpf, neutrophil numbers can be assessed by counting fluorescent cells, visible in association with the site of injury. Neutrophil numbers act as a measure of the magnitude of the inflammatory response because they are its main cellular component. Neutrophils are recruited early, with numbers peaking at ∼ 6 hours,26 followed by spontaneous resolution of this neutrophilic inflammation over 24 hours (Figure 1A). Macrophages are recruited later and persist longer.37
DMOG delays resolution of neutrophilic inflammation. (A-B) Fluorescent neutrophil numbers in the mpx:GFP line were counted at 6, 24, and 48 hpi in anesthetized embryos. Six hpi is the time point of maximal neutrophil recruitment. By 24 hpi, neutrophilic inflammation has resolved in wild-type embryos. At 4 hpi, fish were treated with 100μM DMOG (B) or with DMSO as vehicle control (A). Data shown are individual embryos for each line, n = 18 performed as 3 independent experiments. (C) Fluorescence photomicrographs of 8 zebrafish tails from control (DMSO; top panel) and DMOG-treated (bottom panel) larvae. Imaged at 24 hpi, original magnification ×2 on a TE2000U inverted microscope (Nikon) at constant exposure. (D-E) Overlaid fluorescence and brightfield photomicrographs of injured 3 dpf embryos at 6 and 24 hpi (D) or uninjured 3 and 4 dpf embryos (E) after treatment at 3 dpf with DMSO and DMOG. Imaged at original magnification ×2 on a TE2000U inverted microscope (Nikon) at constant exposure. (F) The 6-hpi time point neutrophil counts, in DMSO- and DMOG-treated zebrafish embryos. Data shown are mean ± SEM, n = 18 performed as 3 independent experiments. (G) Resolution of the cellular component of inflammation is decreased in DMOG-treated embryos, expressed as percentage of change in neutrophil number between 6 and 24 hpi. Data shown are mean ± SEM, n = 18 performed as 3 independent experiments. P values were calculated using 1-way ANOVA and Bonferroni multiple comparison test, *P < .05, **P < .01, and ***P < .001. (H) Photomicrograph of a typical tracking experiment, with arrows indicating the path of neutrophil movement over a 1-hour time lapse during the recruitment phase of inflammation (1-2 hpi). No difference was observed in the speed of neutrophil migration (I) or meandering index (displacement/path length; J) in DMOG-treated larvae compared with DMSO controls. Data shown are mean ± SEM, n = 18 performed as 3 independent experiments. (K) TUNEL and TSA colocalization shows the percentage of neutrophils at the injury site undergoing apoptosis. Data shown are mean ± SEM, n = 3 performed as independent experiments each containing 35 to 40 embryos/treatment group.
DMOG delays resolution of neutrophilic inflammation. (A-B) Fluorescent neutrophil numbers in the mpx:GFP line were counted at 6, 24, and 48 hpi in anesthetized embryos. Six hpi is the time point of maximal neutrophil recruitment. By 24 hpi, neutrophilic inflammation has resolved in wild-type embryos. At 4 hpi, fish were treated with 100μM DMOG (B) or with DMSO as vehicle control (A). Data shown are individual embryos for each line, n = 18 performed as 3 independent experiments. (C) Fluorescence photomicrographs of 8 zebrafish tails from control (DMSO; top panel) and DMOG-treated (bottom panel) larvae. Imaged at 24 hpi, original magnification ×2 on a TE2000U inverted microscope (Nikon) at constant exposure. (D-E) Overlaid fluorescence and brightfield photomicrographs of injured 3 dpf embryos at 6 and 24 hpi (D) or uninjured 3 and 4 dpf embryos (E) after treatment at 3 dpf with DMSO and DMOG. Imaged at original magnification ×2 on a TE2000U inverted microscope (Nikon) at constant exposure. (F) The 6-hpi time point neutrophil counts, in DMSO- and DMOG-treated zebrafish embryos. Data shown are mean ± SEM, n = 18 performed as 3 independent experiments. (G) Resolution of the cellular component of inflammation is decreased in DMOG-treated embryos, expressed as percentage of change in neutrophil number between 6 and 24 hpi. Data shown are mean ± SEM, n = 18 performed as 3 independent experiments. P values were calculated using 1-way ANOVA and Bonferroni multiple comparison test, *P < .05, **P < .01, and ***P < .001. (H) Photomicrograph of a typical tracking experiment, with arrows indicating the path of neutrophil movement over a 1-hour time lapse during the recruitment phase of inflammation (1-2 hpi). No difference was observed in the speed of neutrophil migration (I) or meandering index (displacement/path length; J) in DMOG-treated larvae compared with DMSO controls. Data shown are mean ± SEM, n = 18 performed as 3 independent experiments. (K) TUNEL and TSA colocalization shows the percentage of neutrophils at the injury site undergoing apoptosis. Data shown are mean ± SEM, n = 3 performed as independent experiments each containing 35 to 40 embryos/treatment group.
Because of the known effects of the pan-hydroxylase inhibitor DMOG on neutrophil apoptosis, we hypothesized that DMOG would delay inflammation resolution in the zebrafish tailfin model. Agents delaying neutrophil apoptosis have been shown to delay inflammation resolution in this model when added once neutrophils have been recruited.28 We therefore added 100μM DMOG at 4 hpi and then observed the effects on inflammation resolution. Treatment with DMOG caused a significant increase in neutrophil numbers at 24 hpi (Figure 1B-D) compared with DMSO-treated controls (Figure 1A,C-D) from the same clutch of embryos, but numbers had decreased to basal levels by 48 hpi, despite persistence of Hif signaling at 48 hpi, as assessed by in situ hybridization expression of the target gene phd-3 (supplemental Figure 1A). There were no additional effects of DMOG on neutrophil distribution throughout the embryo (Figure 1E), nor any increase in total neutrophil number (supplemental Figure 1B). DMOG dose and timing of administration were assessed and found to be optimal (supplemental Figure 1C-D).
Recruitment of neutrophils was assessed at 6 hpi and was unchanged by DMOG treatment (Figure 1F), confirming that the differences seen in percentage change in neutrophil number from 6 to 24 hours (a measure of inflammation resolution) in DMOG-treated embryos is independent of effects on neutrophil recruitment (Figure 1G).
Neutrophil counts do not take account of changes in the migration behavior of neutrophils, which may be affected by hydroxylase inhibition. We therefore assessed the speed and meandering of neutrophil migration toward the site of tail transection by in vivo tracking of mpx:GFP neutrophils over 1 hour during the recruitment phase using 3D time-lapse videomicroscopy (Figure 1H). This tracking technique was sensitive enough to detect changes in neutrophil recruitment after treatment with the chemoattractant N-formyl-l-methionyl-l-leucyl-l-phenylalanine (fMLP; supplemental Figure 2A-B). After DMOG treatment, however, no significant difference in neutrophil speed (Figure 1I) or meandering index (Figure 1J) was observed compared with vehicle-only controls during the recruitment phase.
DMOG treatment decreases neutrophil apoptosis and retains neutrophils at the inflammatory site
DMOG treatment increased the number of neutrophils at the site of tail transection at 24 hpi, but the mechanisms behind this delay in resolution remained unknown. Neutrophils were never seen to leave the zebrafish body through the wound margin or elsewhere, and it is reasonable to conclude that loss of neutrophils from the larva is not important in this model. We therefore assessed the effects of DMOG both on levels of neutrophil apoptosis at the transection site, and on migration of neutrophils away from it.
Neutrophil apoptosis was quantified using TUNEL staining to identify apoptotic cells, in combination with a fluorescent histochemical stain to identify neutrophils, as reported previously.28,29,38 Postmortem TSA staining was used in preference to GFP imaging, because we have previously shown GFP fluorescence is lost during neutrophil apoptosis.28 In DMOG-treated larvae, there was a significant decrease in apoptotic neutrophils at the transection site compared with that of DMSO-treated larvae at 3 dpf (Figure 1K). The rates of apoptosis seen are comparable to those in other studies of zebrafish neutrophil apoptosis,28 and to published rates of detectable apoptosis in other models.39 Because levels of detectable neutrophil apoptosis are low, these events are scarce in fixed embryos at a single time point, presumably reflecting the short life span of an apoptotic neutrophil in vivo. Therefore, this experimental result was confirmed at 10 dpf where numbers of neutrophils are higher using both TUNEL and anti–active caspase-3 staining (supplemental Figure 3A-B).
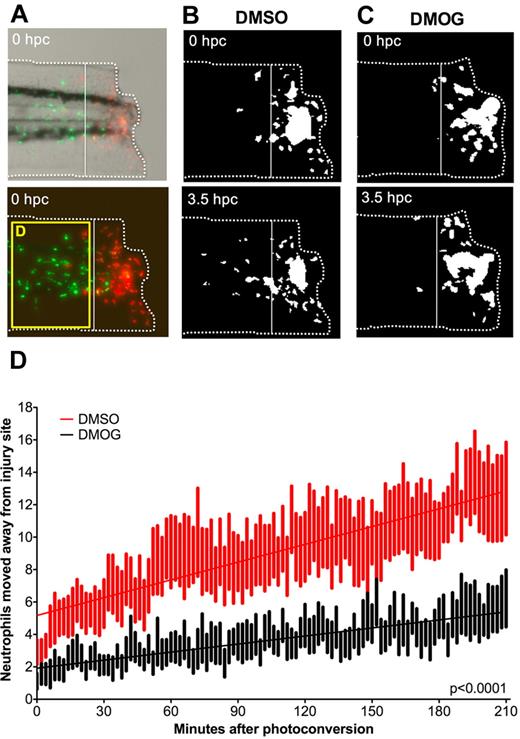
To investigate the migration of neutrophils away from the transection site, lyz:Kaede transgenic fish were used. lyz:Kaede fish use the lysozyme C promoter to drive GAL4, which drives expression of Kaede, a photoconvertible protein that fluoresces in the red channel after exposure to 405-nm excitation.40 This allows individual cells, or cells within a certain area, to be converted from green to red fluorescence and tracked over time. Tailfin transection was performed in lyz:Kaede transgenic zebrafish at 3 dpf, and at 6 hpi the neutrophils present at the transection site were photoconverted to red fluorescence and tracked over the next 3.5 hours (Figure 2A). Embryos treated with DMOG had significantly fewer red neutrophils moving away from the injury site than DMSO controls (Figure 2B-D). Not only were more neutrophils in DMSO–treated embryos moving away from the injury site, but they were moving further away than those treated with DMOG (supplemental Figure 4A).
DMOG retains neutrophils in the region of tissue injury. (A) Photomicrographs of a 3-dpf photoconverted, DMSO-treated, lyz:Kaede embryo at 0 hours postconversion (hpc). The white dashed line indicates the border of the site of transection area. The cells to the right of the line were photoconverted, leaving them with red fluorescence rather than green. The bottom fluorescent panel shows the area of interest where neutrophils migrated away from the injury site shown in a subsequent figure (D). (B) Photomicrographs of the same embryo as in panel A at 0 and 3.5 hpc. The red channel only is shown, as a binary image. Between 0 and 3.5 hpc, photoconverted cells have migrated away from the site of transection. (C) Photomicrographs of a DMOG-treated embryo at 0 and 3.5 hpc converted into black and white. Fewer photoconverted leukocytes have migrated away from the site of transection. (D) Plot showing the number of photoconverted leukocytes leaving the area of transection over 3.5 hpc in DMSO- and DMOG-treated embryos. Data shown are mean ± SEM, n = 9 performed as 2 independent experiments. Line of best fit shown is calculated by linear regression. P value shown is for the difference between the 2 slopes.
DMOG retains neutrophils in the region of tissue injury. (A) Photomicrographs of a 3-dpf photoconverted, DMSO-treated, lyz:Kaede embryo at 0 hours postconversion (hpc). The white dashed line indicates the border of the site of transection area. The cells to the right of the line were photoconverted, leaving them with red fluorescence rather than green. The bottom fluorescent panel shows the area of interest where neutrophils migrated away from the injury site shown in a subsequent figure (D). (B) Photomicrographs of the same embryo as in panel A at 0 and 3.5 hpc. The red channel only is shown, as a binary image. Between 0 and 3.5 hpc, photoconverted cells have migrated away from the site of transection. (C) Photomicrographs of a DMOG-treated embryo at 0 and 3.5 hpc converted into black and white. Fewer photoconverted leukocytes have migrated away from the site of transection. (D) Plot showing the number of photoconverted leukocytes leaving the area of transection over 3.5 hpc in DMSO- and DMOG-treated embryos. Data shown are mean ± SEM, n = 9 performed as 2 independent experiments. Line of best fit shown is calculated by linear regression. P value shown is for the difference between the 2 slopes.
To further investigate neutrophil movement at the injury site after DMOG treatment, a small area of between 5 and 10 neutrophils located at the dorsal side of the midline near the wound was photoconverted at 6 hpi (Figure 3A). It was observed that DMSO-treated neutrophils tended to migrate away from the injury site, whereas DMOG-treated neutrophils favored migration along the injury edge (Figure 3B). This was independent of measurements of speed and meandering, which remained unchanged after treatment (Figure 3C). Over 3.5 hours of tracking, neutrophils migrated out of the photoconverted region at the same rate in both DMSO- and DMOG-treated individuals (Figure 3D). Fewer neutrophils migrate away from the injury site in DMOG-treated individuals than in DMSO controls (Figure 3E), whereas more neutrophils in DMOG-treated embryos moved down the injury site ventral of the photoconverted region (Figure 3F). This is consistent with DMOG-treated neutrophils patrolling the injury site, rather than moving away.
DMOG treatment causes a change in direction of neutrophil movement independent of neutrophil speed and meandering. (A) Photomicrographs of a 3-dpf photoconverted, DMSO-treated, lyz:Kaede embryo at 0 hpc. The white box indicates the area of photoconverted neutrophils. The bottom panel shows the regions to which neutrophils migrated in subsequent panels (D-F). (B) Photomicrographs of examples of DMSO (top panel)– and DMOG (bottom panel)–treated embryos with the tracks of neutrophils over the first 3.5 hpc shown. The dashed line indicates the outline of the embryo. (C) Tracking of neutrophils over the first 3.5 hpc in the resolution phase of inflammation showed no difference in the speed of neutrophil migration (top panel) or meandering index (displacement/path length; bottom panel) in DMOG-treated larvae compared with DMSO controls. (D) Leukocytes leave the photoconversion site at the same rate in DMOS- and DMOG-treated embryos over 3.5 hpc. (E) Number of photoconverted leukocytes moving away from the wound is shown over 3.5 hpc in DMSO- and DMOG-treated embryos. (F) More neutrophils remain and patrol the injury site in DMOG-treated embryos, because a greater number of leukocytes move within the injury region, ventrally away from the patch area. (D-F) Data shown are mean ± SEM, n = 14 performed as 3 independent experiments. Line of best fit shown is calculated by linear regression. P values shown are the differences between the 2 slopes.
DMOG treatment causes a change in direction of neutrophil movement independent of neutrophil speed and meandering. (A) Photomicrographs of a 3-dpf photoconverted, DMSO-treated, lyz:Kaede embryo at 0 hpc. The white box indicates the area of photoconverted neutrophils. The bottom panel shows the regions to which neutrophils migrated in subsequent panels (D-F). (B) Photomicrographs of examples of DMSO (top panel)– and DMOG (bottom panel)–treated embryos with the tracks of neutrophils over the first 3.5 hpc shown. The dashed line indicates the outline of the embryo. (C) Tracking of neutrophils over the first 3.5 hpc in the resolution phase of inflammation showed no difference in the speed of neutrophil migration (top panel) or meandering index (displacement/path length; bottom panel) in DMOG-treated larvae compared with DMSO controls. (D) Leukocytes leave the photoconversion site at the same rate in DMOS- and DMOG-treated embryos over 3.5 hpc. (E) Number of photoconverted leukocytes moving away from the wound is shown over 3.5 hpc in DMSO- and DMOG-treated embryos. (F) More neutrophils remain and patrol the injury site in DMOG-treated embryos, because a greater number of leukocytes move within the injury region, ventrally away from the patch area. (D-F) Data shown are mean ± SEM, n = 14 performed as 3 independent experiments. Line of best fit shown is calculated by linear regression. P values shown are the differences between the 2 slopes.
DMOG-induced delay in resolution is blocked by arnt-1 morpholino and by dominant-negative hif-1αb
Given the potential for nonspecific effects of competitive hydroxylase inhibition by DMOG and the lack of selective inhibitors of individual PHD enzymes, we sought to investigate the HIF-1α dependence of hydroxylase inhibition. ARNT-1 (or HIF-1β) is a crucial binding partner of HIF-1α, and arnt-1 morpholino31 will block Hif signaling. In the morpholino experiments, tail transection was performed at 2 dpf, to increase the likelihood that the morpholino would remain active during the experiment. The 2-dpf tail transection model retains the same features as the 3-dpf model used in other studies,26,28,41 and was used for all morpholino and RNA injection experiments described here. In control morpholino–injected larvae, DMOG increased neutrophil numbers at 24 hpi. Treatment with DMOG after arnt-1 morpholino injection, however, caused no significant increase in neutrophil numbers at 24 hpi (Figure 4A), supporting the Hif dependence of the DMOG effect.
DMOG-induced delay in inflammation resolution is blocked by genetic inhibition of the Hif-1α pathway. (A) The 24 hpi neutrophil counts in the mpx:GFP line at 2 dpf after injection with control and arnt-1 morpholinos. DMSO and DMOG treatment was performed at 4 hpi. Data shown are mean ± SEM, n = 12 performed as 2 independent experiments. (B-E) We injected 177 pg of dominant-negative hif-1α RNA into the 1-cell-stage zebrafish mpx:GFP embryos, and then neutrophil counts were performed at 24 hpi after tail transection at 2 dpf. (B) Injection of dominant-negative hif-1αb abrogated the increase in neutrophil number at the site of injury at 24 hpi seen with DMOG treatment, whereas embryos injected with phenol red as a negative control, or dominant-negative hif-1αa alone exhibited a significant increase in neutrophil number after DMOG treatment. Data shown are mean ± SEM, n = 24 performed as 2 independent experiments. P values were calculated using 1-way ANOVA and Bonferroni multiple comparison test, where P < .05, **P < .01, and ***P < .001. (C) Injection of dominant-negative hif-1α variants led to no significant difference in total neutrophil number at 2 pf compared with phenol red–injected negative control embryos. Data shown are mean ± SEM, n = 36 performed as 3 independent experiments. (D) Injection of dominant-negative hif-1α caused no significant change in percentage of neutrophils at the injury site colabeling with TUNEL stain (12 hpi injured at 2 dpf). Data shown are mean± SEM, n = 4 performed as independent experiments containing 5 to 25 embryos/injection group/repeat. (E-F) Dominant-negative hif-1α can block the increased neutrophil retention at the site of injury caused by DMOG treatment. Embryos were injected with dominant-negative hif-1αb, grown to 3 dpf, and imaged 3.5 hours after photoconversion, with only the red channel shown, as a binary image. (E, left panels) In DMSO-treated larvae, red-labeled neutrophils have migrated away from the site of transection. (E, right panel) In DMOG-treated larvae, red-labeled neutrophils also move away from the site of transection. (F) The number of red (photoconverted) lysozyme C–labeled cells leaving the area of transection at 6 hpi over a time period of 3.5 hpc in DMSO- and DMOG-treated embryos at 2 dpf that were injected at the 1-cell stage with dominant-negative hif-1αb. Data shown are mean ± SEM, n = 14 performed as 3 independent experiments. Line of best fit shown is calculated by linear regression. P value shown is the difference between the 2 slopes.
DMOG-induced delay in inflammation resolution is blocked by genetic inhibition of the Hif-1α pathway. (A) The 24 hpi neutrophil counts in the mpx:GFP line at 2 dpf after injection with control and arnt-1 morpholinos. DMSO and DMOG treatment was performed at 4 hpi. Data shown are mean ± SEM, n = 12 performed as 2 independent experiments. (B-E) We injected 177 pg of dominant-negative hif-1α RNA into the 1-cell-stage zebrafish mpx:GFP embryos, and then neutrophil counts were performed at 24 hpi after tail transection at 2 dpf. (B) Injection of dominant-negative hif-1αb abrogated the increase in neutrophil number at the site of injury at 24 hpi seen with DMOG treatment, whereas embryos injected with phenol red as a negative control, or dominant-negative hif-1αa alone exhibited a significant increase in neutrophil number after DMOG treatment. Data shown are mean ± SEM, n = 24 performed as 2 independent experiments. P values were calculated using 1-way ANOVA and Bonferroni multiple comparison test, where P < .05, **P < .01, and ***P < .001. (C) Injection of dominant-negative hif-1α variants led to no significant difference in total neutrophil number at 2 pf compared with phenol red–injected negative control embryos. Data shown are mean ± SEM, n = 36 performed as 3 independent experiments. (D) Injection of dominant-negative hif-1α caused no significant change in percentage of neutrophils at the injury site colabeling with TUNEL stain (12 hpi injured at 2 dpf). Data shown are mean± SEM, n = 4 performed as independent experiments containing 5 to 25 embryos/injection group/repeat. (E-F) Dominant-negative hif-1α can block the increased neutrophil retention at the site of injury caused by DMOG treatment. Embryos were injected with dominant-negative hif-1αb, grown to 3 dpf, and imaged 3.5 hours after photoconversion, with only the red channel shown, as a binary image. (E, left panels) In DMSO-treated larvae, red-labeled neutrophils have migrated away from the site of transection. (E, right panel) In DMOG-treated larvae, red-labeled neutrophils also move away from the site of transection. (F) The number of red (photoconverted) lysozyme C–labeled cells leaving the area of transection at 6 hpi over a time period of 3.5 hpc in DMSO- and DMOG-treated embryos at 2 dpf that were injected at the 1-cell stage with dominant-negative hif-1αb. Data shown are mean ± SEM, n = 14 performed as 3 independent experiments. Line of best fit shown is calculated by linear regression. P value shown is the difference between the 2 slopes.
A truncated form of human HIF-1α has been shown previously to block HIF-1α signaling in cell culture.32 We generated analogous constructs for the zebrafish homologs, hif-1αa and hif-1αb. These were expressed in all cells of the embryo by RNA injection at the 1-cell stage. We showed dominant-negative hif-1α was able to block activation of the HIF pathway by DMOG as late as 56 hpf, indicated by a decrease in phd-3 expression (supplemental Figure 5).42 Dominant-negative hif-1α abrogated the DMOG-induced increase in neutrophil numbers at the transection site at 24 hpi (Figure 4B), with no effect on wholebody neutrophil numbers (Figure 4C). Dominant-negative hif-1αb alone was sufficient to block the DMOG delay in resolution of neutrophilic inflammation (Figure 4B), with hif-1αa having no effect on inflammation (Figure 4B).
TUNEL staining showed that there was no significant difference in levels of neutrophil apoptosis in embryos injected with dominant-negative hif-1α (Figure 4D).
To investigate the effect on neutrophil retention at the inflammatory site, lyz:Kaede embryos were injected with dominant-negative hif-1αb RNA and neutrophils at the inflammatory site were photoconverted. Neutrophil movement away from the wound was investigated at 6 hpi after tail transection at 2 dpf. In phenol red–injected negative control embryos, DMOG treatment caused fewer neutrophils to leave the site of transection (supplemental Figure 4B), as was observed at 3 dpf. However, in embryos injected with dominant-negative hif-1αb, DMOG-treated embryos had similar numbers of neutrophils migrating away from the site of transection as the DMSO-treated individuals (Figure 4E-F). The neutrophils that did move away moved similar distances away from the injury site in treatment groups (supplemental Figure 4C). Therefore, dominant-negative hif-1α is able to negate the DMOG-induced delay in resolution partially by restoring the normal pattern of neutrophil movement away from the wound edge.
Dominant-active hif-1αb delays resolution of neutrophilic inflammation
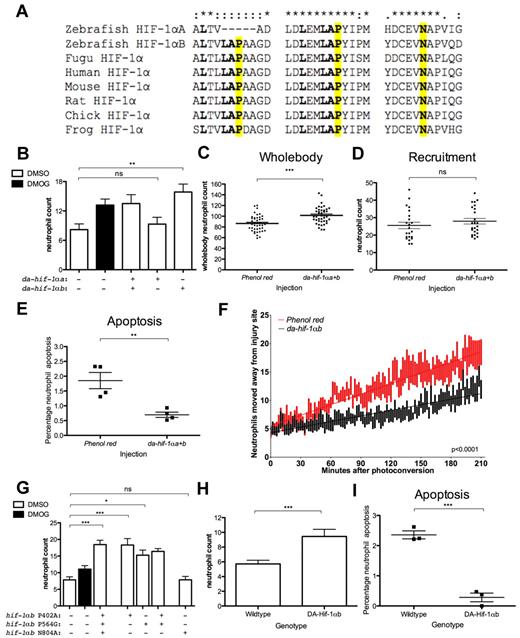
Dominant-active forms of Hif-1αa and Hif-1αb were generated by mutation of conserved proline hydroxylation sites for Phd and the asparagine hydroxylation site for Fih (Figure 5A). In mammalian studies, these mutations render HIF-1α resistant to hydroxylation by oxygen-sensitive hydroxylases, leading to stabilization of HIF-1α.20,33 In zebrafish larvae expressing dominant-active hif-1α by RNA injection, Hif signaling was up-regulated, shown by increased phd-3 expression (supplemental Figure 5). In addition, an increase in neutrophil number at 24 hpi was observed in dominant-active hif-1α/DMSO-treated embryos, with neutrophil numbers comparable to DMOG-treated/mock-injected siblings (Figure 5B). As with dominant-negative hif-1α, dominant-active hif-1αb alone recapitulated the effects of DMOG on 24 hpi neutrophil numbers present at the transection site (Figure 5B). Neither dominant-active nor -negative hif-1αa had any effect in these assays. In situ hybridization expression of wild-type hif-1αa and hif-1αb showed similar expression patterns, although in general hif-1αa seemed to be expressed at a lower level than hif-1αb (supplemental Figure 6).
Dominant-active hif-1αb delays resolution of neutrophilic inflammation. (A) Partial protein alignment showing the conserved hydroxylation sites of Hif-1α. Dominant-active hif-1α has the following nonhydroxylatable mutations: Hif-1αb P402A, P564G, N804A and Hif-1αa P493G, N678A (Hif-1αa lacks the first proline hydroxylation residue). (B-F) Dominant-active forms of hif-1α RNA (177 pg) were injected into the 1-cell-stage zebrafish mpx:GFP embryos, tailfin transection was performed at 2 dpf, and neutrophils counted at 24 hpi. (B) Dominant-active hif-1α caused a significant increase in neutrophil number in the absence of DMOG treatment compared with phenol red–injected negative controls. Dominant-active hif-1αb alone was able to recapitulate the DMOG phenotype, whereas dominant-active hif-1αa homolog did not. Data shown are mean ± SEM, n = 24 performed as 2 independent experiments. P values were calculated using 1-way ANOVA and Bonferroni multiple comparison test, where P < .05, **P < .01, and ***P < .001. (C) Injection of dominant-active hif-1α variants led to a significant increase in total neutrophil numbers at 2 dpf. Data shown are mean ± SEM, n = 36 performed as 3 independent experiments. (D) Injection of dominant-active hif-1α variants did not alter the recruitment of neutrophils to the injury site after 6 hpi when the tail was transected at 2 dpf. Data shown are mean ± SEM, n = 24 performed as 2 independent experiments. P values were calculated using 1-way ANOVA and Bonferroni multiple comparison test. (E) Injection of dominant-active hif-1α led to a significant decrease in percentage of neutrophils at the injury site colabeled with TUNEL apoptosis staining 12 hpi when injured at 2 dpf. Data shown are mean ± SEM, n = 4 performed as independent experiments containing 10 to 25 embryos/injection group/repeat. (F) Number of red (photoconverted) lysosyme C–labeled cells leaving the area of transection at 6 hpi over a time period of 3.5 hpc in phenol red– and dominant-active hif-1α–injected embryos at 2 dpf. Data shown are mean ± SEM, n = 14 performed as 3 independent experiments. Line of best fit shown is calculated by linear regression. P value shown is the difference between the 2 slopes. (G) Injection of hif-1αb with each hydroxylation site mutated individually shows that the asparagine hydroxylation site of Fih is not required for resolution of neutrophilic inflammation. Mutation of either proline hydroxylation site of Hif-1αb was sufficient to lead to the decrease in neutrophil inflammation resolution. Data shown are mean ± SEM, n = 24 performed as 2 independent experiments. (H) Tg(lyz:Gal4)i252;Tg(UAS:da-hif-1αb-IRES-GFP)i218 positive embryos and wild-type siblings underwent tailfin transection at 3 dpf. Data shown are 24 hpi TSA-positive neutrophil counts, mean ± SEM, n = 30 performed as 3 independent experiments. (I) Tg(lyz:Gal4)i252;Tg(UAS:da-hif-1αb-IRES-GFP)i218 positive embryos had a lower rate of neutrophil apoptosis analyzed by TUNEL staining at 12 hpi after tail transection at 3 dpf. Data shown are mean ± SEM, n = 3 performed as independent experiments containing 9 to 40 embryos/treatment group/repeat.
Dominant-active hif-1αb delays resolution of neutrophilic inflammation. (A) Partial protein alignment showing the conserved hydroxylation sites of Hif-1α. Dominant-active hif-1α has the following nonhydroxylatable mutations: Hif-1αb P402A, P564G, N804A and Hif-1αa P493G, N678A (Hif-1αa lacks the first proline hydroxylation residue). (B-F) Dominant-active forms of hif-1α RNA (177 pg) were injected into the 1-cell-stage zebrafish mpx:GFP embryos, tailfin transection was performed at 2 dpf, and neutrophils counted at 24 hpi. (B) Dominant-active hif-1α caused a significant increase in neutrophil number in the absence of DMOG treatment compared with phenol red–injected negative controls. Dominant-active hif-1αb alone was able to recapitulate the DMOG phenotype, whereas dominant-active hif-1αa homolog did not. Data shown are mean ± SEM, n = 24 performed as 2 independent experiments. P values were calculated using 1-way ANOVA and Bonferroni multiple comparison test, where P < .05, **P < .01, and ***P < .001. (C) Injection of dominant-active hif-1α variants led to a significant increase in total neutrophil numbers at 2 dpf. Data shown are mean ± SEM, n = 36 performed as 3 independent experiments. (D) Injection of dominant-active hif-1α variants did not alter the recruitment of neutrophils to the injury site after 6 hpi when the tail was transected at 2 dpf. Data shown are mean ± SEM, n = 24 performed as 2 independent experiments. P values were calculated using 1-way ANOVA and Bonferroni multiple comparison test. (E) Injection of dominant-active hif-1α led to a significant decrease in percentage of neutrophils at the injury site colabeled with TUNEL apoptosis staining 12 hpi when injured at 2 dpf. Data shown are mean ± SEM, n = 4 performed as independent experiments containing 10 to 25 embryos/injection group/repeat. (F) Number of red (photoconverted) lysosyme C–labeled cells leaving the area of transection at 6 hpi over a time period of 3.5 hpc in phenol red– and dominant-active hif-1α–injected embryos at 2 dpf. Data shown are mean ± SEM, n = 14 performed as 3 independent experiments. Line of best fit shown is calculated by linear regression. P value shown is the difference between the 2 slopes. (G) Injection of hif-1αb with each hydroxylation site mutated individually shows that the asparagine hydroxylation site of Fih is not required for resolution of neutrophilic inflammation. Mutation of either proline hydroxylation site of Hif-1αb was sufficient to lead to the decrease in neutrophil inflammation resolution. Data shown are mean ± SEM, n = 24 performed as 2 independent experiments. (H) Tg(lyz:Gal4)i252;Tg(UAS:da-hif-1αb-IRES-GFP)i218 positive embryos and wild-type siblings underwent tailfin transection at 3 dpf. Data shown are 24 hpi TSA-positive neutrophil counts, mean ± SEM, n = 30 performed as 3 independent experiments. (I) Tg(lyz:Gal4)i252;Tg(UAS:da-hif-1αb-IRES-GFP)i218 positive embryos had a lower rate of neutrophil apoptosis analyzed by TUNEL staining at 12 hpi after tail transection at 3 dpf. Data shown are mean ± SEM, n = 3 performed as independent experiments containing 9 to 40 embryos/treatment group/repeat.
Dominant-active hif-1α injection was associated with increased wholebody neutrophil numbers at 2 dpf (Figure 5C), suggesting activation of Hif signaling might regulate total neutrophil numbers in vivo. Because HIF is activated by infection, this effect might contribute to the increase in neutrophils seen during infections; however, this increase in wholebody neutrophil number did not cause an increase in neutrophil recruitment to the injury site at 6 hpi (Figure 5D). Like DMOG, dominant-negative and dominant-active forms of hif-1α caused no significant changes in neutrophil speed or meandering index during the recruitment phase (supplemental Figure 2C-D).
TUNEL staining showed that there was a significant decrease in levels of neutrophil apoptosis in embryos injected with dominant-active hif-1α (Figure 5E), similar to that observed in DMOG (Figure 1K). Neutrophil migration away from the injury site was assessed as with lyz:Kaede. Fewer neutrophils move out of the injury site in dominant-active hif-1α–injected embryos compared with phenol red–injected controls (Figure 5F).
Each hydroxylation site of Hif-1αb was mutated individually, and RNA was injected into mpx:GFP larvae to assess their effects on neutrophil retention at 24 hpi. Mutation of either of the 2 Phd target proline residues was sufficient to significantly increase the neutrophil numbers at the site of transection at 24 hpi to the same or greater level as DMOG treatment (Figure 5G). Mutation of the Fih target asparagine had no effect on neutrophil numbers at 24 hpi (Figure 5G).
To confirm the effects of Hif activation act within the neutrophil itself, a stable transgenic line expressing dominant-active hif-1αb under the upstream activating sequence (UAS) promoter was generated (UAS:da-hif-1αb). This line was shown to express active Hif when driven by an injected cmv:GAL4 construct, demonstrated by increased phd-3 expression (supplemental Figure 7). Dominant-active hif-1αb was expressed in neutrophils by crossing UAS:da-hif-1αb with the lyz:Gal4 line described above. After tail transection at 3 dpf, neutrophils were counted at 24 hpi using fluorescent TSA. Neutrophil numbers at 24 hpi are elevated in embryos with the heart marker (ie, da-hif-1α positive) compared with heart marker negative siblings (Figure 5H). As with dominant-active hif-1α RNA experiments, levels of apoptosis are significantly reduced in da-hif-1α positive transgenics compared with controls (Figure 5I).
Discussion
In this study, we investigated the role of the HIF pathway in modulating neutrophilic inflammation in vivo. Although several in vitro studies have shown effects on neutrophil function and life span,14-16,19 there has been no previous study of the effects of HIF signaling on neutrophil behavior during inflammation in vivo. We found recruitment of neutrophils to the injury site was unaffected by hydroxylase inhibition, and in addition, the pattern of neutrophil localization was unaltered in uninjured embryos. Neutrophil migration properties including speed and meandering of neutrophils during the recruitment phase also were not affected. DMOG did, however, cause a delay in the resolution of inflammation in association with an increase in HIF signaling. HIF signaling pathways therefore modulate the resolution rather than recruitment phase of inflammation. The number of neutrophils at the transection site in DMOG-treated embryos had reached basal levels by 48 hpi, suggesting that the abrogation of hydroxylase activity delays rather than prevents the resolution of inflammation.
There are 2 plausible explanations for the decrease in inflammation resolution observed in DMOG-treated zebrafish embryos: neutrophils are actively retained at the site of injury or neutrophil apoptosis is suppressed. Movement of neutrophils away from sites of injury and infection, by reverse migration, has been reported several zebrafish studies.8-10 A similar phenomenon has been reported in some mammalian systems, although the underlying mechanisms are less well understood.7 Cell tracking experiments were performed to assess movement of neutrophils away from the wound region. In DMOG-treated embryos, fewer labeled neutrophils left the site of transection over a 3.5-hour period during the resolution phase of inflammation, and neutrophils traveled a shorter distance away from the wound zone. These effects of DMOG were blocked by dominant-negative and replicated by dominant-active Hif constructs, indicating Hif signaling retained neutrophils in the wound zone during the resolution phase. By labeling a small population of cells at the injury site, we were able to show that the same number of neutrophils remains in the labeled region in DMOG-treated embryos in the resolution phase and that neutrophils migrate with the same speed and meandering index. The difference between these populations is purely a difference in the direction of the migrating neutrophils. Blocking hydroxylases led to more neutrophils patroling the injury site rather than moving away from the injury site. This change in migration direction may reflect a differential response of neutrophils to existing chemotactic gradients, perhaps as a result of altered chemokine receptor expression.43 It is unlikely that global activation of Hif signaling causes a change in the chemical gradients at the injury site, because neutrophil-specific expression of dominant Hif-1α led to persisting inflammation. The decrease in reverse migration after Hif activation is probably responsible, in part, for the decrease in the resolution of neutrophilic inflammation. To our knowledge, this is the first report of the modulation of a genetic pathway directly altering the migratory behavior of neutrophils specifically during inflammation resolution. Whether this represents a global effect of HIF signaling on inflammation resolution, or whether it is specific to zebrafish, will require careful experimentation in emerging models of in vivo inflammation in mammalian systems.
In addition, DMOG treatment caused a modest but significant decrease in the levels of neutrophil apoptosis in the resolution phase of inflammation. This is also likely to contribute to the delay in resolution observed after hydroxylase inhibition. This effect was shown to be Hif-dependent because dominant-active Hif down-regulated apoptosis. Expressing dominant-active Hif specifically in neutrophils was able to decrease neutrophil apoptosis showing this effect is mediated by the neutrophils themselves. Up-regulated hypoxia has been demonstrated previously to decrease neutrophil apoptosis in mammalian systems14-16,19 ; however, this is the first time this effect has been observed in vivo in a whole organism. Dominant-negative Hif expression did not detectably increase apoptosis levels compared with controls. This probably indicates that levels of Hif are low during the normal process of inflammation resolution; therefore, further inhibition of this pathway does not significantly affect neutrophil apoptosis.
It is difficult to assess the relative contribution of apoptosis versus reverse migration to the clearance of neutrophils after Hif activation, because the number of apoptotic cells identified depends on unknown variables, such as the duration for which an apoptotic neutrophil can be detected before it is degraded after macrophage ingestion. However, these data suggest both apoptosis and reverse migration probably play an important role in the clearance of neutrophils.
Given the potential for nonspecific effects of competitive hydroxylase inhibition by DMOG, and the lack of selective inhibitors of individual PHD enzymes, there were multiple possible modes of action of DMOG in vivo. DMOG has targets other than the HIF hydroxylases and PHD enzymes themselves have HIF-independent effects, including on the nuclear factor-κB pathway.44 By manipulating gene expression, we have been able to establish a direct link between the effects of hydroxylase inhibition and Hif-1α. Previous reports have shown that an arnt-1 translation blocking morpholino is able to block Hif-1α signaling.31 We found that morpholino mediated knockdown of Arnt-1, an obligate binding partner of Hif-1α, is able to block the delay in inflammation resolution caused by DMOG. In addition, we found that the proinflammatory effects of DMOG were replicated by dominant-active hif-1αb and blocked by dominant-negative hif-1αb. Dominant-active hif-1αb delayed the resolution of inflammation to a similar degree to that of DMOG treatment alone. Interestingly, dominant-active hif-1αb also increased the whole-body neutrophil number. This might be a feature of suppression of neutrophil apoptosis during hematopoiesis, or of an increase in myeloid cell production after hypoxic stimulation. This effect is not seen with either DMOG or hypoxia itself (supplemental Figure 1B; data not shown), but prolonged exposure of developing larvae to DMOG or to direct hypoxia is toxic to embryos (data not shown). This agrees with the findings in vhl mutants, in which increased Hif signaling leads to increased leukocyte number.42 By developing a stable UAS:da-hif-1αb transgenic line, we were able to drive the expression of da-hif-1αb specifically in lyz-expressing leukocytes. We were able to demonstrate that overactivation of the Hif-1α pathway in neutrophils alone was able to induce the delay in the resolution of neutrophilic inflammation observed when Hif-1α was up-regulated across all tissues of the embryo.
The 2 zebrafish homologs of human HIF-1α had different effects on neutrophilic inflammation. Hif-1αb was able to modulate the resolution of neutrophilic inflammation: dominant-active Hif-1αb was able to replicate the effect of hydroxylase inhibition, whereas dominant-negative Hif-1αb almost completely blocked the DMOG effect. This is consistent with Hif-1αb being the hypoxia transcription factor responsible for the timely resolution of neutrophilic inflammation. Hif-1αa, by contrast, had no significant effect on neutrophil behavior in the assays we performed. Interestingly, Hif-1αa has less sequence homology to human of HIF-1α than Hif-1αb.45 In particular, Hif-1αa lacks the first conserved proline site for Phd hydroxylation, although it does have the second conserved proline site, and the Fih asparagine. The loss of complete Phd hydroxylation in Hif-1αa may indicate a divergence of the role of Hif-1αa in evolution away from a role in transducing the hypoxic signal in inflammatory cells.
To assess the role of hydroxylation at different sites in Hif-1α, we assayed inflammation resolution in zebrafish larvae injected with a range of dominant Hif-1αb constructs, in which Phd and Fih hydroxylation sites were mutated alone and in combination. Mutation of either Phd hydroxylation site reproduced the delay of inflammation resolution phenotype, consistent with a stabilization of Hif, suggesting that either site may function independently, in keeping with the recent in vitro crystal structure of hydroxyproline recognition by pVHL.46 These data are consistent with Phd hydroxylation of Hif-1αb being required for the timely resolution of neutrophilic inflammation. Although in vitro FIH/HIF interaction has been proposed to partially determine oxygen sensitivity, mutation of the Fih hydroxylation site, either alone or in combination with mutation of the Phd sites, had no detectable effect on inflammation resolution. This suggests that in zebrafish neutrophils, Phd enzymes are the critical regulators of Hif activity.
Our data show that Hif-1α delays the resolution of inflammation in a whole-organism in vivo model. We show for the first time that this delay in resolution is caused by suppression of the movement of neutrophils away from the site of inflammation in favor of patrolling the inflamed area, and by a decrease in neutrophil apoptosis. Furthermore, we find that hydroxylation of Hif-1αb by Phd enzymes is required for the timely resolution of neutrophilic inflammation. By developing the stable UAS:da-hif-1αb transgenic line, we are able to show that this depends on Hif signaling within the neutrophil. These data have important implications in understanding the role of HIF-hydroxylase signaling in innate immune responses and, in addition, provide an illustration of how zebrafish models might elucidate key regulators of inflammatory signaling pathways with relevance to human health.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Catherine Loynes for assistance in establishing the techniques used in this study.
This work was funded by a project grant from the Wellcome Trust (WT082909MA), an MRC Senior Clinical Fellowship (G0701932; S.A.R.) and by A*STAR (Singapore). S.R.W. holds a Wellcome Intermediate Fellowship (WT078244AIA). Microscopy studies were supported by a Wellcome Trust grant to the Molecular Biology and Biotechnology/Biomedical Science Light Microscopy Facility (GR077544AIA), and the work was supported by an MRC Center grant (G0700091).
Wellcome Trust
Authorship
Contribution: F.J.v.E., S.R.W., M.K.B.W., and S.A.R. conceived the study and designed the experiments with P.M.E.; P.W.I. and X.W. generated new transgenic reagents; P.M.E., F.J.v.E., P.W.I., M.K.B.W., S.R.W., and S.A.R. wrote the manuscript; C.C.R.-A. assisted and advised on image processing; and P.M.E. and G.D. performed the experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for C.C.R.-A. is Biomedical Engineering Research Group, School of Engineering and Design, University of Sussex, Brighton, United Kingdom.
Correspondence: Stephen A. Renshaw, MRC Centre for Developmental and Biomedical Genetics, University of Sheffield, Western Bank, Sheffield, S10 2TN, United Kingdom; e-mail: s.a.renshaw@sheffield.ac.uk.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal