Abstract
In this age of promise of new therapies for cancer, immunotherapy is emerging as an exciting treatment option for patients. Vaccines and cytokines are being tested extensively in clinical trials, and strategies using monoclonal antibodies and cell transfer are mediating dramatic regression of tumors in patients with certain malignancies. However, although initially advocated as being more specific for cancer and having fewer side effects than conventional therapies, it is becoming increasingly clear that many immunotherapies can lead to immune reactions against normal tissues. Immunotoxicities resulting from treatment can range from relatively minor conditions, such as skin depigmentation, to severe toxicities against crucial organ systems, such as liver, bowel, and lung. Treatment-related toxicity has correlated with better responses in some cases, and it is probable that serious adverse events from immune-mediated reactions will increase in frequency and severity as immunotherapeutic approaches become more effective. This review introduces immunotherapeutic approaches to cancer treatment, provides details of toxicities arising from therapy, and discusses future potential ways to avoid or circumvent these side effects.
Introduction
Immunotherapy holds much promise for the treatment of cancer. The immune system is capable of dramatic and decisive responses against infectious disease, which is accomplished with exquisite specificity against antigen. Components of immunity are seen as potentially more specific weapons to direct against tumors than chemotherapy or radiation. With our expanding knowledge of tumor-associated antigens (TAAs), there are many different approaches being developed to direct immunity against transformed cells.
Immunotherapies may involve the active generation of immunity to TAA, via vaccination with peptides or peptide-pulsed dendritic cells.1 In addition, administration of immune modulators, such as cytokines, can boost existing antitumor immunity and target immune effector cells to sites of tumor growth.2 Monoclonal antibodies harness both innate and adaptive immune mechanisms and direct them against tumor cells.3 In addition, the effector functions of cytotoxic T lymphocytes have proven them to be particularly useful in targeting TAA in adoptive immunotherapeutic protocols.4
Some TAAs are tumor specific, whose expression is entirely limited to tumors, examples of which include viral antigens expressed on cells in which viral oncogenes have contributed to cellular transformation. In these cases, immunotherapy can be used with fine specificity and very little toxicity against normal tissues.5 However, most TAAs are expressed by some cells of normal tissues and the potential exists for on-target toxicity against these tissues. These on-target toxicities can be assigned to 2 broad categories. First, they can comprise “true” autoimmunity, involving a fundamental induction of endogenous immunity against self-antigens, and we refer to this type as “autoimmunity.” Second, they can be more “drug-like” in nature, where damage is mediated directly by the immunomodulatory agent, and these toxicities are referred to as “immune-mediated.” Toxicities have been described in a proportion of patients using a range of immunotherapeutic approaches. As immune-based cancer therapies become more potent, it is probable that autoimmune and immune-mediated toxicities will become more severe. Indeed, these toxicities can be associated with better antitumor responses resulting from immunotherapy.
In this review, we introduce various immunotherapeutic approaches and provide details of toxicity to normal tissues resulting from these approaches, as well as describe potential ways to overcome these toxicities.
Autoimmunity associated with adoptive immunotherapy
Adoptive immunotherapy is a very promising approach to treating cancer that involves the isolation of leukocytes and their activation and expansion in vitro followed by infusion into patients. Advantages of this type of approach include the opportunity to manipulate and activate lymphocytes away from the in vivo immunosuppressive environment, and their expansion to vast numbers, thereby circumventing many regulatory checkpoints and delivering “instant” immunity. Adoptive immunotherapy has been demonstrated to induce regression of established tumors, often complete, in both mouse models of disease and in patients.4 However, apart from Epstein-Barr virus–associated malignancies, this form of therapy targets antigens expressed on some normal tissues besides tumor cells, and immune-mediated toxicity has been observed in the treatment of both mice and humans (Table 1; Figure 1).
Immune-mediated toxicities associated with adoptive immunotherapy of cancer
| Cell type . | Species . | Target antigen . | Tumor . | Toxicities . | Reference(s) . |
|---|---|---|---|---|---|
| CTL | Mouse (or rat) | Pnma-1 | Paraneoplastic syndrome (rat) | CNS inflammation | 80 |
| CTL | Recoverin | Fibrosarcoma | Retinal dysfunction | 81 | |
| CTL with IL-2 and vaccine | Various | Melanoma | Melanocyte destruction, ocular toxicity | 8,82 | |
| CD4+ T cells | TRP-1 | Melanoma | Vitiligo | 83,84 | |
| Immune T cells | CEA | Colon | Colitis | 85 | |
| Transgenic T cells | Telomerase | Prostate | Reduction in B cells | 86 | |
| TCR transgenic T cells | Various | None | Pancreatitis, colitis | 11 | |
| CAR-modified T cells | CD19 | Lymphoma | Depletion of normal B cells | 87,88 | |
| CAR-modified T cells | VEGF-R2 | Various | Various organs, probably the result of cytokine-induced hypotension | 89 | |
| TIL and IL-2 | Human | Various | Melanoma | Autoimmune thyroiditis, systemic and ocular autoimmunity | 90,–92 |
| TIL with lymphodepletion and IL-2 | Various | Melanoma | Vitiligo, uveitis | 9,93 | |
| CTL | MART-1 | Melanoma | Melanocyte destruction | 94 | |
| TCR gene–modified T cells | MART-1, gp100 | Melanoma | Melanocyte destruction | 10 | |
| CAR-modified T cells | CAIX | RCC | Liver toxicity (grade 2-4) in 3 of 7 patients; lower grade in 4 of 7 patients with reduced dosing | 14 | |
| CAR-modified T cells | CD19 | Lymphoma | B-cell depletion | 15 | |
| CAR-modified T cells | Her-2 | Colorectal cancer | Lung toxicity | 17 | |
| CAR-modified T cells | CEA | Colorectal cancer | Colitis | 19 |
| Cell type . | Species . | Target antigen . | Tumor . | Toxicities . | Reference(s) . |
|---|---|---|---|---|---|
| CTL | Mouse (or rat) | Pnma-1 | Paraneoplastic syndrome (rat) | CNS inflammation | 80 |
| CTL | Recoverin | Fibrosarcoma | Retinal dysfunction | 81 | |
| CTL with IL-2 and vaccine | Various | Melanoma | Melanocyte destruction, ocular toxicity | 8,82 | |
| CD4+ T cells | TRP-1 | Melanoma | Vitiligo | 83,84 | |
| Immune T cells | CEA | Colon | Colitis | 85 | |
| Transgenic T cells | Telomerase | Prostate | Reduction in B cells | 86 | |
| TCR transgenic T cells | Various | None | Pancreatitis, colitis | 11 | |
| CAR-modified T cells | CD19 | Lymphoma | Depletion of normal B cells | 87,88 | |
| CAR-modified T cells | VEGF-R2 | Various | Various organs, probably the result of cytokine-induced hypotension | 89 | |
| TIL and IL-2 | Human | Various | Melanoma | Autoimmune thyroiditis, systemic and ocular autoimmunity | 90,–92 |
| TIL with lymphodepletion and IL-2 | Various | Melanoma | Vitiligo, uveitis | 9,93 | |
| CTL | MART-1 | Melanoma | Melanocyte destruction | 94 | |
| TCR gene–modified T cells | MART-1, gp100 | Melanoma | Melanocyte destruction | 10 | |
| CAR-modified T cells | CAIX | RCC | Liver toxicity (grade 2-4) in 3 of 7 patients; lower grade in 4 of 7 patients with reduced dosing | 14 | |
| CAR-modified T cells | CD19 | Lymphoma | B-cell depletion | 15 | |
| CAR-modified T cells | Her-2 | Colorectal cancer | Lung toxicity | 17 | |
| CAR-modified T cells | CEA | Colorectal cancer | Colitis | 19 |
CTL indicates cytotoxic T lymphocyte; CNS, central nervous system; IL-2, interleukin-2; and TIL, tumor-infiltrating lymphocytes.
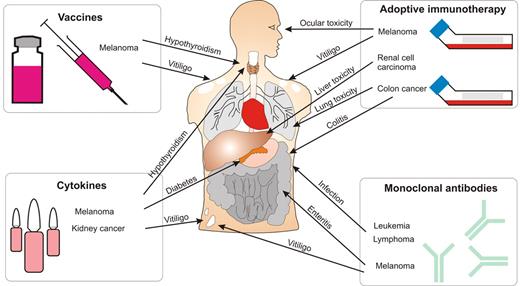
Immune toxicity associated with immunotherapy of cancer. Therapeutic strategies, including vaccines, adoptive immunotherapy, cytokines, and antibodies, can induce immunity against tumor antigens. However, these immune responses can also cause damage to a variety of organ systems as shown.
Immune toxicity associated with immunotherapy of cancer. Therapeutic strategies, including vaccines, adoptive immunotherapy, cytokines, and antibodies, can induce immunity against tumor antigens. However, these immune responses can also cause damage to a variety of organ systems as shown.
Tumor-infiltrating lymphocytes and melanoma
Tumor inhibition has been described using adoptive immunotherapy in various animal models over the past 50 years, but antigen specificity and its expression on normal tissue were largely undefined in earlier models.6,7 More recently, mouse tumor models with known tumor antigens also expressed on self-tissues have become available and effective antitumor responses after adoptive immunotherapy has been observed.8 However, immune-mediated toxicity has been observed in these mouse models targeting normal tissues, including skin, eye, colon, and the B-cell compartment as summarized in Table 1. In the following discussion, we focus on these toxicities following adoptive immunotherapy in humans.
One of the most promising applications of adoptive immunotherapy in the clinic involves the use of tumor-infiltrating lymphocytes to treat melanoma. Lymphocytes derived from tumors can be expanded to yield many billions of cells that are reactive with a range of melanoma antigens, including gp100, MART-1, and tyrosinase.
After demonstrations that prior lymphodepletion could lead to enhanced persistence and activity of transferred T cells, adoptive immunotherapy protocols were modified to include preconditioning regimens to deplete cells of the hematopoietic system. Depletion regimens ranged from nonmyeloablative, using cyclophosphamide and fludarabine, to fully ablative regimens using chemotherapy and whole body irradiation, together with stem cell support. Objective response rates of adoptive immunotherapy reached 70% with these modifications, but immune-mediated toxicities were also observed, with reports of vitiligo and occasional reports of ocular toxicity, involving responses against melanin-containing cells important in retina function.9
In cases where endogenous tumor-infiltrating lymphocytes are difficult to obtain, tumor-specific T cells can be generated from peripheral blood lymphocytes by genetic modification with genes encoding specific T-cell receptor (TCR) α- and β-chains. In a 2009 trial, melanoma patients treated with gene-modified T cells reactive with melanoma/melanocyte antigens experienced destruction of normal melanocytes in the skin (27 of 36 patients), as well as responses against normal cells of the eye and ear, which occurred in approximately 50% of 20 patients receiving T cells modified with a highly reactive TCR.10 These patients were administered steroids to inhibit T-cell activity, which led to resolution of both uveitis and hearing loss.
Interestingly, in addition to the aforementioned on-target immune toxicities, the potential for immune-mediated off-target toxicity using adoptive transfer of TCR gene-modified T cells has been demonstrated in mice. In a study using mouse T cells genetically engineered to express TCR α- and β-chains specific for ovalbumin, severe toxicity was observed, including cachexia, anemia, pancreatitis, and colitis.11 Reactivity against these normal tissues was thought to be the result of mispairing of introduced and endogenous TCR chains leading to T cells with neo-specificities. These findings were also extended to include T cells modified to express other TCRs, including those specific for gp100, simian virus 40 large T antigen, TRP-2, and influenza virus nucleoprotein, although the incidence of lethal toxicity varied depending on the TCR. The propensity of TCR mispairing to induce immune-mediated toxicity in humans has yet to be determined fully, but it has not been observed to date in clinical trial.12
Genetically redirected T cells in adoptive immunotherapy
By far the greatest application of adoptive immunotherapy has been in the melanoma setting as described in “Tumor-infiltrating lymphocytes and melanoma.” This is largely because of the availability of specific T cells, and extension to other common cancers is restricted by a lack of availability of endogenous T cells of appropriate specificity. However, T cells reactive with a range of common cancers can be generated by genetic modification of peripheral blood lymphocytes with chimeric antigen receptors (CARs), whose specificity is derived from monoclonal antibodies specific for cell surface TAA. CAR-modified T cells have been used in clinical trials for a range of cancers, including ovarian cancer, neuroblastoma, colon cancer, and lymphoma.13 The use of CAR-modified T cells is in its infancy, and only limited antitumor effects have been described to date. Nevertheless, in a phase 1 study for renal cell carcinoma (RCC) targeting the TAA carbonic anhydrase IX, adoptive transfer of gene-modified T cells led to grade 3 to 4 liver toxicity in 3 of 7 patients treated. Toxicity was thought to be the result of T cells targeting the CAIX antigen also present on bile ducts.14 Toxicity was resolved in this study after cessation of adoptive T-cell transfer or administration of steroids.
CAR-modified T-cell activity against normal cells was also observed in a clinical study targeting CD19 for the treatment of follicular lymphoma.15 The CAR was composed of a single-chain anti-CD19 antibody linked to CD28 and the ζ-chain of the CD3-TCR complex. In this study, dramatic regression of malignant cells was observed, but a prolonged depletion of normal B cells was also observed, leading to greatly decreased levels of serum immunoglobulin. Although low levels of serum antibody are concerning, administration of exogenous immunoglobulin can correct for this deficiency, thereby providing protection against infection. In another study targeting CD19 with CAR-modified T cells, this time for chronic lymphocytic leukemia, treatment was well tolerated in 3 patients receiving T-cell transfer in the absence of prior lymphodepletion, with only transient fevers experienced. However, a patient receiving T cells after lymphodepletion developed hypotension, dyspnea, and renal failure and died 4 days after treatment.16 Death in this case was not thought to be the result of treatment and was attributed to sepsis because of infection.
More recently, adoptive transfer of CAR-modified T cells, specific for the TAA human epidermal growth factor receptor-2 (Her-2), led to death of a patient receiving treatment for colon cancer.17 Toxicity involved respiratory distress and may have been the result of CAR-expressing T-cell activity against normal Her-2–expressing cells of the lung. Increased levels of serum cytokines, including IFN-γ, GM-CSF IL-6, and TNF-α, were observed, together with hypotension, brachycardia, and gastrointestinal bleeding, which led to cardiac arrest. In another study targeting Her-2, no toxicity was observed,18 although comparisons between this study using autologous cytotoxic T lymphocyte clones and the CAR-expressing study are difficult because of significant differences in the studies, including the use of lymphodepleting conditioning and greater numbers of T cells in the CAR study. Thus, many different organ systems can become targets of immune-mediated damage after adoptive immunotherapy, a recent example of which involved severe, yet transient, colitis in 3 of 3 patients receiving T cells specific for the colon cancer-associated antigen, carcinoembryonic antigen.19
In the aforementioned examples, the target tumor antigens were also expressed by a range of normal tissues. However, antigens with greater tumor specificity are emerging as more desirable targets. The cancer/testes antigen, NY-ESO-1, represents such a target that is expressed on a range of tumors, but whose normal tissue expression is limited to testes.20 In a phase 1 study, > 50% of patients with either melanoma or synovial cell sarcoma experienced objective clinical responses in the absence of any immune-mediated toxicity.21 Similarly, in a phase 1 study targeting the carbohydrate antigen GD2 with genetically redirected T cells, no T cell–related adverse events were observed, despite apparent antitumor activity demonstrated in 4 of 8 evaluable patients.22 GD2 is an attractive target because it is expressed on neuroblastoma and many melanomas, but normal tissue expression is largely limited to brain and peripheral nerves.
Autoimmunity associated with antibody therapy
There is much promise and excitement in the use of monoclonal antibodies for immunotherapy, with 10 approved by the Food and Drug Administration for the treatment of cancer (reviewed by Weiner et al23 ). These antibodies are specific for a variety of molecular targets expressed on a range of cancers, including lymphomas, leukemias, breast cancer, and colorectal cancer. A variety of effector mechanisms are used by antibodies against tumor cells, which include antagonizing growth factors and their receptors, or inducing their degradation. Alternatively, antibodies may activate antibody-dependent cell-mediated cytotoxicity (ADCC) or the complement pathway. Finally, antibodies may also be used to antagonize receptors, such as cytotoxic T lymphocyte antigen-4 (CTLA-4), which normally down-regulate immune responses.
Clinical trials are currently under way to test the potential of antibodies to mediate tumor regression. However, just as spontaneously arising tumor-specific antibodies have been shown to induce autoimmune pathologies in paraneoplastic neurologic disorders,24 toxicities against normal tissues have also been observed in a proportion of patients receiving exogenous antibody (Table 2).
Immune-mediated toxicities associated with antibody therapy of cancer
| Antibody regimen . | Target antigen . | Cancer type . | Toxicities . | Reference(s) . |
|---|---|---|---|---|
| Ipilimumab | CTLA-4 | Hodgkin disease, myeloma, AML, CML, CLL, NHL | Arthritis, hyperthyroidism, pneumonitis | 29 |
| Ipilimumab | CTLA-4 | Melanoma | Colitis, bowel perforation, vitiligo, hypophysitis | 33 |
| Tremelimumab or ipilimumab | CTLA-4 | Melanoma | Colitis, dermatitis | 30,32,95 |
| Anti-CTLA-4 + TAA peptides | CTLA-4 | Melanoma | Colic, dermatitis, uveitis, enterocolitis, hepatitis, hypophysitis, vitiligo, pulmonary leukocyte infiltration | 28,96,,–99 |
| Anti-CTLA-4 (with vaccines and cytokines) | CTLA-4 | Melanoma (mouse and human) | Melanocyte destruction, enteritis | 31,100,–102 |
| Anti-CTLA-4, irradiated tumor cells, GM-CSF | CTLA-4 | C2 prostate cancer (mouse) | Prostatitis, destruction of prostate epithelium | 103 |
| MDX-1106 | PD-1 | Melanoma, RCC, NSCLC, prostate | Colitis | 34 |
| Alemtuzumab | CD52 | CLL | Destruction of normal leukocytes, leading to susceptibility to infections | 42,44,104 |
| Gemtuzumab | CD33 | AML | Infection, sepsis, pneumonia | 36,–38 |
| Rituxumab | CD20 | Lymphoma | Suppression of B cells leading to deficiency in immunoglobulin and infections | 48,49,105,106 |
| Antibody regimen . | Target antigen . | Cancer type . | Toxicities . | Reference(s) . |
|---|---|---|---|---|
| Ipilimumab | CTLA-4 | Hodgkin disease, myeloma, AML, CML, CLL, NHL | Arthritis, hyperthyroidism, pneumonitis | 29 |
| Ipilimumab | CTLA-4 | Melanoma | Colitis, bowel perforation, vitiligo, hypophysitis | 33 |
| Tremelimumab or ipilimumab | CTLA-4 | Melanoma | Colitis, dermatitis | 30,32,95 |
| Anti-CTLA-4 + TAA peptides | CTLA-4 | Melanoma | Colic, dermatitis, uveitis, enterocolitis, hepatitis, hypophysitis, vitiligo, pulmonary leukocyte infiltration | 28,96,,–99 |
| Anti-CTLA-4 (with vaccines and cytokines) | CTLA-4 | Melanoma (mouse and human) | Melanocyte destruction, enteritis | 31,100,–102 |
| Anti-CTLA-4, irradiated tumor cells, GM-CSF | CTLA-4 | C2 prostate cancer (mouse) | Prostatitis, destruction of prostate epithelium | 103 |
| MDX-1106 | PD-1 | Melanoma, RCC, NSCLC, prostate | Colitis | 34 |
| Alemtuzumab | CD52 | CLL | Destruction of normal leukocytes, leading to susceptibility to infections | 42,44,104 |
| Gemtuzumab | CD33 | AML | Infection, sepsis, pneumonia | 36,–38 |
| Rituxumab | CD20 | Lymphoma | Suppression of B cells leading to deficiency in immunoglobulin and infections | 48,49,105,106 |
CTLA-4 indicates cytotoxic T lymphocyte antigen-4; AML, acute myeloid leukemia; CML, chronic myeloid leukemia; CLL, chronic lymphocytic leukemia; NHL, non-Hodgkin lymphoma; and NSCLC, non–small cell lung cancer.
Toxicities arising from antibody administration can occur in various ways. First, toxicity can follow the induction of potent endogenous autoimmunity against both tumor antigens and other self-antigens, resulting in both on- and off-target toxicities. Second, toxicities can involve on-target depletion of normal cell subsets, compromising normal tissue function. In this case, cell depletion and resulting toxicity are generally transient, persisting only during antibody administration, after which normal cells are replaced from precursors. Nevertheless, even transient depletion can have severe consequences.
Some therapeutic antibodies mediate their antitumor activity by blocking growth factor receptors. Antibodies in this category include bevacizumab, cetuximab, and trastuzumab, which target VEGF-R, EGF-R, and Her-2, respectively. Toxicities against a range of normal tissues, including gastrointestinal tract, skin, and lung, have been observed using these antibodies for cancer therapy. However, because these antibodies mediate their effects largely by blocking the signaling ability of growth factor receptors, which is reminiscent of the action of drugs rather than immunity, we have not discussed these antibodies here but cite references as information for the reader.25-27
Autoimmunity involving on- and off-target toxicities
An immune response is a complex process that is subject to many controls and balances, including the involvement of inhibitory molecules on lymphocytes that down-regulate responses (on elimination of antigen) or prevent inappropriate activity against self-antigens. CTLA-4 is one such regulatory molecule expressed by T cells that transmits an inhibitory signal to T cells on binding to CD80 and CD86 on antigen-presenting cells. The targeting of this inhibitory receptor in immunotherapy has been used to break immune tolerance of T cells to TAAs, resulting in the expansion of T cells that elicit antitumor effects.28 However, in addition to tumor regression, anti–CTLA-4 antibodies, such as ipilimumab and tremelimumab, have been associated with autoimmunity affecting tissues, including the thyroid, lung, joints, gastric mucosa, and liver (Table 2). In a clinical study using ipilimumab to treat hematopoietic malignancy after recurrence after allogeneic hematopoietic cell transplantation, complete remissions were observed in 2 Hodgkin disease patients (2 of 29) and a partial remission in a patient with mantle cell lymphoma. However, grade 2 to 4 autoimmune events, thought to be treatment-related observed in 4 patients in this study, included arthritis, hyperthyroidism, and recurring pneumonitis.29
Toxicities can be common and sometimes severe, as seen in a study using ipilimumab alone or together with vaccine for the treatment of melanoma or RCC. In this study, 21% of 198 patients developed grade 3 to 4 enterocolitis, with 4 patients having colonic perforation leading to 2 deaths and one colectomy.30 Enterocolitis resolved in most patients after administration of corticosteroids, providing further evidence of immune involvement. However, it was not clear whether lymphocyte responses were directed against endogenous antigens from normal bowel or antigens derived from intestinal microflora. Gastrointestinal toxicity was also observed in another study, where 11 of 71 patients receiving ipilimumab developed grade 3 to 4 diarrhea, and gastrointestinal bleeding required colectomy in one patient.31
Of significant interest is that autoimmunity has been demonstrated to be associated with clinical response, suggesting that the greater the immune dysregulation mediated by anti–CTLA-4, the greater the antitumor effect. In some of these cases, specific T cells have been identified that were associated with both antitumor activity and autoimmunity, where Melan-A–specific cytotoxic T cells were observed infiltrating tumors and skin rashes together with a 30-fold increase in circulating Melan-A–specific cytotoxic T cells after treatment with ipilimumab.32
Further randomized clinical studies continue to determine whether the benefits of anti–CTLA-4 therapy justify the risk of autoimmune side effects. In a recent phase 3 study involving 676 melanoma patients, ipilimumab (with or without gp100 vaccine) was demonstrated to significantly improve survival of patients compared with patients receiving vaccine alone (10.1 months median survival vs 6.4 months).33 Complete or partial tumor responses were observed in 9.4% of patients receiving treatment that included ipilimumab compared with 1.5% responses in patients not receiving the antibody. Immune-related adverse events occurred in 60% of ipilimumab-treated patients, with approximately 15% of patients experiencing grade 3 or 4 immune-related adverse events. Toxicity was largely reversed with appropriate treatment, although 7 deaths occurred that were thought to be immune-related because of conditions, including colitis, bowel perforation, liver failure, and 1 patient with Guillain-Barré syndrome.
A range of measures can be taken to manage immune-mediated toxicities, including the use of corticosteroids, which can decrease the severity of toxicity. Interestingly, antitumor responses have been observed to be maintained in the presence of steroid treatment, suggesting that antitumor activity can be separated from autoreactivity, at least in some circumstances.30,31
Anti–CTLA-4 antibodies have induced antitumor responses most markedly against RCC and melanoma, which suggests that these 2 cancers are exceptionally immunogenic.28 Induction of autoimmunity by anti–CTLA-4 may occur through dysregulation of a preexisting immune response to self-antigens, which was held in check by CTLA-4 or through a de novo expansion of self-reactive T cells from naive precursors in the absence of the CTLA-4 checkpoint.
Immune checkpoints are attractive targets for immunotherapies, and much interest is being expressed in targeting immune inhibitory pathways other than CTLA-4. Programmed death-1 (PD-1) is expressed by activated T cells, and its ligand is expressed by a range of cell types, including antigen-presenting cells and sometimes by tumor cells themselves. Interaction of PD-1 with its ligand inhibits T-cell responses and down-regulates immunity.
In a clinical study, MDX-1106, a PD-1–blocking antibody, was used for the treatment of a range of malignancies, including melanoma, renal cell carcinoma, non–small cell lung carcinoma, and prostate cancer. Results from the first 39 patients indicate that 3 patients had objective tumor responses.34 Treatment was well tolerated, with a single case of colitis the only grade 3 to 4 adverse event reported potentially related to antibody administration. A more detailed knowledge of immune checkpoints may lead to effective and safe immunotherapies.
On-target toxicities from depletion of normal cell subsets
To apply effector mechanisms of ADCC and complement against malignant cells, the identification of cell surface markers is necessary. However, most molecular targets of antibodies against hematologic cancers are lineage-specific rather than tumor-specific; consequently, immune-mediated toxicity is induced against normal blood cells, which can compromise immunity or hematopoiesis. Fortunately, target markers can sometimes be selected that are not expressed on stem cells or lineage precursors; therefore, normal cells can often be reconstituted after cessation of therapy. Nevertheless, toxicities can occur because of lowered immunity after immune-mediated depletion of normal leukocytes, as seen when using antibodies for the treatment of some hematologic malignancies.
Gemtuzumab ozogamicin (Mylotarg) is an antibody-calicheamicin conjugate that targets the CD33 surface antigen. Gemtuzumab treatment has been successful in the treatment of CD33-positive AML, after internalization of the conjugate.35 However, the expression of CD33 on normal cells (myeloid progenitor cells and monocytes) has resulted in a large proportion of patients experiencing neutropenia (> 90%) resulting in pneumonia and infections (∼ 20%), which can be life-threatening.36,37 Another serious effect of gemtuzumab is the development of hepatotoxicity, such as vascular obstructive disease, which damages the hepatic sinusoidal epithelium.38 This is thought to occur via receptor-mediated uptake of the antibody-calicheamicin complex through CD33 expression on liver cell populations, such as Kupffer cells (up to 48% of patients in some studies).39 Although CD33 is still a target of interest for treating acute myeloid leukemia and some patients can benefit from treatment with gemtuzumab,40 Mylotarg was voluntarily withdrawn from the market in June 2010 because no significant benefit of the antibody conjugate in combination with chemotherapy was demonstrated above chemotherapy alone.
Alemtuzumab (MabCampath) provides another example where subsets of normal leukocytes are depleted after treatment of hematologic malignancy. Alemtuzumab is used in the treatment of CLL and binds to CD52, which is present on both normal and malignant B and T lymphocytes, as well as monocytes, macrophages, and eosinophils.41 The use of alemtuzumab has yielded promising results, with objective response rates of up to 90% in CLL and encouraging results against other malignancies, such as T-cell prolymphocytic leukemia and peripheral T-cell lymphoma.42,43 As a result of expression of CD52 on normal leukocytes, alemtuzumab also depletes normal immune cell populations, which can result in severe infections that can be fatal.42,44
On-target depletion of normal leukocytes has also been observed using rituximab, which is specific for CD20 expressed on a variety of non-Hodgkin lymphomas and normal mature B cells.45 Rituximab has been used in the treatment of malignancies, including follicular and diffuse large B-cell lymphoma.46,47 It has been shown to mediate lysis of lymphoma cells through a complement-dependent mechanism, and it has substantially increased the survival rates of patients with B-cell lymphomas since its approval by the FDA in 1997. However, the use of this antibody has been associated with toxicities, including tumor lysis syndrome, infections, and reactivation of viruses, such as hepatitis B and herpes simplex virus.48,49 Although antibody-producing plasma cells are not depleted with rituximab, several other cell subsets, including mature B cells, are depleted, which is thought to be responsible for increased rates of infection. Interestingly, depletion of neutrophils has also been observed after the use of rituximab.50 The extent of neutropenia did not generally have clinical significance in these cases; and although the mechanism of neutrophil depletion was not clear, the production of antineutrophil antibodies and disruption of neutrophil homeostasis resulting from B-cell depletion have been proposed as potential contributing causes.
Autoimmunity from cytokine administration
A range of cytokines are being tested in the clinic for their ability to recruit immunity against tumor cells by inducing the activation, proliferation, and survival of tumor-specific lymphocyte populations.2 The complexity of cytokine interactions with the immune system means that often the outcomes of their use cannot be fully predicted. It is therefore not entirely unexpected that autoimmune pathologies have been encountered in a number of cytokine trials to date (Table 3).
Autoimmunity associated with cytokine administration in cancer in humans
| Cytokine administered . | Cancer targeted . | Autoimmune events . | Reference(s) . |
|---|---|---|---|
| IFN-α | Chronic myeloid leukemia | Thyroditis, sarcoidosis, systemic lupus erythematosus associated with autoantibodies, autoimmune thrombocytopenic purpura, acute pancreatitis, hyperthyroidism, aggravation of psoriasis | 51,107,,–110 |
| IFN-α | Cutaneous T-cell lymphoma | Psoriasis | 111 |
| IFN-α | Mid-gut carcinoid tumors | Systemic lupus erythematosus, pernicious anemia, thyroid disease | 112 |
| IFN-α2b | Melanoma | Hypothyroidism | 113 |
| IFN-α2b and piroxicam | Melanoma | Vitiligo, pulmonary interstitial fibrosis | 114 |
| GM-CSF with gp100 and tyrosinase peptides | Melanoma | Vitiligo | 115 |
| IL-2 | RCC | Hypothyroidism, myasthenia gravis, diabetes mellitus (insulin-dependent), and myositis | 62,–64,116 |
| IL-2 | Melanoma | Vitiligo | 67 |
| IL-2 | Colorectal cancer | Diabetes mellitus | 117 |
| IL-2 and IFN-γ | RCC | Autoantibodies against erythrocytes | 65 |
| IL-2 and IFN-α2b | RCC, melanoma | Antithyroid antibodies, hypothyroidism, autoimmune thyroiditis | 63,118,119 |
| Cytokine administered . | Cancer targeted . | Autoimmune events . | Reference(s) . |
|---|---|---|---|
| IFN-α | Chronic myeloid leukemia | Thyroditis, sarcoidosis, systemic lupus erythematosus associated with autoantibodies, autoimmune thrombocytopenic purpura, acute pancreatitis, hyperthyroidism, aggravation of psoriasis | 51,107,,–110 |
| IFN-α | Cutaneous T-cell lymphoma | Psoriasis | 111 |
| IFN-α | Mid-gut carcinoid tumors | Systemic lupus erythematosus, pernicious anemia, thyroid disease | 112 |
| IFN-α2b | Melanoma | Hypothyroidism | 113 |
| IFN-α2b and piroxicam | Melanoma | Vitiligo, pulmonary interstitial fibrosis | 114 |
| GM-CSF with gp100 and tyrosinase peptides | Melanoma | Vitiligo | 115 |
| IL-2 | RCC | Hypothyroidism, myasthenia gravis, diabetes mellitus (insulin-dependent), and myositis | 62,–64,116 |
| IL-2 | Melanoma | Vitiligo | 67 |
| IL-2 | Colorectal cancer | Diabetes mellitus | 117 |
| IL-2 and IFN-γ | RCC | Autoantibodies against erythrocytes | 65 |
| IL-2 and IFN-α2b | RCC, melanoma | Antithyroid antibodies, hypothyroidism, autoimmune thyroiditis | 63,118,119 |
GM-CSF indicates granulocyte-macrophage colony-stimulating factor; and IL-2, interleukin-2.
IFN-α
IFN-α is a prominent and sometimes first-line therapy against many cancers, including melanoma, RCC, cutaneous T-cell lymphoma, chronic myeloid leukemia, bladder cancer, and ovarian cancer. The receptor for IFN-α is almost ubiquitously expressed and can signal to leukocytes and tumors in a variety of ways. It can have pro- or antiproliferative effects, pro- or antiapoptotic effects, modulate cell immunogenicity, promote immune responses, and inhibit angiogenesis.
An extensive range of autoimmune complications have been reported after IFN-α therapy for cancer (Table 3).51 Adverse events include diabetes and vitiligo, as well as the aggravation of preexisting autoimmune diseases. Prospective studies have indicated that autoimmunity in patients correlates with prolonged relapse-free and overall survival.52 This provides evidence that in humans IFN-α is working against cancer, at least in part, by boosting the immune response. The mechanisms by which IFN-α induces cancer regression remain the subject of ongoing investigation. It has been shown to act both on host leukocytes and directly on tumor cells.53,54
Both B and T cell–mediated autoimmunities have been reported in response to IFN-α, which is consistent with known activities of type I interferons. Type I interferons can promote skewing of T cells to a cytotoxic phenotype by stimulating their expression of the IL-12R.55 They complement this by also inducing genes coding for MHC-I and TAAs on tumor and nontumor populations.56 IFN-α may indirectly promote the proliferation of memory T-cell populations57 and also promotes B-cell switching to the IgG2a isotype. IgG2a is capable of targeting cells for phagocytosis and triggering complement-mediated cytotoxicity.58
In summary, type I IFNs are powerful modulators of antitumor immunity. The information available regarding their effects on leukocytes, host stroma, and tumor cells serves as a further example of the pleiotropic effects that cytokines can mediate.
IL-2
IL-2 has been widely applied in the clinic in the treatment of melanoma, RCC, and AML and is one of only a few cytokines that has progressed to FDA approval in the treatment of cancer. Used alone in the treatment of metastatic melanoma, it has produced objective response rates of up to 16%.59 The antitumor effect of IL-2 stems, at least in part, from its ability to act as a growth factor for T cells and to enhance the cytolytic activity of NK cells.
Nonimmune systemic toxicities associated with high-dose IL-2 therapy are well appreciated. IL-2 therapy induces systemic inflammation, the exact mechanism of which is poorly understood, and the main symptomatic outcomes are hypotension and capillary leak syndrome, accompanied by flu-like symptoms, vomiting, and diarrhea. Despite its toxicity, advances in managing symptoms and the resolution of toxicity after cessation of administration have led to its continued use in the cancer setting.60,61
In addition to these systemic toxicities, IL-2 therapy is known to both exacerbate autoimmunity and trigger it de novo (Table 3). In a notable case study, both of these outcomes were detected within the same patient after high-dose IL-2 therapy for RCC, with worsening of diabetes and induction of myasthenia gravis and polymyositis.62 There is evidence that IL-2 can augment humoral responses specific for autoantigens and drive expansion of NK and T cells.63-65 Thus, both humoral and cell-mediated immune mechanisms may contribute to autoimmunity resulting from cytokine therapy.66 Interestingly, similar to observations using antibodies or adoptive immunotherapy for cancer treatment as discussed in “Autoimmunity associated with antibody therapy,” antitumor responses in patients receiving cytokines can correlate with autoimmunity.63,64
The best evidence that high-dose IL-2 can induce on-target (tumor antigen) autoimmunity stems from a prospective study of patients treated for metastatic melanoma and RCC.67 Here, vitiligo (autoimmune destruction of melanocytes) was observed in 11 of 74 of patients treated for melanoma but 0 of 104 treated for RCC. Development of vitiligo in melanoma patients correlated with objective responses to therapy, indicating that immune targeting of melanoma differentiation antigens was underpinning both outcomes. Other autoimmune toxicities reported in conjunction with IL-2 therapy include diabetes mellitus and hypothyroidism (Table 3). These are presumed to represent examples of off-target autoimmunity in the absence of reported antigen sharing between tumors and the affected tissues.
Autoimmunity associated with cancer vaccines
Vaccines are a major focus of cancer immunotherapy, and lessons learned from successful vaccines against infectious disease are being applied to the treatment of malignant disease. Antigen formulations used in cancer vaccines include peptides and whole proteins in combination with adjuvants to improve immunogenicity or pulsed directly onto dendritic cells to facilitate antigen presentation. In addition, antigen has been encoded in recombinant viruses in attempts to generate robust immune responses to TAAs in parallel to viral antigens.
The vaccination approach has shown considerable promise in preclinical murine models, although breaking of tolerance toward TAAs expressed by both the tumor and healthy peripheral tissues of mice has been observed to result in autoimmune disease in some cases (summarized in Table 4). In humans, cancer vaccines have been studied intensively, particularly in the melanoma setting. Increased frequencies of antigen-specific circulating T cells have often been observed after vaccine administration, although overall clinical responses rates of only 2.6% have been achieved.68 Perhaps in keeping with the low response rate, there have been few reports of autoimmunity arising in patients after vaccination with TAAs. An evaluation of autoimmunity in melanoma patients treated with IL-2 and vaccines reported the occurrence of autoimmune thyroiditis and autoimmune insulitis, but these events were infrequent.69 Perhaps as cancer vaccines become more potent as they can be in animal models, we might see a concomitant increase in autoimmune sequelae.
Autoimmunity associated with cancer vaccines
| Vaccine . | Malignancy (species) . | Autoimmune events . | Reference(s) . |
|---|---|---|---|
| TRP-1 encoded by virus | Melanoma (mouse) | Vitiligo | 120,121 |
| CD20 + adjuvant | Lymphoma (mouse) | Depletion of normal B cells | 122 |
| Her-2 encoded by plasmids | Breast cancer–expressing Her-2 (mouse) | Impaired lactation, accelerated involution of mammary gland | 123,124 |
| DCs (peptide pulsed) CD40 and IL-2 (fibroblasts) | Lymphoblastic leukemia (mouse) | Systemic autoimmunity, immunity against fibroblasts, hepatomegaly, splenomegaly, fur loss, cachexia, | 125 |
| DCs (peptide-pulsed) β-gal and LCMV model antigens | EL-4 thymoma (mouse) | Diabetes, myocarditis | 126 |
| Pulsed DCs or tumor cells and either GM-CSF and adjuvant or GM-CSF, adjuvant, and peptides | Melanoma (human) | Vitiligo, diabetes, thyroiditis, ocular toxicity | 69 |
| Vaccine . | Malignancy (species) . | Autoimmune events . | Reference(s) . |
|---|---|---|---|
| TRP-1 encoded by virus | Melanoma (mouse) | Vitiligo | 120,121 |
| CD20 + adjuvant | Lymphoma (mouse) | Depletion of normal B cells | 122 |
| Her-2 encoded by plasmids | Breast cancer–expressing Her-2 (mouse) | Impaired lactation, accelerated involution of mammary gland | 123,124 |
| DCs (peptide pulsed) CD40 and IL-2 (fibroblasts) | Lymphoblastic leukemia (mouse) | Systemic autoimmunity, immunity against fibroblasts, hepatomegaly, splenomegaly, fur loss, cachexia, | 125 |
| DCs (peptide-pulsed) β-gal and LCMV model antigens | EL-4 thymoma (mouse) | Diabetes, myocarditis | 126 |
| Pulsed DCs or tumor cells and either GM-CSF and adjuvant or GM-CSF, adjuvant, and peptides | Melanoma (human) | Vitiligo, diabetes, thyroiditis, ocular toxicity | 69 |
DCs indicates dendritic cells; LCMV, lymphocytic choriomeningitis virus; and GM-CSF, granulocyte-macrophage colony-stimulating factor.
Conclusions and future perspective
Although immunotherapy holds great promise for the treatment of a range of malignancies, many of these therapies can be associated with autoimmunity against normal self-tissues (Figure 1). Mechanisms involved in immune-mediated damage of normal tissues are varied, and a greater understanding of these mechanisms will enable enhanced and more specific forms of immunotherapy for cancer.
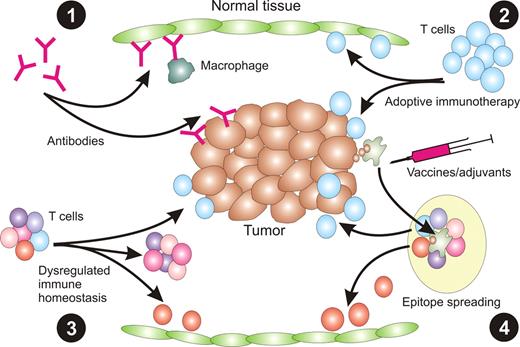
The most obvious means of toxicity results from expression of TAAs on normal tissue (Figure 2). Thus, antibodies raised against these antigens can react against normal cells either by inducing complement-mediated lysis or facilitating ADCC by innate leukocytes. Adoptively transferred T cells specific for TAAs can likewise react directly against normal tissues expressing those antigens as part of their normal proteome. However, toxicity can also result from de novo induction of immune responses against other antigens on normal tissues. For example, inhibition of CTLA-4 can dysregulate normal lymphocyte homeostasis, leading to expansion of self-reactive T cells able to respond against normal tissues that do not express TAAs.70 Similarly, immunotherapy can lead to epitope spreading in which immune responses can be raised against additional molecular targets, including those expressed on normal tissues.71
Mechanisms of immune toxicity associated with immunotherapy. (1) The delivery of exogenous antibody specific for TAA expressed on both tumor and normal tissue can result in damage to normal tissue mediated by complement or ADCC mediated by innate immune cells, such as macrophages. (2) Similarly, transfer of TAA-specific T cells can lead to destruction of normal tissue if the TAA is also expressed on cells of normal tissue. (3) Alternate ways of inducing immune reactivity toward normal tissue include dysregulation of normal immune homeostasis using anti–CTLA-4, resulting in expansion of self-reactive T cells. (4) It is also possible that induction of immunity to tumor cells could lead to epitope spreading whereby T cells reactive with self-antigens are generated.
Mechanisms of immune toxicity associated with immunotherapy. (1) The delivery of exogenous antibody specific for TAA expressed on both tumor and normal tissue can result in damage to normal tissue mediated by complement or ADCC mediated by innate immune cells, such as macrophages. (2) Similarly, transfer of TAA-specific T cells can lead to destruction of normal tissue if the TAA is also expressed on cells of normal tissue. (3) Alternate ways of inducing immune reactivity toward normal tissue include dysregulation of normal immune homeostasis using anti–CTLA-4, resulting in expansion of self-reactive T cells. (4) It is also possible that induction of immunity to tumor cells could lead to epitope spreading whereby T cells reactive with self-antigens are generated.
As our knowledge of the human proteome and the cancer genome increases, opportunities are arising to refine the choice of antigens for immunotherapy. Next-generation genome sequencing allows cataloging of all the mutations within any given tumor cell.72 This could allow the rapid identification of truly tumor-unique antigens and greater personalization of vaccine and adoptive immunotherapies in the near future. Already anti-idiotype vaccines are showing promise for the specific targeting of malignant B-cell lymphomas.73,74
Methods to reduce immune-mediated toxicity in adoptive immunotherapy may involve identifying multiple antigens that together constitute a tumor signature or “barcode.”75 Gene constructs encoding several antigen receptors can be developed where the threshold for cytotoxic activity is only reached if the complete “barcode” is recognized. Alternatively, constructs can be designed which shut down T-cell activity if a barcode indicative of normal healthy tissue is encountered.
In the case of cytokine therapy, systemic toxicities and the deregulation of immune responses may also be reduced through more targeted delivery of cytokines to the tumor site, rather than systemically.76
In antibody therapy, it is useful to consider that not all antibodies for a specific target molecule are equal in their immune side effects, as seen using TGN1412, a monoclonal antibody specific for the T-cell costimulatory molecule CD28. TGN1412 was thought to hold promise as a treatment for cancer and rheumatoid arthritis, but a safety trial performed in 6 healthy volunteers demonstrated severe toxicity, including hypotension and respiratory distress.77 Toxicity was thought to be the result of high levels of serum cytokines in response to CD28 ligation occurring within hours of antibody administration. TGN1412 differed from other anti-CD28 antibodies in that it had superagonist qualities, not possessed by all anti-CD28 antibodies. Similarly, individual anti-CTLA-4 antibodies can differ in their capacity to induce autoimmunity, as demonstrated in mice engineered to express human CTLA-4. In this mouse model, several antibodies varied in their relative abilities to induce autoimmunity and protection against tumor.78 An antibody was identified that produced the strongest antitumor activity and the least autoimmunity. Thus, it may be possible to separate antitumor activity from autoimmunity by antibody selection.
Selective down-regulation of autoimmunity may also be possible using immunosuppressants. The use of corticosteroids to counteract severe immune-mediated toxicity has surprisingly indicated that steroid treatment may not always signal the end of an immunotherapy's beneficial impact, with tumor responses being maintained after resolution of autoimmunity using steroids.31,79 A further consideration is whether it is possible to kinetically separate antitumor immunity from autoimmunity. Regulatory mechanisms on healthy tissues may allow them to withstand immune toxicity for a period of time.
Immunotherapy is an exciting and increasingly effective treatment option for cancer. However, it is becoming increasingly clear that cancer immunotherapy is a balancing act between antitumor immunity and immune toxicity. The association between immune toxicity and increased antitumor effects after immunotherapy highlights the need for strategies that can mitigate the risk of these toxicities during immunotherapy while preserving activity against malignancy.
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia, Cancer Council of Victoria, and the Susan G. Komen Breast Cancer Foundation. M.H.K. is supported by a Senior Research Fellowship from the National Health and Medical Research Council of Australia. P.K.D. is supported by a National Health and Medical Research Council of Australia Career Development Award. S.M.A. is supported by a Cancer Council of Victoria Postgraduate Cancer Research Scholarship.
Authorship
Contribution: S.M.A., C.P.M.D., J.A.W., D.S.R., P.K.D., and M.H.K. researched and wrote the paper; and R.P.J. assisted in writing the adoptive immunotherapy section and critically reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael H. Kershaw, Cancer Immunology Research Program, Peter MacCallum Cancer Centre, St Andrews Place, Melbourne, Victoria, Australia 3002; e-mail: michael.kershaw@petermac.org.
References
Author notes
S.M.A. and C.P.M.D. contributed equally to this study as first authors.
P.K.D. and M.H.K. contributed equally to this study as senior authors.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal