Vascular endothelial growth factor A (VEGF-A) regulates many aspects of blood vessel physiology including angiogenesis. In this issue of Blood, Ballmer-Hofer et al report that the VEGFR2 receptor tyrosine kinase binding to different VEGF-A isoforms and neuropilin-1 can program different endothelial responses such as angiogenesis.1
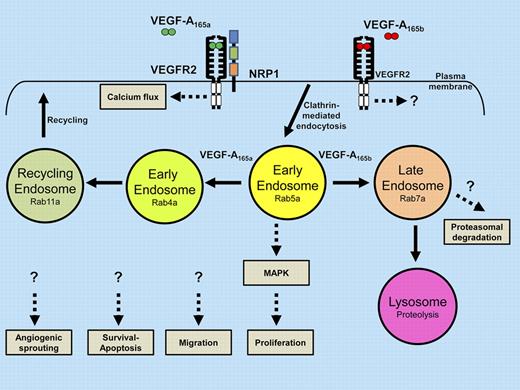
Alternative pathways of VEGFR2 signaling, trafficking, and proteolysis influence decisions on cell migration, proliferation, survival, apoptosis, and vessel sprouting.
Alternative pathways of VEGFR2 signaling, trafficking, and proteolysis influence decisions on cell migration, proliferation, survival, apoptosis, and vessel sprouting.
In multicellular organisms that use a vascular network, spatial and temporal control of blood vessel sprouting is needed to mobilize cells, molecules, and lipid particles for normal and pathophysiologic responses. Vascular endothelial growth factor receptors (VEGFR1-3) and neuropilins (NRP1, NRP2) bind VEGF-related ligands to respond to the changing extracellular milieu. In this way, an organism is able to build a 3-D vascular network, regulate physiology, and respond to pathologic insults, for example, injury or hypoxia. The existence of neuropilins as VEGF coreceptors and the abundance of VEGF-A splice isoforms (more than 8) provide additional levels of mechanistic complexity.
VEGF-A binding to endothelial VEGFR2 triggers proangiogenic signals that activate mitogen-activated protein kinase (MAPK), the key serine/threonine protein kinase c-Akt, and endothelial nitric oxide synthase (eNOS). Such signaling modulates cell migration, survival, proliferation, apoptosis, and new blood vessel sprouting (angiogenesis).2 How can these different outcomes be fine-tuned by a single interaction between VEGFR2 and VEGF-A? This mechanism is further complicated by the finding that VEGFR2 can form homo- and heteromeric complexes with VEGFR1 and NRP1 that can mediate signaling to regulate prostacyclin synthesis, which in turn regulates vasodilation.3
Ballmer-Hofer and colleagues now provide evidence that endothelial VEGFR2 binding to either the VEGF-A165a or VEGF-A165b isoforms triggers different trafficking, signaling, and cellular outcomes.1 Trafficking within the endocytic pathway is regulated by the Ras-related small GTP-hydrolysing enzymes such as Rab4a, Rab5a, Rab7a, and Rab11a. Depending on the VEGF-A isoform and coreceptor (NRP1) bound to VEGFR2, different signals are generated. VEGFR2 complexes that bind to the more abundant VEGF-A165a isoform triggers assembly of a VEGF-A165a–VEGFR2-NRP1 heteromeric complex that undergoes trafficking through Rab4a-, Rab5a-, and Rab11a-associated endosomes. Importantly, the VEGF-A165b isoform failed to recruit NRP1 to VEGFR2 but promoted trafficking via a late endosome-to-lysosome route regulated by the Rab7a GTPase. Depending on the expression of wild-type or mutant NRP1, VEGFR2 activation, signaling, proteolysis, and endothelial sprouting in vitro were differentially affected.
Quiescent VEGFR2 undergoes clathrin-mediated endocytosis and recycling via endosomes but activated VEGFR2 undergoes proteolysis and terminal degradation in lysosomes.4,5 In the schematic diagram (see figure), the balance between VEGFR2 membrane trafficking and proteolysis via the endosome-lysosome system could generate unique signaling outputs to different VEGF-A isoforms. Importantly, activated VEGFR2 can signal from the plasma membrane via phospholipase Cγ1 and from endosomes via p42/44 MAPK, respectively.4,5 Interestingly, there is still debate about VEGFR2 endosome-to-plasma membrane recycling: a Rab-independent c-Src–dependent pathway has been postulated whereas Rab4a-dependence was later noted, including this recent study.1,6,7
How can we reconcile flexibility in vascular physiology control with VEGF activation of membrane receptors? One possibility is that this is because of the remarkable diversity of VEGF-related protein isoforms encoded by the mammalian VEGF gene family that includes VEGFA, B, C, D and placental growth factor (PlGF). The VEGFA gene alone undergoes alternative RNA splicing to generate more than 8 protein isoforms including the antiangiogenic VEGF-A165b isoform. For example, replacement of the terminal 6 amino acids in a VEGF-Axxxb isoform could double the number of splice variants encoded by this remarkable gene to further modulate the angiogenic response.8 Thus a mixture of pro-and antiangiogenic signals in endothelial cells can be generated by differential occupancy of VEGFR2 by VEGF-A165a or VEGF-A165b isoforms. Importantly, vascular tubule length and sprout efficiency could thus be fine-tuned by the presence of both pro- and antiangiogenic VEGFs each with a distinct property in influencing VEGFR activation, trafficking, and proteolysis.
An increasingly important aspect of VEGF biology is its function in nonvascular cells and tissues. VEGFs and their cognate receptors are also linked to epithelial and neural physiology in both healthy and diseased states. One intriguing possibility is that the 3-D phenomenon of vascular tubulogenesis has evolved from a 2-D cell-cell communication system used to develop and maintain neural networks in simple multicellular organisms.9 Such a view has significant implications for neuro-vascular control and communication including the evidence for VEGF-linked dysfunction in many neurologic diseases.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal