Abstract
A primary HCMV infection or virus reactivation may cause severe disease in hosts with a deficient immune system. The virus can disturb both innate and adaptive immunity by targeting dendritic cell (DC) functions. Monocytes, the precursors of DCs in vivo (MoDCs), are the primary targets of HCMV; they can also harbor latent virus. The DCs generated from infected monocytes (CMV-MoDCs) have an altered phenotype and functional defects. We have shown that CMV-MoDCs do not secrete IL-12 in response to lipopolysaccharide stimulation, cannot ingest dead cells, induce TH1 differentiation, or the proliferation of naive allogeneic CD4+ T cells. We found that the GM-CSF signaling in an entire population of CMV-MoDCs was impaired, although only half of the cells were productively infected, and that IL-6 secretion and suppressors of cytokine signaling 3 induction contributed to this bystander effect. We also showed that MoDCs derived ex vivo from monocytes of viremic patients had the same altered phenotype as CMV-MoDCs, including decreased STAT5 phosphorylation, indicating defective GM-CSF signaling. We have thus described a new mechanism of HCMV-induced immunosupression, indicated how infection may disturb both GM-CSF–dependent physiologic processes and proposed GM-CSF–based therapeutic approaches.
Introduction
Human CMV (HCMV) is a β-herpesvirus that infects most of the human population during their lifetime. HCMV opportunistically exploits its host immune defects to replicate and spread, so causing severe disease. Transplant recipients, fetuses, and newborns are all at risk of disease or death because of primary infections or virus reactivation. HCMV also encodes a variety of proteins that disrupt TCD4+ and TCD8+ antiviral responses; the latter are critical for controlling the spread of virus and preventing associated diseases (for a review see Crough and Khanna1 ). Dendritic cells (DCs) are the sites of primary infection and are essential for capturing the virus Ags needed to mould an appropriate CD8+ T-cell repertoire against the virus. Thus, DCs are main targets of virus subversion. DCs infected with HCMV have defects in all stages of Ag presentation, migration to secondary lymphoid organs, and T-cell activation (reviewed in Varani et al2 ). We have developed a working hypothesis that these virus-induced changes can be overcome by uninfected DC cross-presenting Ags to CD8+ T cells.3 Monocytes are primary targets of HCMV, can harbor latent virus, and are a source of inflammatory DCs in vivo.4-6 We therefore assumed that DC dysfunction was because of abnormalities arising during the differentiation of their monocyte precursors. DCs derived from infected monocytes in vitro and in patients who received a transplant experiencing an active infection7,8 all have abnormal phenotypes and function that may contribute to immunosuppression. However, the molecular and cellular mechanisms underlying these defects are poorly documented. We have obtained evidence that the infection of monocytes with HCMV results in defective GM-CSF signaling, with inhibition of STAT5 phosphorylation that leads to dysfunctional derived DCs. These DCs had lost critical features such as the ability to potentiate TH1 differentiation and proliferation because of secreted factors such as IL-6 that is essential for suppressors of cytokine signaling 3 (SOCS3) synthesis. We have therefore provided new insights into the way HCMV infections cause immunosuppressive damage and the ensuing side effects.
Methods
Generation and culture of monocyte-derived DCs
DCs were generated from monocytes, and cell cultures were phenotyped as described in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Viruses
The endothelial cell–adapted strain HCMVTB40/E GFP was provided by M. Messerle (Hanover, Germany) and HCMVVHLE was provided by C. Sinzger (Tubingen, Germany). HCMV BACTB40/E GFP (green fluorescent protein) was designed and generated by E. Borst (Hanover, Germany) from a HCMV BACTB40/E genome9 in which the US1 to US7 genomic fragment was replaced by a GFP cassette under the control of mouse MIEP.
Viruses were amplified on MRC5 fibroblasts and collected by centrifugation at 100 000g for 35 minutes. Virus titers (PFU/mL) were determined on MRC5 cells. Viruses were inactivated by UV irradiation for 20 minutes at 0.17 Amp with the use of a Spectroline device.
Monocyte infection and treatment
Monocytes in differentiation medium were either mock-infected (medium) or infected with live or UV-inactivated virus at a MOI of 5. Alternatively, culture medium was supplemented with human recombinant IL-6 (25-50 ng/mL; Ozyme), anti–IL-6 or anti–IL-6R blocking Abs, or IgG isotype control (15 μg/mL; Gen-Probe Diaclone), and phosphonoacetic acid (PAA; 250 μg/mL; Sigma-Aldrich). CMV-MoDC defines cells produced by the differentiation of infected monocytes and IL-6-MoDC defines cell supplemented with IL-6. In experiments with conditioned medium, the culture medium was a mixture of fresh differentiation medium and the medium to be tested (1/1; vol/vol). When applicable, culture media were cleared of infectious virus by centrifugation (100 000g for 35 minutes).
Flow cytometric analysis
Cells were treated, stained, and analyzed by flow cytometry on a FACSCalibur cytometer (BD Biosciences; supplemental Methods).
Western blot analysis
Electrophoresis of cell lysates and protein analysis by immunoblotting was performed with standard procedures (supplemental Methods).
RT-PCR analysis
PCRs were performed on reverse-transcribed total RNA as detailed in supplemental Methods.
Phagocytosis assays
MRC5 cells were stained with PKH26 (Sigma-Aldrich) and cultured with TNF-α (1 μg/mL) and cycloheximide (25 μg/mL; Sigma-Aldrich) in complete DMEM medium for 24 hours to obtain apoptotic bodies. Apoptotic MRC5 cells were cocultured with DCs (1 DC for 2 MRC5 cells). Cells were harvested and stained with an FITC-conjugated anti–HLA-DR Ab, and double-positive cells were quantified on a FACSCalibur device. The percentage of PKH26+/HLA-DR+ MoDCs was considered as 100% of phagocytic activity. Alternatively, the Vybrant phagocytosis assay kit (Escherichia coli bioparticles; Invitrogen) was used.
Cytokine quantification
Secreted inflammatory cytokines were detected with a dedicated Ab array from Raybiotech. TNF-α, IL-6, and IL-12p70 were measured with a cytometric bead array assay (CBA; BD Biosciences) and the soluble form of GM-CSF receptor (sGMRα) by ELISA (Gen-Probe Diaclone).
Small interfering RNA transfection
Purified monocytes were treated with Lipofectamine RNAiMax (Invitrogen) and 50nM RNAi was directed against SOCS1 (3′-UCGAAGAGGCAGUCGAAGCUCUCGC-5′) or SOCS3 (3′-AGUAGAUGUAAUAGGCUCUUCUGGG-5′) mRNA (Invitrogen) or control RNAi (Invitrogen). Corresponding protein expressions were checked by Western blot analysis with the use of anti-SOCS3 or anti-SOCS1 Abs (Abcam).
Isolation of naive CD4+ T cells and polarization experiments
Naive CD4+CD45RA+ T cells were isolated from PBMCs with the use of a magnetic bead separation system (naive CD4+ T-cell isolation kit; Miltenyi Biotec). Allogeneic stimulators were infected with HCMV or were treated with anti–IL-6 or isotypic Abs during differentiation and then with ultrapure lipopolysaccharide (LPS; Invivogen) for 24 hours, washed with culture medium, and cocultured for 5 days in 96-well plates with T cells (95%-98% purity) at a ratio of 1:10 (1 × 104 allogeneic stimulators for 1 × 105 T cells/well in 200 μL). IL-2 (50 U/mL; PeproTech) was then added to each well, and culture was continued for a further 3 days. For TH2 polarization experiments, IL-4 (5 ng/mL; PeproTech) and anti–IFN-γ Ab (10 μg/mL; BD Biosciences) were added to cocultures. Intracellular cytokines were assayed on day 8 (see supplemental Methods). In CFSE experiments, naive T cells were stained with CFSE (Invitrogen), cocultured for 5 days at a ratio of 1 stimulator to 10 T cells as above, and T-cell proliferation was analyzed.
Patients
The project was approved by the ethics committees of the Rangueil Hospital (Toulouse) and Besançon Hospital (Besançon), and all patients who provided samples gave their written informed consent in accordance with the Declaration of Helsinki. PBMCs were isolated from patients' blood samples with the use of a Ficoll density gradient, and CD14+ cells were sorted as described in supplemental Methods: “Generation and culture of monocytes derived DCs.” Cells were differentiated to MoDCs for 5 days, and their CD1a and pSTAT5 contents were determined. Secreted IL-6 in blood plasma and MoDC supernatants were assayed with CBA. HCMV DNA in patients' blood was quantified by real-time PCR.10
Statistical analysis
Values are expressed as the means ± SDs of ≥ 3 independent experiments. Data were analyzed for significance with the Mann-Whitney U test or Student t test, and a P value ≤ .05 was considered significant. All data were analyzed with Prism Version 5 (GraphPad) and FlowJo Version 7.6.5 (TreeStar) software.
Results
Phenotype of DCs differentiated from HCMV-infected monocytes
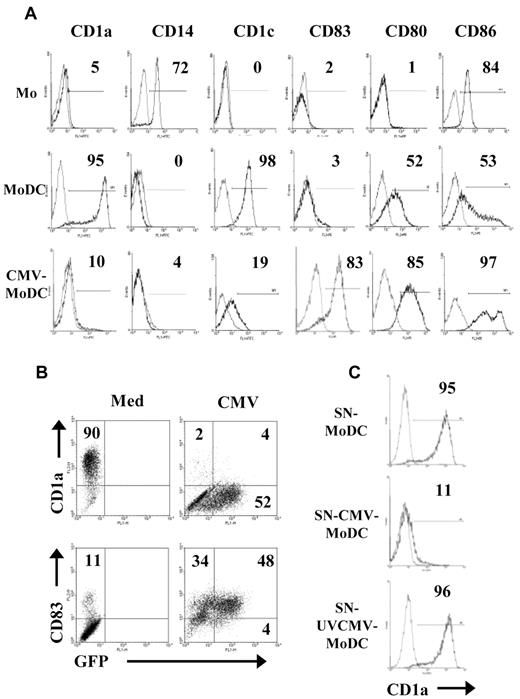
Adherent monocytes obtained from the peripheral blood of healthy donors were either mock-infected or infected with HCMV (VHLE; MOI of 5). The resulting MoDCs and CMV-MoDCs were obtained by differentiation in a culture medium containing GM-CSF and IL-13 for 5 days. IL-4 treatment always generated the same phenotype as IL-13 (not shown). The MoDCs had the phenotype of immature DCs bearing CD1a (95%) and CD1c (98%), CD80 (52%) and CD86 (53%), but no CD14 (0%) or CD83 (3%). Only 10% of infected MoDCs expressed CD1a, and only 19% had CD1c, whereas most of them had the maturation marker CD83 (83%) and had increased CD80 (85%) and CD86 (97%; Figure 1A). Identical results were obtained when monocytes were positively selected from PBMCs with the use of CD14 magnetic beads (data not shown). Alternatively, the HCMV inoculum was UV-inactivated before adding it to monocytes to determine whether the phenotype modifications required live virus. UVCMV-MoDCs had the regular MoDC phenotype (not shown), indicating that de novo synthesis of virus proteins was necessary for production of the modified phenotype. Surprisingly, monocytes are poorly permissive to HCMV, but most CMV-MoDCs were modified. We therefore measured the percentage of infected cells with an HCMV BAC construct encoding a GFP. Approximately 50% of CMV-MoDCs contained GFP-labeled fluorescence, whereas 82% of them were positive for CD83 and 6% were positive for CD1a compared with 11% (CD83) and 90% (CD1a) of MoDCs (Figure 1B). These data suggest that infected cells inhibited their uninfected counterparts by secreting soluble factors. We therefore cultured monocytes for 5 days in standard medium (GM-CSF, IL-13) supplemented with supernatants recovered from 5-day cultures of MoDCs, CMV-MoDCs, and UVCMV-MoDC. Most of the cells generated from monocytes that had been exposed to medium conditioned by CMV-MoDCs expressed no CD1a (11% of CD1a+ cells), whereas those exposed to supernatants from UVCMV-MoDCs were unchanged (Figure 1C). Thus, monocytes infected with live HCMV secrete soluble factors that alter their differentiation into standard immature DCs.
Abnormal phenotype of DCs derived from HCMV-infected monocytes results from a paracrine effect. Monocytes (Mo) were differentiated into DCs after mock-infection (MoDC) or infection with HCMV-VHLE (CMV-MoDC) by culture for 5 days with GM-CSF and IL-13. (A) Surface markers (CD1a, CD1c, CD14, CD83, CD80, and CD86) were analyzed by flow cytometry, and the percentages of positive cells was determined as indicated. (B) Monocytes were infected with BAC-TB40E/GFP and checked for GFP fluorescence and by flow cytometry after labeling with anti-CD1a and anti-CD83 Abs. (C) Monocytes were differentiated by culture for 5 days with GM-CSF and IL-13 standard medium supplemented with supernatants recovered from MoDC (SN-MoDC), MoDC infected with live CMV (SN-CMV-MoDC) or UV-inactivated CMV (SN-UVCMV-MoDC). Cells were labeled with PE-anti-CD1a Abs, and the percentage of CD1a+ cells was determined as indicated. Representative of ≥ 3 independent experiments.
Abnormal phenotype of DCs derived from HCMV-infected monocytes results from a paracrine effect. Monocytes (Mo) were differentiated into DCs after mock-infection (MoDC) or infection with HCMV-VHLE (CMV-MoDC) by culture for 5 days with GM-CSF and IL-13. (A) Surface markers (CD1a, CD1c, CD14, CD83, CD80, and CD86) were analyzed by flow cytometry, and the percentages of positive cells was determined as indicated. (B) Monocytes were infected with BAC-TB40E/GFP and checked for GFP fluorescence and by flow cytometry after labeling with anti-CD1a and anti-CD83 Abs. (C) Monocytes were differentiated by culture for 5 days with GM-CSF and IL-13 standard medium supplemented with supernatants recovered from MoDC (SN-MoDC), MoDC infected with live CMV (SN-CMV-MoDC) or UV-inactivated CMV (SN-UVCMV-MoDC). Cells were labeled with PE-anti-CD1a Abs, and the percentage of CD1a+ cells was determined as indicated. Representative of ≥ 3 independent experiments.
Paracrine inhibition of STAT5 phosphorylation in CMV-MoDCs
We checked that monocytes that differentiated in the absence of GM-CSF gave rise to DCs bearing no CD1a, as previously reported11 (Figure 2A). We then investigated whether CMV-MoDC phenotype could be because of, at least in part, a defect in GM-CSF signaling. Because GM-CSF triggers STAT5 activation,12 which requires the phosphorylation of tyrosine at position 694, we measured the phosphorylated STAT5 (pSTAT5) in CMV-MoDCs by Western blot analysis. The pSTAT5/total STAT5 ratio in DCs generated in the absence of GM-CSF (ΔGM-MoDCs) was lower than that of standard MoDCs (Figure 2B). This was also true for CMV-MoDCs and monocytes differentiated in the presence of CMV-MoDC supernatants. The altered phenotype of CMV-MoDCs could therefore be because of a blockage of GM-CSF signaling, perhaps by soluble factors secreted by infected monocytes during their differentiation. STAT5 phosphorylation was not inhibited in cells treated with UVCMV, confirming that blocking STAT5 activation requires live CMV. We substantiated the impairment of STAT5 activation in CMV-MoDCs by flow cytometry to detect pSTAT5 in cells infected with BAC-GFP (Figure 2C). This again pointed to HCMV impairing monocyte differentiation via a paracrine effect. Inhibiting virus replication with PAA, which inhibits late viral protein synthesis (supplemental Figure 1), did not reverse the inhibition of STAT5 phosphorylation by HCMV, suggesting that immediate early (IE) or early (E) virus products are involved (Figure 2D).
Paracrine inhibition of STAT5 phosphorylation in CMV-MoDCs. Monocytes were differentiated into DCs by culture with (MoDC) or without GM-CSF (ΔGM-MoDC) and IL-13. (A) The percentage of MoDCs (Med) and ΔGM-MoDC (ΔGM) cells bearing CD1a and CD83 was determined by flow cytometry with PE-Abs. (B) Total and phosphorylated STAT5 (pSTAT5) analyzed by Western blotting of cell lysates recovered from MoDCs (Med), ΔGM-MoDCs (ΔGM), cells issued from monocytes infected with live CMV (CMV), or UV-inactivated CMV (UVCMV), or monocytes incubated with supernatants from MoDC (SN-MoDC), CMV-MoDC (SN-CMV-MoDC), and UVCMV-MoDC (SN-UVCMV-MoDC). β-actin was used as control. The histograms below represent the quantification of 3 independent experiments. (C) The percentage of cells containing pSTAT5 after infection with BAC-TB40E/GFP was determined by flow cytometry after sequential labeling with a mouse monoclonal Ab specific for pSTAT5 (P-Y694) and PE-labeled anti–mouse Ab. (D) Total and phosphorylated STAT5 proteins were assayed by Western blotting of MoDCs (Med) and CMV-MoDCs (CMV) treated (+) or not (−) with PAA (250 μg/mL) during differentiation. The histogram (right) represents the quantification of 3 independent experiments. Representative of ≥ 3 independent experiments (A,C).
Paracrine inhibition of STAT5 phosphorylation in CMV-MoDCs. Monocytes were differentiated into DCs by culture with (MoDC) or without GM-CSF (ΔGM-MoDC) and IL-13. (A) The percentage of MoDCs (Med) and ΔGM-MoDC (ΔGM) cells bearing CD1a and CD83 was determined by flow cytometry with PE-Abs. (B) Total and phosphorylated STAT5 (pSTAT5) analyzed by Western blotting of cell lysates recovered from MoDCs (Med), ΔGM-MoDCs (ΔGM), cells issued from monocytes infected with live CMV (CMV), or UV-inactivated CMV (UVCMV), or monocytes incubated with supernatants from MoDC (SN-MoDC), CMV-MoDC (SN-CMV-MoDC), and UVCMV-MoDC (SN-UVCMV-MoDC). β-actin was used as control. The histograms below represent the quantification of 3 independent experiments. (C) The percentage of cells containing pSTAT5 after infection with BAC-TB40E/GFP was determined by flow cytometry after sequential labeling with a mouse monoclonal Ab specific for pSTAT5 (P-Y694) and PE-labeled anti–mouse Ab. (D) Total and phosphorylated STAT5 proteins were assayed by Western blotting of MoDCs (Med) and CMV-MoDCs (CMV) treated (+) or not (−) with PAA (250 μg/mL) during differentiation. The histogram (right) represents the quantification of 3 independent experiments. Representative of ≥ 3 independent experiments (A,C).
Paracrine effect of IL-6 on differentiating monocytes
Cytometric analysis showed that there was more GM-CSF receptors on the cell surface of CMV-MoDCs than on MoDCs at day 2 of differentiation (supplemental Figure 2A). Hence, impaired surface expression of the GM-CSF receptor could not cause the defective STAT5 signaling in CMV-MoDCs.
The secretion of a sGMRα by monocytes can be stimulated by an inflammatory stimulation.13 We therefore measured the sGMRα in the culture media of MoDCs, CMV-MoDCs, and UVCMV-MoDCs by ELISA. The medium from CMV-MoDCs contained much more sGMRα than that from MoDCs. Inactivation of CMV by UV irradiation resulted in the concentration of sGMR being similar to that of controls, again indicating that live virus is required for the modified phenotype of CMV-MoDCs (supplemental Figure 2B). However, the concentration of sGMRα in the supernatants of MoDCs was as high as those of CMV-MoDCs in some experiments, although the phenotype of the cells was unchanged (data not shown), indicating that other factors were involved.
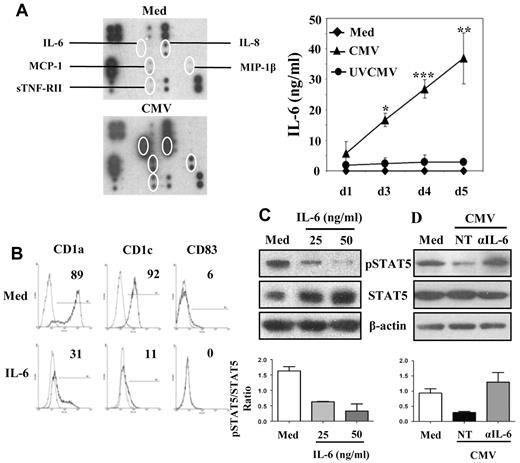
We assayed the inflammatory cytokines in culture supernatants of MoDCs and CMV-MoDCs with the use of an Ab array to investigate the soluble factors responsible for the defective STAT5 activation. Infected monocytes secreted higher concentrations of IL-6 and IL-8 than did MoDCs after 2 days in culture (Figure 3A). Infected monocytes also secreted more MIP-1β, sTNFRII, and MCP-1, although to a lesser extent. The IL-6 in the culture medium of cells allowed to differentiate for 1-5 days was also assayed by CBA. IL-6 was undetectable in supernatant from MoDCs throughout the 5 days and increased slightly in supernatant from UVCMV-MoDCs from day 1 (1.8 ng/mL) to day 4 (2.9 ng/mL). In contrast, the IL-6 in the supernatant from CMV-MoDCs increased dramatically from 5.7 ng/mL (day 1) to 37 ng/mL (day 5). Because IL-6 influences monocyte differentiation and DC function,14,15 we examined its influence on the CMV-MoDC phenotype and function. Monocytes differentiated in standard medium supplemented with IL-6 (IL-6-MoDC) had no CD1a or CD1c on their surfaces (Figure 3B). STAT5 phosphorylation was inhibited in cells cultured in medium containing IL-6 concentrations similar to those in the supernatants of CMV-MoDCs (Figure 3C). We monitored STAT5 phosphorylation in CMV-MoDCs differentiated in medium containing anti–IL-6 Ab to confirm that IL-6 influenced the acquisition of the CMV-MoDC phenotype (Figure 3D). STAT5 was phosphorylated in cells when IL-6 was blocked, indicating that IL-6 is important in the paracrine inhibition of STAT5 signaling in CMV-MoDCs.
Increased IL-6 secretion by CMV-MoDCs and inhibition of STAT5 phosphorylation by IL-6. Monocytes were differentiated into DCs by culture for 5 days with GM-CSF and IL-13 after mock-infection (Med), infection with live (CMV-MoDC), or UV-inactivated CMV (UVCMV). (A) MoDCs (Med) and CMV-MoDCs (CMV) were differentiated by culture for 5 days; their inflammatory cytokines were assayed with a dedicated Ab array (Raybiotech), and IL-6 was quantified by CBA. (B) Monocytes were also differentiated in the presence of recombinant IL-6. The percentages of cells expressing CD1a, CD1c, and CD83 were determined by flow cytometry. (C-D) Total and phosphorylated STAT5 (pSTAT5) were analyzed by Western blotting of cell lysates of IL-6–MoDCs (IL-6) and CMV-MoDCs (CMV) or CMV-MoDCs treated with anti–IL-6 neutralizing Abs (αIL-6; 15 μg/mL). The histograms below their respective Western blots represent the quantification of 3 independent experiments. Error bars indicate the SD. Representative of ≥ 3 independent experiments.
Increased IL-6 secretion by CMV-MoDCs and inhibition of STAT5 phosphorylation by IL-6. Monocytes were differentiated into DCs by culture for 5 days with GM-CSF and IL-13 after mock-infection (Med), infection with live (CMV-MoDC), or UV-inactivated CMV (UVCMV). (A) MoDCs (Med) and CMV-MoDCs (CMV) were differentiated by culture for 5 days; their inflammatory cytokines were assayed with a dedicated Ab array (Raybiotech), and IL-6 was quantified by CBA. (B) Monocytes were also differentiated in the presence of recombinant IL-6. The percentages of cells expressing CD1a, CD1c, and CD83 were determined by flow cytometry. (C-D) Total and phosphorylated STAT5 (pSTAT5) were analyzed by Western blotting of cell lysates of IL-6–MoDCs (IL-6) and CMV-MoDCs (CMV) or CMV-MoDCs treated with anti–IL-6 neutralizing Abs (αIL-6; 15 μg/mL). The histograms below their respective Western blots represent the quantification of 3 independent experiments. Error bars indicate the SD. Representative of ≥ 3 independent experiments.
Influence of SOCS3 on GM-CSF–mediated STAT5 signaling in CMV-MoDCs
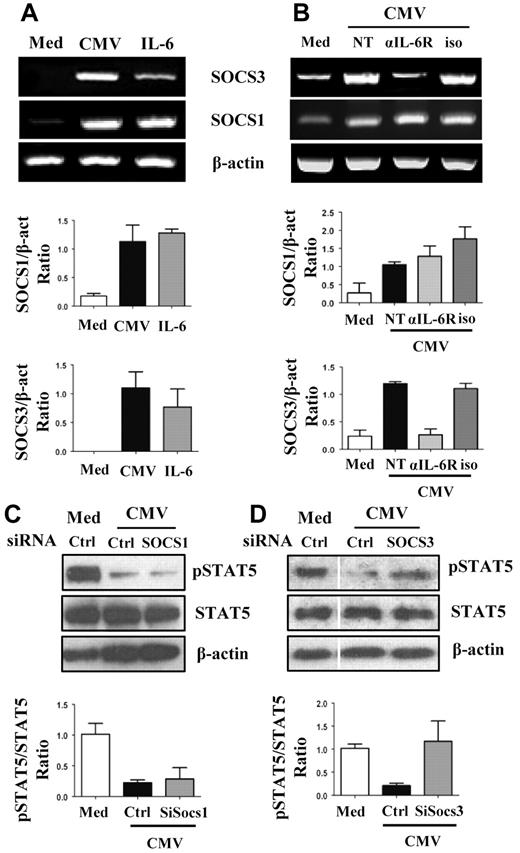
SOCS plays a key role in inhibiting the Jak/Stat pathways.16 We therefore monitored SOCS1 and SOCS3 synthesis in MoDCs, CMV-MoDCs, and IL6-MoDCs. The concentrations of mRNAs encoding SOCS1 and SOCS3 were higher in CMV-MoDCs and in IL-6-MoDCs than in MoDCs (Figure 4A). We then blocked IL-6 signaling with Abs directed against the IL-6 receptor to determine how IL-6 inhibited the GM-CSF–mediated activation of STAT5 in CMV-MoDCs. Secreted IL-6 stimulated the synthesis of SOCS3 but not SOCS1 (Figure 4B). Moreover, treating CMV-MoDCs with SOCS3 small interfering RNA restored the phosphorylation of STAT5, whereas siSOCS1 did not (Figure 4C-D; supplemental Figure 3). These data strongly suggest that the IL-6–induced synthesis of SOCS3 is at least partially responsible for blocking GM-CSF–mediated STAT5 signaling in CMV-MoDCs.
Increased SOCS3 and inhibition of STAT5 phosphorylation in CMV-MoDCs. (A-B) Mock-infected (Med), HCMV-infected (CMV), or IL-6–treated (50 ng/mL) monocytes were differentiated by culture with GM-CSF and IL-13 for 5 days. Alternatively, medium (NT), or neutralizing Ab against IL-6 receptor (αIL-6R; 15 μg/mL) or isotype Ab (iso; 15 μg/mL) was added to infected cells during differentiation. SOCS1, SOCS3, and β-actin mRNAs were analyzed by RT-PCR. (C-D) Total and phosphorylated STAT5 protein (pSTAT5) were assessed by Western blotting of mock-infected (Med) and HCMV-infected (CMV) cells. Cells were transfected with SOCS1, SOCS3, and control small interfering RNA (siRNA; Ctrl) as indicated. β-actin was used as control. The histograms below RT-PCR (A-B) and Western blots (C-D) represent the quantification of 3 independent experiments.
Increased SOCS3 and inhibition of STAT5 phosphorylation in CMV-MoDCs. (A-B) Mock-infected (Med), HCMV-infected (CMV), or IL-6–treated (50 ng/mL) monocytes were differentiated by culture with GM-CSF and IL-13 for 5 days. Alternatively, medium (NT), or neutralizing Ab against IL-6 receptor (αIL-6R; 15 μg/mL) or isotype Ab (iso; 15 μg/mL) was added to infected cells during differentiation. SOCS1, SOCS3, and β-actin mRNAs were analyzed by RT-PCR. (C-D) Total and phosphorylated STAT5 protein (pSTAT5) were assessed by Western blotting of mock-infected (Med) and HCMV-infected (CMV) cells. Cells were transfected with SOCS1, SOCS3, and control small interfering RNA (siRNA; Ctrl) as indicated. β-actin was used as control. The histograms below RT-PCR (A-B) and Western blots (C-D) represent the quantification of 3 independent experiments.
IL-6 and the inhibition of phagocytosis in CMV-MoDCs
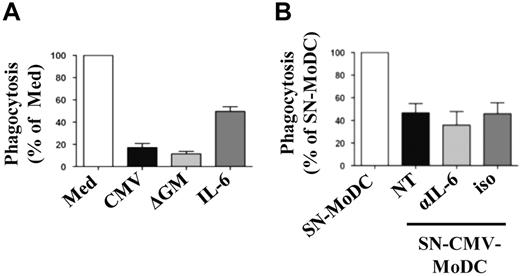
Abnormal GM-CSF activity had been reported to decrease the phagocytic activity of neutrophils17 and MoDCs.18 We have previously shown that the phagocytosis of infected dead cells by MoDCs is crucial for Ag cross-presentation to CD8+ T cells.19 We have now investigated the phagocytic function of CMV-MoDCs by adding PKH26-labeled dead cells to MoDCs, CMV-MoDCs, ΔGM-MoDCs, IL-6–MoDCs, (SN-MoDC)–MoDCs, and (SNCMV-MoDC)–MoDCs. The phagocytic activity of CMV-MoDCs and ΔGM-MoDCs was only 20% that of MoDCs, suggesting that GM-CSF signaling also plays a role in this process (Figure 5A). The CMV-induced IL-6–mediated inhibition of STAT5 accounted at least in part for this defect in CMV-MoDCs because the phagocytic capacity of IL-6–MoDCs was ∼ 50% that of MoDCs. However, although adding conditioned medium from CMV-MoDCs to MoDCs mimicked the effect of IL-6 (50% reduction; Figure 5B), neutralization of IL-6 with anti–IL-6 Abs did not restore phagocytosis. Thus, IL-6 may not be the only secreted factor involved in this effect. Assays of phagocytosis which used E coli bioparticles gave similar results (data not shown). Thus, the defective phagocytosis of dead cells by CMV-MoDCs probably involves both autocrine and paracrine inhibition of STAT5 signaling, some of which involves IL-6.
IL-6 is not crucial for inhibition of phagocytosis in CMV-MoDCs. Monocytes differentiated for 5 days as described below were cocultured with PKH-26–labeled apoptotic fibroblasts for 24 hours. Phagocytic activity was determined as described in “Phagocytosis assays.” (A) Monocytes were differentiated in standard medium (Med), after infection (CMV), with added IL-6 (50 ng/mL), or without GM-CSF (ΔGM). (B) Monocytes were differentiated in the presence of supernatant from MoDCs (SN-MoDC) or CMV-MoDCs (SN-CMV-MoDC) supplemented with medium (NT), IL-6 neutralizing Ab (αIL-6; 15 μg/mL), or isotype control Ab (iso; 15 μg/mL). Results are expressed as the percentage of phagocytic activity relative to MoDCs (100%). Error bars indicate the SD. Representative of ≥ 3 independent experiments.
IL-6 is not crucial for inhibition of phagocytosis in CMV-MoDCs. Monocytes differentiated for 5 days as described below were cocultured with PKH-26–labeled apoptotic fibroblasts for 24 hours. Phagocytic activity was determined as described in “Phagocytosis assays.” (A) Monocytes were differentiated in standard medium (Med), after infection (CMV), with added IL-6 (50 ng/mL), or without GM-CSF (ΔGM). (B) Monocytes were differentiated in the presence of supernatant from MoDCs (SN-MoDC) or CMV-MoDCs (SN-CMV-MoDC) supplemented with medium (NT), IL-6 neutralizing Ab (αIL-6; 15 μg/mL), or isotype control Ab (iso; 15 μg/mL). Results are expressed as the percentage of phagocytic activity relative to MoDCs (100%). Error bars indicate the SD. Representative of ≥ 3 independent experiments.
IL-6 secretion and blockage of allogeneic T-cell proliferation and TH1 polarization by CMV-MoDCs
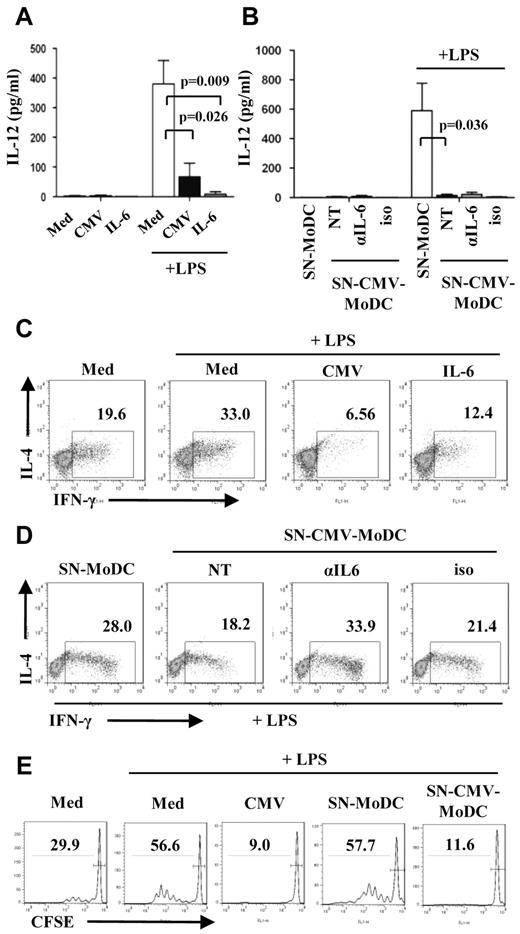
DCs and their environment help determine the programming of T cells toward TH1 or TH2 subsets. We have examined how the polarization of naive CD4+ T cells is influenced by CMV-MoDCs. One of the mechanisms by which DCs drive TH1 differentiation involves IL-12p70. It is generally agreed that the secretion of IFN-γ by differentiated T cells correlates with the amount of IL-12 secreted by stimulated MoDCs2 in mixed lymphocyte reactions (MLRs),20 whereas IL-4 production promotes TH2 differentiation. We measured the amount of IL-12p70 secreted by MoDCs, CMV-MoDCs, and IL-6–MoDCs that had been stimulated with LPS, a known activator of IL-12 secretion. CMV-MoDCs were much less responsive (67 pg/mL) than MoDCs (380 pg/mL), whereas IL-6–MoDCs produced almost no detectable IL-12p70 (Figure 6A). No DC population secreted any IL-12p70 without LPS stimulation. The production of IL-12p70 by stimulated MoDCs was inhibited by adding CMV-MoDC supernatants. However, neutralization of IL-6 did not restore IL-12p70 production, suggesting that IL-6 is not the only factor involved (Figure 6B). We used MLR experiments to study TH polarization by DCs. Unactivated and LPS-activated MoDCs, CMV-MoDCs, and IL-6-MoDCs were washed and cocultured for 8 days with allogeneic naive T cells. The TH1 and TH2 responses were assessed by flow cytometry by measuring the IL-4 and IFN-γ produced by T cells. MoDCs promoted the production of IFN-γ in 19.6% and LPS-stimulated MoDCs in 33.0% of T cells (Figure 6C), correlating the ability of MoDCs to potentiate the TH1 response with IL-12p70 secretion. In contrast, LPS-stimulated CMV-MoDCs induced IFN-γ secretion in very few T cells (6.56%). Few T cells secreted IFN-γ when incubated with LPS-unstimulated CMV-MoDCs, suggesting that CMV-MoDCs are intrinsically unable to prime a TH1 response (data not shown). IL-6–MoDCs treated with LPS also showed a reduced capacity to prime TH1 (Figure 6C), suggesting that IL-6 alters the ability of MoDCs to polarize T cells toward TH1. T cells stimulated with MoDCs that had been treated with CMV-MoDC conditioned medium (SN-CMV-MoDCs) and LPS formed fewer IFN-γ–producing T cells (18%) than did those stimulated with supernatants from MoDCs (28.0%). The rate of TH1 formation was restored (33.9%) by blocking the IL-6 in these supernatants (Figure 6D). The IL-6 secreted by CMV-MoDCs during differentiation therefore prevents them from stimulating T cells to form TH1. None of the DC populations induced detectable IL-4– (Figure 6) or IL-17– (data not shown) secreting cells after stimulation with LPS. This indicates that CMV-MoDCs do not stimulate naive T cells to form either TH2 or TH17, although they were able to differentiate toward TH2 when stimulated with MoDCs treated with IL-4 and anti–IFN-γ Abs (supplemental Figure 4). Besides impaired TH1 commitment, incubation of naive T cells with LPS-stimulated CMV-MoDCs or MoDCs conditioned with SN-CMV-MoDCs blocked their proliferation (Figure 6E). Thus, the infection of monocytes with CMV causes profound changes in the ability of CMV-MoDCs to promote TH1 proliferation and activation, partly because of secreted IL-6.
Blockage of allogenic T-cell proliferation and TH1 polarization by CMV-MoDCs is partially because of IL-6 secretion. (A,C) Monocytes were either mock-infected (Med) or infected with CMV (CMV) or supplemented with IL-6 (50 ng/mL), differentiated in the presence of GM-CSF and IL-13 for 5 days and treated (or not) with ultrapure LPS (200 ng/mL) for 24 hours. (B,D) Supernatant from MoDCs (SN-MoDC) or CMV-MoDCs (SN-CMV-MoDC) were added to monocytes before differentiation in the presence (αIL-6; 15 μg/mL) or not (NT) of anti–IL-6 neutralizing Abs or isotypic Ab as control (iso; 15 μg/mL). (A-B) IL-12 was quantified by CBA on day 6 of culture. (C-D) DCs derived from monocytes as described above were cocultured with naive allogenic T cells for 8 days after adding IL-2 (50 U/mL) on day 5. Cells were fixed, permeabilized, double-labeled with anti–IL-4 and anti–IFN-γ Abs, and analyzed by flow cytometry. (E) Monocytes were either mock-infected (Med), or infected with CMV (CMV), or treated with supernatant (SN) as indicated before differentiation. Naive allogenic T cells were stained with CFSE and cocultured for 5 days with monocyte-derived DCs as indicated. The proliferation of T cells was analyzed by flow cytometry. Error bars represent SD. Representative of ≥ 3 independent experiments. P values were obtained with Student t test.
Blockage of allogenic T-cell proliferation and TH1 polarization by CMV-MoDCs is partially because of IL-6 secretion. (A,C) Monocytes were either mock-infected (Med) or infected with CMV (CMV) or supplemented with IL-6 (50 ng/mL), differentiated in the presence of GM-CSF and IL-13 for 5 days and treated (or not) with ultrapure LPS (200 ng/mL) for 24 hours. (B,D) Supernatant from MoDCs (SN-MoDC) or CMV-MoDCs (SN-CMV-MoDC) were added to monocytes before differentiation in the presence (αIL-6; 15 μg/mL) or not (NT) of anti–IL-6 neutralizing Abs or isotypic Ab as control (iso; 15 μg/mL). (A-B) IL-12 was quantified by CBA on day 6 of culture. (C-D) DCs derived from monocytes as described above were cocultured with naive allogenic T cells for 8 days after adding IL-2 (50 U/mL) on day 5. Cells were fixed, permeabilized, double-labeled with anti–IL-4 and anti–IFN-γ Abs, and analyzed by flow cytometry. (E) Monocytes were either mock-infected (Med), or infected with CMV (CMV), or treated with supernatant (SN) as indicated before differentiation. Naive allogenic T cells were stained with CFSE and cocultured for 5 days with monocyte-derived DCs as indicated. The proliferation of T cells was analyzed by flow cytometry. Error bars represent SD. Representative of ≥ 3 independent experiments. P values were obtained with Student t test.
Correlation of the phenotype of cells from patients with HCMV viremia and CMV-MoDCs
We investigated the relevance of these observations in vivo with the use of monocytes from patients who received a transplant experiencing CMV viremia (Table 1). The monocytes were differentiated into DCs (MoDCs). The MoDCs from viremic patients formed cell clusters (Figure 7A), a feature indicating cell activation as observed in cultured CMV-MoDCs. The medium recovered after 5 days of differentiation contained no detectable viral genome, suggesting the absence of virus replication. Significantly fewer MoDCs from CMV-positive patients bore CD1a than those from CMV-negative patients (Figure 7B). IL-6 was quantified in the growth medium and blood plasma, and the phosphorylation of STAT5 in MoDCs was analyzed. Patients 7 and 8 had very high virus loads (480 000 and 340 000 copies/mL, respectively); these were correlated with large amounts of IL-6 secreted by their MoDCs and in their plasma (Figure 7C-D) and few CD1a+ cells (Figure 7A). Furthermore, the MoDCs derived from all the viremic patients lost their ability to phosphorylate STAT5 on differentiation (Figure 7E). These data reinforce the in vitro findings of the role of IL-6 and the subsequently defective GM-CSF signaling in DCs derived from infected monocytes.
Demographic, immunosuppressive regimen, and virologic data from organ recipients
| Patients . | Sex . | Age, y . | Transplanted organ . | Time from transplantation, mo . | Immunossupressive regimen . | CMV serologic status before transplantation . | CMV viremia, copies/mL . |
|---|---|---|---|---|---|---|---|
| CMV− | |||||||
| 1 | M | 58 | Heart | 156 | CsA/MPA/CS | R+ | ND |
| 2 | M | 45 | Kidney | 168 | Tac/MPA | R+ | ND |
| 3 | M | 63 | Liver | 1 | Tac/MPA/CS | R+ | ND |
| 4 | F | 63 | Kidney | 6 | Tac/MPA/CS | R+ | ND |
| 5 | M | 67 | Kidney | 26 | Tac/MPA/CS | R− | ND |
| CMV+ | |||||||
| 6 | M | 50 | Kidney | 240 | MPA/CS | R+ | 36 000 |
| 7 | M | 56 | Kidney | 8 | Tac/MPA/CS | R− | 480 000 |
| 8 | M | 49 | Kidney | 2 | Jak3i/MPA/CS | R+ | 341 000 |
| 9 | M | 20 | Kidney | 12 | Tac/MPA/CS | R+ | 67 000 |
| Patients . | Sex . | Age, y . | Transplanted organ . | Time from transplantation, mo . | Immunossupressive regimen . | CMV serologic status before transplantation . | CMV viremia, copies/mL . |
|---|---|---|---|---|---|---|---|
| CMV− | |||||||
| 1 | M | 58 | Heart | 156 | CsA/MPA/CS | R+ | ND |
| 2 | M | 45 | Kidney | 168 | Tac/MPA | R+ | ND |
| 3 | M | 63 | Liver | 1 | Tac/MPA/CS | R+ | ND |
| 4 | F | 63 | Kidney | 6 | Tac/MPA/CS | R+ | ND |
| 5 | M | 67 | Kidney | 26 | Tac/MPA/CS | R− | ND |
| CMV+ | |||||||
| 6 | M | 50 | Kidney | 240 | MPA/CS | R+ | 36 000 |
| 7 | M | 56 | Kidney | 8 | Tac/MPA/CS | R− | 480 000 |
| 8 | M | 49 | Kidney | 2 | Jak3i/MPA/CS | R+ | 341 000 |
| 9 | M | 20 | Kidney | 12 | Tac/MPA/CS | R+ | 67 000 |
CsA indicates cyclosporin A; MPA, mycophenolic acid; CS, corticosteroid; R, recipient; ND, not detectable; Tac, tacrolimus; and JAK3i, JAK3 inhibitor.
Reduced CD1a and pSTAT5 in MoDCs from viremic patients who had received a transplant. Monocytes were isolated from the blood of patients who had received a transplant with the use of Ficoll gradients followed by sorting with anti-CD14 magnetic separation beads. Cells were differentiated into MoDCs in culture medium containing GM-CSF and IL-13 for 5 days. (A) Light microscopy of monocytes after 3 days in culture (×200; Zeiss, Telaval 31). Images were acquired with a Nikon Eclipse TE 2000_v; 20×/0.35 objective; culture medium 37°C; camera: Nikon DX71200C; Adobe Photo-shop 9.02. (B) The percentage of MoDCs expressing CD1a was determined by flow cytometry. (C-D) Amounts of IL-6 secreted by MoDCs (C) and in plasma of patients who received a transplant (D) were determined by CBA. (E) Total and phosphorylated STAT5 (pSTAT5) was assessed by Western blotting of MoDC extracts with β-actin as control. The histogram below the Western blots represents quantification of proteins from all the patients. Lines represent median values. Mann-Whitney U tests were used to determine statistical significance.
Reduced CD1a and pSTAT5 in MoDCs from viremic patients who had received a transplant. Monocytes were isolated from the blood of patients who had received a transplant with the use of Ficoll gradients followed by sorting with anti-CD14 magnetic separation beads. Cells were differentiated into MoDCs in culture medium containing GM-CSF and IL-13 for 5 days. (A) Light microscopy of monocytes after 3 days in culture (×200; Zeiss, Telaval 31). Images were acquired with a Nikon Eclipse TE 2000_v; 20×/0.35 objective; culture medium 37°C; camera: Nikon DX71200C; Adobe Photo-shop 9.02. (B) The percentage of MoDCs expressing CD1a was determined by flow cytometry. (C-D) Amounts of IL-6 secreted by MoDCs (C) and in plasma of patients who received a transplant (D) were determined by CBA. (E) Total and phosphorylated STAT5 (pSTAT5) was assessed by Western blotting of MoDC extracts with β-actin as control. The histogram below the Western blots represents quantification of proteins from all the patients. Lines represent median values. Mann-Whitney U tests were used to determine statistical significance.
Discussion
Infection by HCMV is known to disturb both innate and adaptive immune responses. DCs are main virus targets to prevent the stimulation of antiviral T cells. However, this is always incomplete because the virus immune evasion functions are circumvented by the host.3 Because monocytes are the precursors of DCs in vivo and HCMV may infect them to further establish latency, we examined the effects of infection on monocyte differentiation and DC dysfunction.
Monocytes were differentiated into DCs by the coordinated action of GM-CSF and IL-13, which models the phenotype and function of inflammatory and migratory DCs in vivo.21 MoDCs infected by HCMV lost their standard CD1a+ immature phenotype and acquired the maturation marker CD83, probably because of the bystander effect of TNF-α, a known inducer of DC maturation, secreted by infected cells in the early phase of differentiation. Discrepancies with published data7 about both cell maturation and the requirement for live virus could be partly because of the use by others of the laboratory strain AD169 that has a 19-kbp genome deletion not found in clinical strains. CD1a and CD1c bind and present lipid Ags to T cells,22 in addition to being DC phenotype markers. Their expressions are subnormal in CMV-MoDCs and in infected DCs,23 but their role in controlling HCMV infection remains to be studied. We have found evidence for a powerful paracrine mechanism that leads to modification of the phenotype of the whole population of CMV-MoDCs. Even though only a fraction of the cells contained the IE/E virus proteins, this viral protein expression seems to be critical for initiating this process. We considered the possibility that there was a failure of GM-CSF signaling in CMV-MoDCs because GM-CSF is needed to generate inflammatory DCs from monocytes in vivo21 and because there are functional and phenotypic defects (CD1a−) in MoDCs generated in the absence of GM-CSF.11 We showed that STAT5 activation was inhibited in the whole population of CMV-MoDCs, which may block GM-CSF signaling, and that this was because of soluble factors secreted by a fraction of infected monocytes.
Our first hypothesis was that the soluble GM-CSF receptor sGMRα blocked the binding of GM-CSF to its receptor. The significantly increased sGMRα concentration in some preparations of CMV-MoDCs pointed to its implication in impairing STAT5 signaling. However, individual variations in the concentrations of constitutive sGMRα in monocytes and our inability to neutralize sGMRα in culture supernatants have made it impossible to decide on the effect of sGMRα at this stage. One of the inflammatory cytokines induced by HCMV, IL-6, is suspected to be a key factor in the failure of organ and BM transplantations.24 IL-6 has also been identified in the HCMV secretome of endothelial cells as a mediator of angiogenesis and cell survival25 and in tumor cells as a paracrine factor in tumor progression and vascularization through activation of the STAT3 cascade.26 We find that DCs derived from the differentiation of monocytes supplemented with IL-6 bore no CD1a or CD1c. Moreover, neutralizing IL-6 during the differentiation of infected monocytes restored phosphorylation of STAT5, suggesting that IL-6 blocks GM-CSF receptor signaling. Previous data have shown that IE1 increases the activity of the IL-6 promoter,27 which may explain why UV-inactivated virus barely increased IL-6 secretion and had no effect on STAT5 phosphorylation. Thus, the secretion of IL-6 by UVCMV is initiated by the activation of NF-κB but cannot be maintained because of blockade of IE1 transcription after UV irradiation.
The inhibition of STAT5 phosphorylation in DCs generated from monocytes involves SOCS3 and SOCS1,18 and SOCS1 can abolish GM-CSF signal transduction in DC precursors28 but not in conditions of viral infection. Furthermore, induction of SOCS3 by IL-6 is crucial for the regulation of the inflammatory response, whereas the IL-6–mediated induction of SOCS3 could inhibit insulin receptor signaling.16,29 We have now obtained evidence that IL-6 secreted by CMV-MoDCs is crucial for the induction of SOCS3, which in turn leads to the inhibition of STAT5 phosphorylation that may reflect blockage of the GM-CSF receptor signaling by SOCS3 but not SOCS1.
The influence of STAT5 blockage by SOCS on the phagocytic activity of MoDCs was deduced18 from the inability of cells to internalize dextran. Defects in the phagocytosis of dextran and yeast particles by CMV-MoDCs have been reported,7 but they have little physiologic relevance. The sequestration of tumor and viral Ags through phagocytosis of dead cells by DCs is a key step in T-cell activation.30,31 We examined the capacity of CMV-MoDCs to ingest apoptotic and necrotic bodies prepared from infected fibroblasts because this phagocytosis is a critical step in the cross-presentation of HCMV Ags to CD8+ T cells.19 The inhibition of this process involved soluble factors secreted by CMV-MoDCs, but IL-6 was not the only factor involved. It has been reported that IL-6 can cause monocytes to differentiate to macrophages rather than DCs,15 but no information was provided about their phagocytic capacities. IL-6 can also reactivate latent HCMV in a model of primary monocytes infected in vitro,32 which makes our findings also relevant to the differentiation of latently infected monocytes. Giving GM-CSF to patients with carcinoma improved their clearance of apoptotic cells,33 suggesting a link between the activation of STAT5 and the expression of genes promoting phagocytosis. Because PU.1, a transcription factor induced by pSTAT5, drives such genes,34 we looked for PU.1 mRNA and protein in CMV-MoDCs, but their expressions were similar to those in MoDCs (data not shown). CD36 and αvβ5 integrin could be involved in the uptake of apoptotic cells by DCs,31 but we observed no alteration in CD36 in DCs (data not shown). The αvβ5 integrin remains to be explored. Because inhibition of phagocytosis could provide the virus with a new way of escaping the host immune response, it might be worth exploring the capacity of CMV-MoDCs to cross-present viral Ag to CD8+ T cells.
We performed MLRs with CMV-MoDCs as stimulators because IL-6 influences T-cell activation and the commitment of naive T cells to form TH1 or TH2. Cells were treated with LPS, which induces APCs to secrete IL-12 and is usually a prerequisite for naive cells to produce IFN-γ. Our data showing that HCMV inhibited IL-12p70 secretion by LPS-activated CMV-MoDCs extend previous reports that used infected DCs from adults35 and neonates.36 The defect in IL-12p70 secretion by CMV-MoDCs was not because of IL-6, even though SOCS1, a negative regulator of both IL-12 synthesis37,38 and TH1 promotion,39 was induced. Nevertheless, the secretion of IFN-γ by T cells was restored when the IL-6 in supernatants of CMV-MoDCs was neutralized before adding it to T cells, suggesting the dissociation of IL-12p70 secretion from TH1 commitment as reported in experiments that implicated Notch signaling.40 We have demonstrated the capacity of HCMV to impair the commitment of T cells to form TH1, by a mechanism that may disturb the overall host response. Protein array studies have reported that CMV-MoDCs secreted more MIP-1β and IL-6 than did MoDCs. The chemokine MIP-1β reduces the polarization of T cells to TH1 when added to LPS-stimulated MoDCs in MLRs.20 Thus, HCMV may interfere with the polarization of naive T cells via MIP-1β and the resulting ERK activation, but this needs further investigation. The negative regulation of GM-CSF receptor signaling by a Src-like adaptor41 and the disruption of DC metabolism because of SOCS3 blocking M2 pyruvate kinase42 have yet to be investigated. PPARγ activation may be involved in the alterations in DC phenotype and function43 ; this is being investigated because we have shown that HCMV activates PPARγ.44
The relevance of our in vitro observations is supported by data obtained from naturally infected MoDCs derived from viremic patients whose high virus loads were correlated with the absence of CD1a, confirming previous observations.8 The blockage of STAT5 phosphorylation in MoDCs and the high IL-6 contents of both MoDC supernatants and patients' blood plasma further suggest abnormal GM-CSF signaling because of HCMV. Because MoDCs from patients produced no virus suggests that the ability of monocytes infected in vivo to differentiate into standard DCs was modified. HCMV may enhance the host's susceptibility to secondary bacterial infections, because GM-CSF is essential for neutrophil function and proliferation. These findings provide new insights into how HCMV infection impairs the overall innate and adaptive responses, and these should be taken into account when using immunomodulatory therapies that are based on GM-CSF to treat infectious diseases in solid-organ transplant recipients,45 as in prophylaxis against sepsis in preterm neonates46 and in fighting cancer.47
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all the patients who donated blood, S. Chavanas, E. Champagne, and K. Leghmari for critically reading the manuscript, and F. L'faqihi and V. Duplan for technical assistance in flow cytometry. The English text was edited by Dr Owen Parkes.
This work was supported by institutional grants from Inserm. J.C. is supported by a Fondation pour la Recherche Médicale fellowship.
Authorship
Contribution: J.C. designed and performed experiments, analyzed data, and wrote the manuscript; H.M. designed experiments; B.M. prepared HCMV-BAC; B.R. analyzed data; C.M. performed virologic diagnosis; H.W. collected patients and analyzed data; A.C., C.V., P.R., N.K., L.R., and G.H. collected patients; C.D. designed experiments, analyzed data, and wrote the manuscript; and all the authors approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christian Davrinche, Inserm 1043, CPTP Bat B, CHU Purpan, Toulouse, 31024 France; e-mail: christian.davrinche@inserm.fr.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal