Abstract
The ability to form anastomoses with the host circulation is essential for vascular networks incorporated within cell-seeded bioengineered tissues. Here, we tested whether and how rapidly human endothelial colony forming cell (ECFC)/mesenchymal progenitor cell (MPC)–derived bioengineered vessels, originally perfused in one mouse, could become reperfused in a secondary mouse. Using in vivo labeling with a systemically injected mixture of human- and murine-specific lectins, we demonstrate that ECFC/MPC blood vessels reconnect and are perfused at day 3 after transplantation. Furthermore, we quantified the longitudinal change in perfusion volume in the same implants before and after transplantation using contrast-enhanced micro-ultrasonic imaging. Perfusion was restored at day 3 after transplantation and increased with time, suggesting an important new feature of ECFC/MPC blood vessels: the bioengineered vessels can reconnect with the vasculature when transplanted to a new site. This feature extends the potential applications of this postnatal progenitor cell-based technology for transplantable large tissue-engineered constructs.
Introduction
Bioengineering has emerged as a promising approach to replace dysfunctional tissues and organs. In addition to integrity and function, bioengineered constructs must have a vascular supply for gas exchange, nutrient delivery, and waste product elimination. Bioengineered constructs in clinical use to date are limited to thin and/or avascular tissues, such as skin1,2 and cartilage,2,3 wherein diffusion of oxygen and nutrients is sufficient.
Significant progress has been made toward building preassembled networks using human endothelial cells from sources, including umbilical vein,4 embryonic stem cells,5 bone marrow,6 blood,7 and adipose tissue.8 However, few studies have focused on the ability of preassembled networks to connect with the host vasculature. In 1975, Ausprunk et al studied the fate of endogenous blood vessels in tumor, adult and embryonic tissues implanted on the chorioallantoic membrane of avian embryos.9 Tumor vessels regressed, but new vessels from the chorioallantois grew (ie, tumor angiogenesis). Adult tissue vessels regressed and were not replaced. In contrast, vessels in the embryonic tissue remained intact and formed anastomoses with the avian vessels. This ability to reconnect with new vasculatures would be highly desirable in bioengineered vascular networks. Therefore, we set out to determine whether human bioengineered blood vessels are akin to the embryonic vessels. We and others showed that human endothelial colony forming cells (ECFCs; called EPCs in our previous papers) combined with perivascular cell precursors, such as mesenchymal progenitor cells (MPCs), form functional blood vessels when coimplanted into immunodeficient mice.7,10-13 We used this approach to design a transplantation model to test whether human ECFC/MPC vessels, formed in a donor mouse, can be disconnected and then reconnected with vasculature in a secondary recipient mouse.
To detect functional anastomoses between bioengineered and host vessels, we implemented 2 new techniques: (1) in vivo labeling by tail vein injection of a mixture of human- and murine-specific fluorescently conjugated lectins, and (2) contrast-enhanced micro-ultrasonic imaging to visualize and quantify perfusion over time in individual mice. Herein, we use these techniques to demonstrate the ability of transplanted bioengineered human vessels to disconnect/reconnect with the recipient murine vasculature and rapidly reestablish perfusion.
Methods
A total of 2 × 106 cells suspended in 200 μL Matrigel at a ratio of 2:3 (ECFCs/MPCs) was injected subcutaneously into each severe combined immunodeficiency (SCID) mouse.11 Matrigel implants were harvested at days 1, 3, 5, and 7 for microvessel density (MVD) analysis or transplanted into secondary mice at day 7. Perfused human and murine vessels were identified by tail-vein injection of a mixture of rhodamine (red)–conjugated Ulex europaeus agglutinin I (UEA-I) and FITC (green)–conjugated Griffonia simplifolia isolectin B4 (GS-IB4), and counted using Leica TCS SP2 Acousto-Optical Beam Splitter confocal system equipped with a DMIRE2 inverted microscope (Diode 405 nm, Argon 488 nm, and HeNe 594 nm; Lecia Microsystems) at room temperature. A 40×/1.25 oil objective (for Figure 1B and E) or 20×/0.7 oil objective (for Figure 1C) was used. Micro-ultrasonic imaging was used to measure perfused vascular volume of implants longitudinally (Vevo 2100 high resolution ultrasound system, VisualSonics Inc). Ultrasound video data were analyzed using Vevo 2100 software. Human endothelial cells were isolated from discarded nonidentified umbilical cord blood or newborn foreskin obtained under an institutional review board-approved protocol. Animal experiments were conducted under a protocol approved by the Institutional Animal Care and Use Committee at Children's Hospital Boston in an Association for Assessment and Accreditation of Laboratory Animal Care-approved facility. Detailed methods are in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Results and discussion
Detection of perfused human and murine vessels in vivo
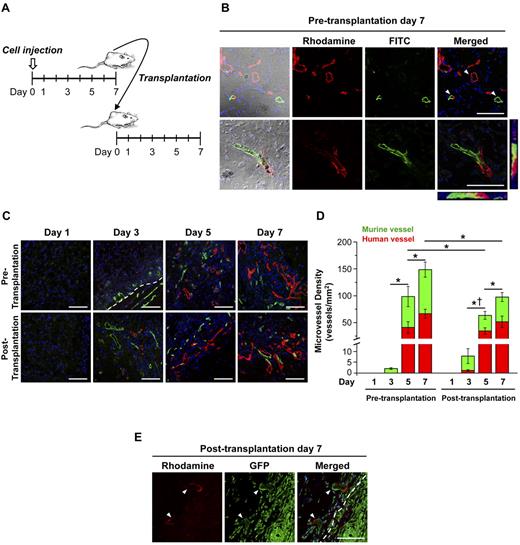
To determine whether ECFC/MPC-vascular networks can rapidly connect to the host vasculature in a new implantation site, we transplanted ECFC/MPC-Matrigel implants from donor to recipient mice (Figure 1A). Perfused vessels before and after transplantation were identified by tail-vein injection of rhodamine-conjugated UEA-I, a lectin specific for human endothelium, and FITC-conjugated GS-IB4, a lectin specific for murine endothelium. Three distinct patterns were observed: UEA-I–positive human vessels, GS-IB4–positive murine vessels, and UEA-I/GS-IB4–double-positive chimeric vessels (Figure 1B). This illustrates several advantages of in vivo labeling by tail-vein injection of the lectin mixture: (1) identifying functional perfused vessels in contrast to dead-end tubular structures, (2) distinguishing human from murine vessels, and (3) visualizing the site of anastomosis. Point 1 is essential to exclude vessels retained within implants after transplantation but not reperfused.
MVD analysis before and after transplantation. Matrigel implants were harvested at multiple time points before and after transplantation. (A) Diagram of transplantation model. (B-E) Perfused human and murine vessels were identified by tail-vein injection of a mixture of rhodamine (red)–conjugated UEA-I and FITC (green)–conjugated GS-IB4. (B) Representative confocal images of 3 distinct patterns of lectin-labeled vessels in the implants at pretransplantation day 7. White arrowheads indicate human, murine, and chimeric vessels. (C) Representative confocal images of lectin-labeled vessels in the implants at multiple time points. White dotted line in the day 3 panel of pretransplantation indicates the border of implant. (D) Graph of MVD before and after transplantation (n = 3-8; mean ± SEM). *Significant difference (P ≤ .05) between groups for total MVD. †Significant difference (P ≤ .05) between groups for human MVD. (E) Representative confocal images of GFP/UEA-I (rhodamine)–positive vessels in the implants at day 7 after transplantation into GFP-transgenic mice. White dotted line indicates the border of implant. Scale bars represent 100 μm.
MVD analysis before and after transplantation. Matrigel implants were harvested at multiple time points before and after transplantation. (A) Diagram of transplantation model. (B-E) Perfused human and murine vessels were identified by tail-vein injection of a mixture of rhodamine (red)–conjugated UEA-I and FITC (green)–conjugated GS-IB4. (B) Representative confocal images of 3 distinct patterns of lectin-labeled vessels in the implants at pretransplantation day 7. White arrowheads indicate human, murine, and chimeric vessels. (C) Representative confocal images of lectin-labeled vessels in the implants at multiple time points. White dotted line in the day 3 panel of pretransplantation indicates the border of implant. (D) Graph of MVD before and after transplantation (n = 3-8; mean ± SEM). *Significant difference (P ≤ .05) between groups for total MVD. †Significant difference (P ≤ .05) between groups for human MVD. (E) Representative confocal images of GFP/UEA-I (rhodamine)–positive vessels in the implants at day 7 after transplantation into GFP-transgenic mice. White dotted line indicates the border of implant. Scale bars represent 100 μm.
Time course of vasculogenesis/anastomosis
Before transplantation, GS-IB4-positive murine vessels were observed at the periphery of implants at day 3, whereas UEA-I–positive human vessels were observed from day 5 (Figure 1C-D). This suggests that infiltration of angiogenic host vessels into the implant was followed by anastomosis with nascent human vessels formed from ECFCs/MPCs. MPCs are known to release VEGF-A,11 providing a possible angiogenic stimulus to the host vessels. It is unclear at present whether the injected human cells form complete vessels, which anastomose with infiltrating host vessels, or whether the human cells individually cooperate with and contribute to the angiogenic elongation of the host vasculature. In vitro studies have shown formation of tubular structures by endothelial cells alone13 or when cocultured with perivascular cells.12 Therefore, we speculate that human ECFCs and MPCs form nascent vessels, which then form anastomoses with infiltrating murine vessels.
Bioengineered human blood vessels reconnect after transplantation
To test whether bioengineered human blood vessels can be disconnected from the donor vasculature and reconnect in secondary recipient mice, we performed in vivo lectin-labeling to detect perfused vessels and quantify MVD. UEA-I–positive human vessels were observed from day 3 after transplantation, 2 days before the appearance of UEA-I–positive human vessels before transplantation (Figure 1C-D). This indicates that the prebuilt vascular network facilitated anastomosis. At posttransplantation day 7, perfused MVD was similar to pretransplantation day 5 (98 ± 8 vs 99 ± 19 vessels/mm2). Perfused human MVD at posttransplantation day 7 (51 ± 11 vessels/mm2) reached 77% of the level observed on pretransplantation day 7 (67 ± 8 vessels/mm2; red bar graph, Figure 1D). Human CD31+ vessel lumens were preserved during the transplantation step (supplemental Figure 2B), suggesting that bioengineered vessels retain their luminal structure until reconnected in the recipient.
Two possibilities should be considered. One is direct reconnection in which human vessels reconnect with recipient vessels. The other possibility is that murine vessel segments in the primary transplants are responsible for reconnection with recipient murine vessels. To address this, green fluorescent protein (GFP)–transgenic SCID mice were used as recipient mice; tail vein injection of UEA-I only was performed at day 7 after transplantation. We observed GFP/UEA-I–positive chimeric vessels (Figure 1E), suggesting that ECFC/MPC-human vessels can reconnect directly with recipient vessels after transplantation.
Perfused vascular volume in implants
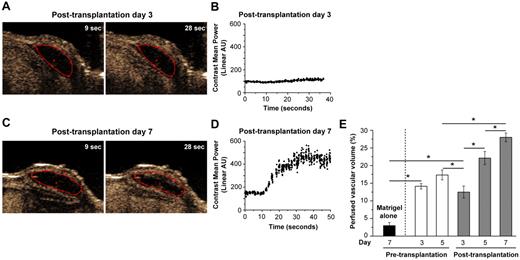
To gain further insight into the extent of perfusion before and after transplantation, we monitored perfusion at days 3 and 5 before transplantation, and days 3, 5, and 7 after transplantation in the same implants using a contrast-enhanced ultrasonic imaging system tuned to detect a microbubble contrast agent introduced to the animal's systemic circulation. This technique enabled us to quantify, by three-dimensional (3D) analysis, the proportion of the whole implant that was perfused by the vasculature.
Figure 2A shows representative raw data images from a low-perfusion implant (posttransplantation day 3, captured from supplemental Video 1), and Figure 2B shows ultrasound signal intensity over time, with contrast agent injected at 10 seconds. The lack of orange pixels within the construct (red outline, Figure 2A) and no signal increase on injection of contrast agent (Figure 2B) indicate very little perfusion. In contrast, representative images, graph, and video from posttransplantation day 7 (Figure 2C-D; supplemental Video 2) showed a dramatic ultrasound signal increase, indicating extensive perfusion.
Perfused vascular volume before and after transplantation. ECFC/MPC/Matrigel suspensions were injected into a SCID mouse to form vascular networks in vivo, and the degree of perfusion in each implant was monitored at days 3 and 5 before transplantation, and days 3, 5, and 7 after transplantation. The 2D images were recorded as video (supplemental Videos 1 and 2) and analyzed. (A) Representative captured 2D image from low-perfused implant (posttransplantation day 3). (B) The plot of nonlinear ultrasound signal intensity (expressed in arbitrary units) integrated over the region of interest of the image plane of Figure 2A (contrast agent was injected at ∼ 10 seconds). (C) Representative captured 2D image from high-perfused implants (posttransplantation day 7). (D) Nonlinear ultrasound signal intensity as described in panel B, integrated over the region of interest of image plane of Figure 2C. (E) Perfused vascular volume was measured by collecting a stack of 2D images from the anterior to posterior ends of the implant (supplemental Videos 3 and 4). The difference in the percentage of voxels returning an ultrasound signal before and after contrast agent injection is perfused vascular volume (percentage), the proportion of implant volume occupied by functional, perfused vessels. *Significant difference (P ≤ .05) between groups (n = 8 for each time point; n = 5 for Matrigel control).
Perfused vascular volume before and after transplantation. ECFC/MPC/Matrigel suspensions were injected into a SCID mouse to form vascular networks in vivo, and the degree of perfusion in each implant was monitored at days 3 and 5 before transplantation, and days 3, 5, and 7 after transplantation. The 2D images were recorded as video (supplemental Videos 1 and 2) and analyzed. (A) Representative captured 2D image from low-perfused implant (posttransplantation day 3). (B) The plot of nonlinear ultrasound signal intensity (expressed in arbitrary units) integrated over the region of interest of the image plane of Figure 2A (contrast agent was injected at ∼ 10 seconds). (C) Representative captured 2D image from high-perfused implants (posttransplantation day 7). (D) Nonlinear ultrasound signal intensity as described in panel B, integrated over the region of interest of image plane of Figure 2C. (E) Perfused vascular volume was measured by collecting a stack of 2D images from the anterior to posterior ends of the implant (supplemental Videos 3 and 4). The difference in the percentage of voxels returning an ultrasound signal before and after contrast agent injection is perfused vascular volume (percentage), the proportion of implant volume occupied by functional, perfused vessels. *Significant difference (P ≤ .05) between groups (n = 8 for each time point; n = 5 for Matrigel control).
Perfused vascular volume (percentage; Figure 2E) increased gradually before transplantation. Transplantation interrupted construct perfusion, but by day 3 perfusion returned to 13% ± 2%. Perfusion was restored to 22% ± 2% on day 5, which was equivalent to that measured on pretransplantation day 5 (17% ± 1%). On posttransplantation day 7, perfused vascular volume was 28% ± 1%. For comparison, renal vascular volume in these animals was 50% ± 2%. At every time point, the experimental implants were significantly more perfused than the Matrigel-alone negative control group.
As shown in Figure 1D, MVD values were similar between pretransplantation day 5 and posttransplantation day 7, whereas perfused vascular volume (Figure 2E) was increased at the latter time point. This may be because MVD is based on 2-dimensional (2D) histologic sectioning, whereas perfused vascular volume is a 3D ultrasound measurement of the whole construct. Furthermore, MVD describes the number of vessels per sectional area and is not expected to be proportional to the volume of blood transmitted by those vessels. The 2 metrics are complementary, with lectin-based MVD valued for allowing the measurement of human and murine vessels, whereas perfused vascular volume is valuable as a more faithful measurement of blood-delivery function.
In conclusion, our goal is to develop a technology for generating vascularized tissue-engineered constructs. In the present study, we aimed to test whether ECFC/MPC-bioengineered human vessels originally perfused at one site could become reperfused in a secondary site. We show, by in vivo staining and ultrasonic imaging analyses, that ECFCs/MPCs rapidly formed anastomoses and increased MVD and perfused vascular volume over 7 days in donor mice. After transplantation, perfusion was reestablished by day 3. Chen et al demonstrated that a 7-day in vitro preassembly step, in which ECFC-derived endothelial cells and fibroblasts were coincubated in a fibrin gel, accelerated anastomosis with host vasculature after implantation.7 In contrast, the present study shows that prebuilt perfused human vessels are transplantable from one in vivo site to another. Perfusion of transplanted human blood vessels, first detected at day 3 and increasing with time to a physiologic range, demonstrates an important new feature of ECFC/MPC-bioengineered blood vessels, the ability to be transplanted and reconnect to the vasculature in a new site and rapidly reestablish perfusion. This feature extends the potential applications of this postnatal progenitor cell-based technology for transplantable tissue-engineered constructs.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by a National Institutes of Health grant (5R01HL94262, J.B.).
National Institutes of Health
Authorship
Contribution: K.-T.K. designed and performed all experiments, interpreted data, and wrote the manuscript; P.A. developed and performed micro-ultrasonic imaging experiments and wrote the manuscript; and J.B. conceived the research plan, contributed to interpretation of data, and assisted with writing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Joyce Bischoff, Children's Hospital Boston, Harvard Medical School, 300 Longwood Ave, Boston, MA 02115; e-mail: joyce.bischoff@childrens.harvard.edu.
References
Author notes
K.-T.K. and P.A. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal