In this issue of Blood, Fazi and colleagues have asked important questions and added several new observations to our understanding of monoclonal B-cell lymphocytosis (MBL) in its role as the precursor state of chronic lymphocytic leukemia or small lymphocytic lymphoma (CLL/SLL).1
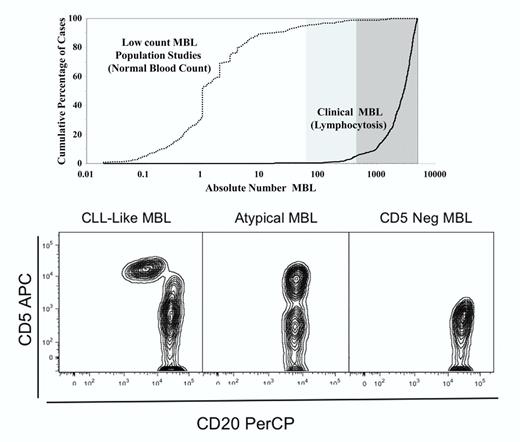
First, it is important to note the role of the absolute B-cell count in distinguishimg low-count, population-screened MBL from clinical MBL with lymphocytosis. Low-count MBL can be designated by a clone size of 50 B cells per μL or less while clinical MBL occurs with a B-cell lymphocytosis of 1500 cells per μL or greater. Rawstron et al have an excellent diagram showing the difference between low-count and clinical MBL (see Figure 12 ). The top panel in the figure here is a modification of the Rawstron diagram. Second, there are 3 common variants of MBL. They are CLL-like MBL (CD5+CD20 dim), atypical MBL (CD5+“bright CD20”), and CD5-negative MBL. (See Vogt et al3 and Marti et al4 for examples of these patterns; the bottom panel in the figure here is from this author's former laboratory.) In a landmark flow cytometric analysis paper, the Primary Health Care Group of Salamanca provided data confirming that the prevalence of MBL is age dependent and 100-fold in excess of CLL.5 Why this large difference, and what are the molecular and biologic features that favor progression to CLL?
Role of absolute B-cell count in MBL and immunophenotype patterns. The top panel shows the absolute number of B cells as a cumulative function of the number of cases dividing low-count MBL (normal white blood cell count, absolute lymphocyte count) from clinical MBL (with lymphocytosis). The bottom panel from left to right shows the 3 major immunophenotypic patterns seen in MBL.
Role of absolute B-cell count in MBL and immunophenotype patterns. The top panel shows the absolute number of B cells as a cumulative function of the number of cases dividing low-count MBL (normal white blood cell count, absolute lymphocyte count) from clinical MBL (with lymphocytosis). The bottom panel from left to right shows the 3 major immunophenotypic patterns seen in MBL.
Briefly, this longitudinal study by Fazi et al is a re-evaluation, follow-up study of population-based, low-count MBL. The investigators had previously found 137 cases of MBL in this genetically isolated rural Italian population. Seventy-six individuals were available for re-evaluation with a median follow-up of 34 months, or almost 3 years. The majority of cases are CLL-like MBL but also include atypical and CD5-negative MBL. Ninety percent of CD5+ CLL-like MBL cases show no progression and are stable. Ten percent of cases could not be confirmed in follow-up; these were originally at the lowest threshold of detection when first identified. There was no statistically significant increase in white blood cell count, absolute lymphocyte count, or clone size from baseline. A greater number of the atypical and CD5-negative cases could not be confirmed at repeat testing. It would now seem that low-count CLL-like MBL can be referred to as persistent MBL and would require no further evaluation. Long-term studies in a blood-donor setting would allow for confirmation of this finding of persistence without progression as well as the transient nature of atypical and CD5-negative MBL.
The second major finding reported by Fazi et al concerns the cytogenetic analysis of MBL. Flow cytometric cell sorting followed by slide-based FISH data are provided on these cases of low-count MBL. They show that 50% of cases display heterozygous and homozygous 13q14 deletions. This is the first extensive report of 13q14 deletions in low-count CLL-like MBL and it is important because, as the authors point out quite convincingly, the acquisition of the CLL-associated genomic abnormalities occur very early in the natural history of MBL/CLL. It follows then that a further event is required for leukemic progression. The Ghia laboratory has already shown that in CLL-like MBL, Ig gene use is more similar to normal B cells than CLL cells.6 Given that in MBL, the Ig gene mutational status is predominately mutated, it would be of interest to reanalyze one more time to ascertain if the Ig gene repertoire of 13q14 del MBL is similar to that found in CLL rather than normal B cells.
The third new finding by Fazi et al shows that 80% of MBL is associated with double-positive T-cell clones (44.4% CD4highCD8low and 36.1% CD8highCD4low) using T-cell receptor Vβ2 (TRBV2; 12/27, 44.4%) and T-cell receptor Vβ8 (TRBV8; 9/27, 33.3%). These findings are new and one would only hope that this type of analysis would be extended to clinical and familial MBL. Are the T-cell clones so-called “cognate clones” associated with a given MBL clone? Does this argue the case for chronic antigen stimulation for both MBL and T-cell clones, or is it a reflection of immune-senescence?
Several questions remain. The overexpression of κ clones in MBL is unexplained. Of the 6 confirmed CLL-like MBL cases that were designated “polyclonal,” did any of these cases show any FISH abnormalities or associated with T-cell clones? Does this represent the early expression of intraclonal heterogenity? Would there be an association with serum free light chains?
In closing, IgM, κ-restricted, mutated, 13q14-deleted, population-based, low-count, CD5+CD20 dim CLL-like MBL is persistent without clinical progression and is associated with double-positive T-cell clones. Almeida et al developed a mathematical algorithm based on 800 to 1200 μL of blood and tested on large volumes of whole blood (50 mL) and AutoMACS-enriched preparations of B cells.7 It would seem that the vast number of healthy adults over the age of 70 years have CLL-like clones. Thus, these low concentrations and frequency in the population may suggest that low-count, CLL-like MBL without cytogenetic abnormalities is the normal physiologic counterpart of CLL; that is, it is the much sought after cell of origin for CLL.8
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal