Abstract
We conducted a 45 patient prospective study of reduced-intensity conditioning (RIC) and transplantation of unrelated umbilical cord blood (UCB) and CD34+ stem cells from a haploidentical family member. Median age was 50 years; weight was 80 kg. Fifty-eight percent had active disease. Neutrophil engraftment occurred at 11 days (interquartile range [IQR], 9-15) and platelet engraftment at 19 days (IQR, 15-33). In the majority of patients, early haploidentical engraftment was replaced by durable engraftment of UCB by 100 days, with regular persistence of minor host and/or haplo-hematopoiesis. Percentage of haplochimerism at day 100 correlated with the haplo-CD34 dose (P = .003). Cumulative incidence of acute GVHD (aGVHD) was 25% and chronic GVHD (cGVHD) was 5%. Actuarial survival at 1 year was 55%, progression-free survival (PFS) was 42%, nonrelapse mortality (NRM) was 28%, and relapse was 30%. RIC and haplo-cord transplantation results in fast engraftment of neutrophils and platelets, low incidences of aGVHD and cGVHD, low frequency of delayed opportunistic infections, reduced transfusion requirements, shortened length of hospital stay, and promising long-term outcomes. UCB cell dose had no impact on time to hematopoietic recovery. Therefore, UCB selection can prioritize matching, and better matched donors can be identified rapidly for most patients. This study is registered at http://clinicaltrials.gov as NCI clinical trial no. NCT00943800.
Introduction
Umbilical cord blood (UCB) is widely used in children and adults as a source of stem cells for those lacking related or unrelated donors (URDs). Despite mismatching, it causes little GVHD and some data suggest less disease recurrence than transplantations from adult donors,1 but the limited numbers of progenitor cells in the UCB unit result in delayed and unpredictable count recovery, particularly in adults. The delayed count recovery is associated with increased transfusion requirements and prolonged length of hospital stay. Delayed neutrophil recovery predisposes to infections, and delayed platelet recovery predicts for high early mortality.2-5 Considerable effort has been devoted to cord blood expansion to address this problem, but the technical obstacles remain considerable.6-8 Using a radically different approach, we conducted a pilot study of combined UCB and haploidentical transplantation.
Methods
The study was approved by the institutional review board of the University of Chicago. All patients and donors provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki and was registered on clinicaltrials.gov.
Patients with hematologic malignancies in need of an allogeneic stem cell transplantation (SCT) who lacked an HLA-identical related donor or URD were eligible. Additional eligibility criteria included Eastern Cooperative Oncology Group (ECOG) performance status ≤ 2, bilirubin ≤ 2 mg/dL, creatinine < 1.5 times the upper limit of normal, preserved heart and lung function, and no evidence of chronic active hepatitis or cirrhosis. HIV negativity was required, and females were not pregnant.
Donors and stem cell processing
Cord blood.
Cord blood units were selected based on HLA typing and cell count. Grafts were matched for at least 4 of 6 HLA loci by the standard cord criteria (ie, low resolution for HLA-A and HLA-B, and high resolution for HLA-DR)1 and contained a minimum cell count of 1 × 107 nucleated cells per kilogram of the recipient's body weight before freezing. In contrast with common practice, we prioritized matching over cell dose. In other words, we selected a closely matched graft with lower nucleated cell content over a less closely matched graft with more nucleated cells as long as the cell dose exceeded the minimum of 1 × 107/kg.
Haploidentical donor.
The preferred haploidentical donor was a nonmaternal HLA haploidentical relative. Maternal donors were avoided because of reported data of a high incidence of graft failure with their use.9 Maternal donors were accepted when they were the only available relative. Related donors underwent stem cell mobilization using filgrastim subcutaneously 5 mcg/kg twice per day (BID; total of 10 mg/kg/d) for 4 consecutive days. Apheresis was started on day 5 and continued daily until at least 5 × 106 CD34+ cells/recipient kg were collected.
After collection and before cryopreservation, haploidentical grafts were T-cell depleted using the Isolex 300i CD34 selection device. The target was to obtain a product containing less than 1 × 104 CD3+ cells/kg of recipient body weight and no more than 3 × 106/kg CD34-positive cells. As of early April 2010, the Isolex 300i CD34 selection device was no longer available and instead the Miltenyi CliniMACS device was used under an investigational new device (IND) from the US Food and Drug Administration.
Donor-directed Abs
As of the 10th patient enrolled on the protocol, UCB and haploidentical donor selection was also based on avoidance of donor-directed HLA Abs.10 Donor-directed Abs were evaluated by Luminex-based solid-phase assays.11 For this purpose, all donors underwent high-resolution HLA typing including DP typing.12 A donor targeted by pre-existing recipient HLA-Abs was avoided.
Preparative regimen
Patients received fludarabine 30 mg/m2/d IV for 5 consecutive days (days −7, −6, −5, −4, −3), rabbit antithymocyte globulin (thymoglobulin, r-ATG) at 1.5 mg/kg every other day for 4 doses (days −7, −5, −3, and −1), and melphalan 70 mg/m2 /d for 2 doses on day −3 and day −2 (Figure 1) based on the regimen initially developed by Giralt et al.13 Acetaminophen, diphenhydramine, and methylprednisolone or hydrocortisone were given to prevent r-ATG reactions. The haploidentical cells were infused on day 0 followed by cord blood later the same day or on day 1.
Posttransplantation immunosuppression
Tacrolimus at 0.03 mg/kg/d IV was given by continuous infusion over 24 hours from day −2 until engraftment or when patient was able to take per oral (PO). Tacrolimus was targeted to maintain levels of 5-15 ng/mL through day 180. Thereafter, tacrolimus was tapered by 20% every week unless there was evidence of acute GVHD (aGVHD) or chronic GVHD (cGVHD). Mycophenolate mofetil 1 gram PO three times a day (TID) was given until day 28, thereafter 1 gram PO BID until day 60.
Supportive care and growth factors
Patients were treated in rooms with high-efficiency, particulate-free (HEPA) air filters and with strict reverse isolation. They received filgrastim 5 mcg/kg/d subcutaneously, starting on day 1 after transplantation until neutrophil recovery. Patients received moxifloxacin 400 mg PO/d until resolution of neutropenia. They also received a broad spectrum azole or echinocandin until day 180. Two trimethoprim/sulfamethoxazole double-strength tablets daily twice a week, were given from engraftment until 1 year after transplantation. No prophylactic intravenous immunoglobulins were given.
All patients who were CMV seropositive or had a CMV-seropositive donor from either graft received ganciclovir 5 mg/kg from day −8 until day −3. They then were given acyclovir 10 mg/kg every 8 hours intravenously until discharge. On discharge, valacyclovir 2000 mg 4 times per day was continued until day 180.14 CMV-seronegative recipients with a seronegative donor received acyclovir 800 mg PO BID to prevent HSV and varicella zoster virus (VZV) reactivation.
Patients were screened weekly for CMV viremia until day 120 and treated with ganciclovir on detection of CMV viremia. All patients including CMV-negative donor/recipient pairs were screened weekly for CMV viremia.15 Valganciclovir or ganciclovir were used for treatment of any CMV viremia occurring before day 100. Rising CMV viremia after day 100 or CMV disease also prompted similar treatment. When clinically indicated, foscarnet or cidofovir was used.
Screening for EBV viremia using a PCR-based method was introduced as of the 10th patient entered on this protocol. Patients were screened weekly until day 100 and at every return clinic visit thereafter. Intervention was considered for rising EBV titer above 10 000 copies/μL or at lower levels if clinical evidence of a posttransplantation lymphoproliferative disorder (PTLD) emerged. Discontinuation of immunosuppression was the first consideration; if this was ineffective or impossible, rituximab at 375 mg/m2 was given for up to a total of 4 weekly doses.16
Antifungal, antiviral, and pneumocystis prophylaxis, as well as screening for CMV and EBV viremia continued indefinitely for those with active GVHD or those on immunosuppressive treatment.
Irradiated and leukodepleted blood products were administered to maintain a hemoglobin level > 8 g/dL and platelet count > 10 × 109/L.
Pre- and posttransplantation evaluation
Comorbidities were recorded prospectively using the hematopoietic cell transplantation–specific comorbidity index.17 Posttransplantation BM and disease restaging were repeated routinely on day 28, day 100, day 180, 1 year, and yearly thereafter. It was also repeated when clinically indicated.
Chimerism was determined by molecular analysis of peripheral blood and BM samples. BM and/or peripheral blood specimens were collected at approximately day 14, day 28, day 100 and day 180 after transplantation. Donor and recipient cells were detected by quantitative analysis of informative microsatellite sequences of DNA as previously described.18
Disease relapse was defined as disease progression from the best response. The diagnosis of disease recurrence was based on clinical and pathologic criteria. Death without disease progression was considered transplantation-related.
Toxicity was scored according to National Cancer Institute–Common Toxicity Criteria (NCI-CTC) Version 3.0. aGVHD was scored according to the criteria proposed by Przepiorka et al, with the caveat that aGVHD could occur after day 100 as set out in the National Institutes of Health (NIH) consensus guidelines.19 cGVHD was scored according to the NIH Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: Diagnosis and Staging Working Report.20
Definitions of engraftment and length of stay
Myeloid engraftment was defined as the first day in which the absolute neutrophil count (ANC) was > 500/mm3 for 3 consecutive days. Platelet engraftment was defined as the first day the platelet count was > 20 000/mm3 without transfusion support for 7 consecutive days.
Failure to engraft was defined as lack of evidence of hematopoietic recovery (ANC < 500/mm3 and platelet count < 20 000/mm3) by day +35, confirmed by a biopsy revealing a marrow cellularity < 5%. Graft failure was also defined as initial myeloid engraftment by day +35, documented to be of donor origin, followed by a drop in the ANC to < 500/mm3 for more than 3 days, independent of any myelosuppressive drugs, severe GVHD, CMV, or other infection. Graft rejection was defined as graft failure with documentation of return of recipient hematopoiesis as determined by cytogenetic and/or chimerism studies. Chimerism studies were used to determine the contribution of cord and haploidentical donor to engraftment.
Length of hospital stay was defined as the number of days spent in the hospital between day of transplantation 0 and day 100. This included time spent in the hospital for any readmission during this time.
Statistical methods
Progression-free survival (PFS; time to relapse or death from any cause) and overall survival were calculated using the Kaplan-Meier product-limit estimate and expressed as probabilities with a 95% confidence interval (CI). Cumulative incidence of disease progression with death before progression as the competing risk21 and cumulative incidence of treatment related mortality with nontreatment related death as the competing risk were also calculated.22 Neutrophil and platelet recovery were calculated using the cumulative incidence function with death before recovery as the competing risk. The incidence of aGVHD and cGVHD were calculated using the cumulative incidence function, with death, relapse, disease progression, and graft failure as competing risks.
Univariate comparisons and multivariate analyses for survival and PFS used Cox proportional hazard regressions.23 Models for relapse and nonrelapse mortality (NRM) integrated competing risks.24 Parameters calculated in the univariate and multivariate analysis included disease status (active disease vs remission), age (< 50 years of age vs ≥ 50 years), performance status at transplantation (0 vs 1 or 2), comorbidity score at transplantation (2 vs > 2), prior transplantation versus not, and cord donor match (4/6 match or not), CD34+ cell number in haploidentical donor, total nucleated cell count in the UCB donor. For the multivariate model, independent variables with P values > .1 were excluded from the models. The relative risks (95% CI) and the associated P values of the remaining variables were reported. All data were analyzed as of June 6, 2011. Statistica software (Statsoft) was used for most analyses. R software was used for calculations of cumulative incidence. All risk factor analysis was considered exploratory and Bonferroni or other corrections were not used.
Results
Patient characteristics
Forty-five patients with hematologic malignancies were enrolled between January 2007 and April 2011 and are reported here. Characteristics of the patients are shown in Table 1. Thirteen patients (29%) belonged to ethnic or racial minorities, and almost half had acute myelogenous leukemia (AML). Twenty-six (58%) had active disease (ie, relapsed and/or refractory) at the time of transplantation. The large majority of patients had received intensive chemotherapy before transplantation, for a median of 3 prior lines of chemotherapy (range 1-8). One patient with profoundly hypocellular myelodysplastic syndrome (MDS) had not received any prior chemotherapy. Four patients, 3 MDS and 1 chronic idiopathic myelofibrosis (CIMF) had only received hypomethylating agents, in 1 case combined with gemtuzumab ozogamicin (Mylotarg).
Baseline patient characteristics
| Characteristic . | No.* . | % . |
|---|---|---|
| Total patients | 45 | |
| Median age, y (range) | 50 (20-69) | |
| Male/female | 30/15 | |
| Median weight, kg (range) | 80 (41-125) | |
| Race | ||
| White | 32 | 71 |
| Black | 9 | 20 |
| Other | 4 | 9 |
| Performance status | ||
| 0 | 30 | 67 |
| 1 | 15 | 33 |
| HCT-CI score | ||
| 0-2 | 34 | 75 |
| 3 or more | 11 | 25 |
| Diagnosis | ||
| AML/MDS | 29 | 64 |
| ALL | 7 | 16 |
| CLL | 2 | 4 |
| Lymphoma | 5 | 11 |
| CML myeloid crisis | 1 | 2 |
| CIMF | 1 | 2 |
| Stage of disease | ||
| CR1 | 14 | 31 |
| CR2 or CR3 | 5 | 11 |
| PR | 5 | 11 |
| Refractory/untreated relapse | 21 | 47 |
| Active disease | 26 | 58 |
| Prior transplantation | 7 | 16 |
| Prior intensive chemotherapy | 40 | 88 |
| Characteristic . | No.* . | % . |
|---|---|---|
| Total patients | 45 | |
| Median age, y (range) | 50 (20-69) | |
| Male/female | 30/15 | |
| Median weight, kg (range) | 80 (41-125) | |
| Race | ||
| White | 32 | 71 |
| Black | 9 | 20 |
| Other | 4 | 9 |
| Performance status | ||
| 0 | 30 | 67 |
| 1 | 15 | 33 |
| HCT-CI score | ||
| 0-2 | 34 | 75 |
| 3 or more | 11 | 25 |
| Diagnosis | ||
| AML/MDS | 29 | 64 |
| ALL | 7 | 16 |
| CLL | 2 | 4 |
| Lymphoma | 5 | 11 |
| CML myeloid crisis | 1 | 2 |
| CIMF | 1 | 2 |
| Stage of disease | ||
| CR1 | 14 | 31 |
| CR2 or CR3 | 5 | 11 |
| PR | 5 | 11 |
| Refractory/untreated relapse | 21 | 47 |
| Active disease | 26 | 58 |
| Prior transplantation | 7 | 16 |
| Prior intensive chemotherapy | 40 | 88 |
HCT-CI indicates hematopoietic cell transplantation–specific comorbidity index; AML, acute myelogenous leukemia; MDS, myelodysplastic syndrome; ALL, acute lymphoblastic leukemia; CLL, chronic lymphocytic leukemia; CIMF, chronic idiopathic myelofibrosis; CR, complete response; and PR, partial response.
No. represents number of patients unless otherwise specified.
Donor and graft characteristics
The haploidentical related donors were recipient's children in 44% and siblings in 45% of cases (Table 2). Only 11% of donors were parents. The median CD34+ cell content postselection of the haplograft was 3.5 × 106/kg (25%-75% interquartile range [IQR] 1.36-4.63), and the median CD3+ cell content was 0.7 × 104/kg (IQR 0.2-1.2). CD3 depletion was much more efficient using the CliniMACS device. The median number of residual CD3-positive cells was 1 × 104/kg (IQR 0.8-1.8) after Isolex depletion and 0.2 × 104/kg (IQR 0.12-0.3) after CliniMACS deple-tion (P < .00001). The devices yielded similar numbers of CD34+ cells.
Characteristics of related haploidentical donors and cord blood units
| Characteristic . | No. of patients (%) . | IQR 25%-75% . | Median . |
|---|---|---|---|
| Relationship of donor to recipient | |||
| Son | 15 (33) | ||
| Daughter | 5 (11) | ||
| Brother | 12 (27) | ||
| Sister | 8 (18) | ||
| Father | 4 (9) | ||
| Mother | 1 (2) | ||
| T-cell depletion device | |||
| Isolex | 26 (58) | ||
| Miltenyi | 19 (42) | ||
| Haploidentical after selection | |||
| Haplo CD34+ cells ≥ 3 × 106/kg | 27 (60) | ||
| Haplo CD3+ cells < 1 × 104/kg | 35 (78) | ||
| Cord match | |||
| 6/6 | 3 (7) | ||
| 5/6 | 33 (73) | ||
| 4/6 | 9 (20) | ||
| Haplo CD34, ×106/kg | 2.4-4.6 | 3.5 | |
| Haplo CD34 total, ×106 | 181-411 | 254 | |
| Haplo CD3 before T depletion, ×104/kg | 10 470-20 623 | 15 083 | |
| Haplo CD3 after T depletion, ×104/kg | 0.2-1.2 | 0.7 | |
| Cord TNC, ×107/kg | 1.24-2.09 | 1.55 | |
| Cord CD34, ×106/kg | 0.04-0.08 | 0.06 |
| Characteristic . | No. of patients (%) . | IQR 25%-75% . | Median . |
|---|---|---|---|
| Relationship of donor to recipient | |||
| Son | 15 (33) | ||
| Daughter | 5 (11) | ||
| Brother | 12 (27) | ||
| Sister | 8 (18) | ||
| Father | 4 (9) | ||
| Mother | 1 (2) | ||
| T-cell depletion device | |||
| Isolex | 26 (58) | ||
| Miltenyi | 19 (42) | ||
| Haploidentical after selection | |||
| Haplo CD34+ cells ≥ 3 × 106/kg | 27 (60) | ||
| Haplo CD3+ cells < 1 × 104/kg | 35 (78) | ||
| Cord match | |||
| 6/6 | 3 (7) | ||
| 5/6 | 33 (73) | ||
| 4/6 | 9 (20) | ||
| Haplo CD34, ×106/kg | 2.4-4.6 | 3.5 | |
| Haplo CD34 total, ×106 | 181-411 | 254 | |
| Haplo CD3 before T depletion, ×104/kg | 10 470-20 623 | 15 083 | |
| Haplo CD3 after T depletion, ×104/kg | 0.2-1.2 | 0.7 | |
| Cord TNC, ×107/kg | 1.24-2.09 | 1.55 | |
| Cord CD34, ×106/kg | 0.04-0.08 | 0.06 |
IQR indicates interquartile range; and TNC total nucleated cell count.
Only 3 of the cord blood units were 6/6 matches, 33 were 5/6, and 9 were 4/6. Infused TNC (postthaw counts) ranged from 1.24 to 2.09 × 107/kg (median 1.55), and the postthaw counts of CD34+cells ranged from 0.04 to 0.08 × 106/kg (median 0.06; Table 2).
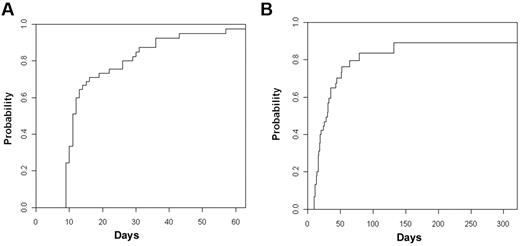
Hematopoietic recovery
The cumulative incidence of neutrophil recovery > 500/mm3 at day +50 was 95% (95% CI, 87%-100%) with a median time to engraftment of 11 days (IQR 9-15 days; Figure 2A). The cumulative incidence of platelet recovery ≥ 20 000/mm3 at day +100 was 83% (95% CI, 69%-97%) with median time to platelet engraftment of 19 days (IQR 15-33 days; Figure 2B). Nine patients died before platelet recovery, 5 from disease recurrence, and 4 from complications of transplantation. There was no correlation between cell dose of UCB graft or haplograft and time to neutrophil or platelet recovery.
Cumulative incidence of neutrophil and platelet engraftment. (A) Neutrophils. (B) Platelets.
Cumulative incidence of neutrophil and platelet engraftment. (A) Neutrophils. (B) Platelets.
There was no difference in time to platelet recovery or neutrophil recovery whether the patients received CD34+ cells selected with the Miltenyi CliniMACS or the Isolex device (data not shown). Time to engraftment of neutrophils and platelets was significantly prolonged for the 4 patients with failure of the haploidentical graft with only delayed engraftment of the UCB cells.
Patients received a median number of 12 platelet transfusions (IQR 6-22) and 7 RBC units (IQR 4-12) after the transplantation. The median number of days of hospitalization during the first 100 days after transplantation was 26 days (IQR 15-43 days).
Chimerism
In the majority of patients, early haploidentical engraftment was replaced by durable engraftment of UCB cells by 100 days. The median percentage of haploidentical cells in unfractionated peripheral blood was 86% on day 30, but declined to 22% by day 100, and to 2% by day 180. Conversely, the median percentage of cells of UCB origin increased from 10% by day 30, to 78% by day 100, and to 95% by day 180 (Figure 3). There was also some re-emergence of host hematopoiesis over time. On day 30 and on day 100, the median percentage of host hematopoiesis was 0% but by day 180, a median of 5% of cells were of host origin. The percentage of host hematopoiesis was higher in CD3 cells with a median of 17.5% of CD3 cells of host origin by day 180 (Figure 3).
The evolution of haplo to cord engraftment during the first 6 months after transplantation. The error bars indicate the 25% to 75% IQR. (A) Unfractionated peripheral blood and (B) CD3-positive cells.
The evolution of haplo to cord engraftment during the first 6 months after transplantation. The error bars indicate the 25% to 75% IQR. (A) Unfractionated peripheral blood and (B) CD3-positive cells.
Several patients had aberrant engraftment patterns. Six patients had persistent haploidentical only chimerism. Donor-specific HLA Abs against UCB but not against the haplodonor were present before transplantation in one case. In 5 other cases, no graft or recipient characteristics could be identified that might have contributed to haplograft failure. Four patients had delayed engraftment with UCB cells only, and never had evidence of haploidentical engraftment. In none of these 4 cases were we able to identify graft or recipient characteristics that might explain the umbilical cord graft failure.
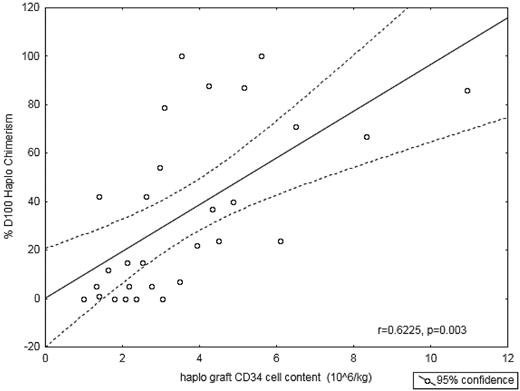
The percentage of day 100 haplodonor chimerism correlated closely with the CD34+ cell content of the haploidentical graft (106/kg; Figure 4; r = 0.6225; P = .0003), but showed no correlation with the CD3 doses of the haploidentical graft. TNC, CD34+ cells, or CD3+ cells of the UCB did not correlate with cord donor chimerism. The correlation between haplo cell dose and day-100 haplochimerism suggests that one may influence the balance between UCB and haplochimerism by manipulating the CD34+ cell dose.
Quantity of haplo CD34+ cells infused versus fraction of haplo chimerism at day 100. A higher number of CD34+ cells infused, correlates with a greater proportion of donor chimerism derived from the haploallograft at day 100.
Quantity of haplo CD34+ cells infused versus fraction of haplo chimerism at day 100. A higher number of CD34+ cells infused, correlates with a greater proportion of donor chimerism derived from the haploallograft at day 100.
One patient with long standing myelofibrosis died of primary graft failure on day 63. Donor -specific HLA Abs at HLA-A, -B, and HLA-DRB1 directed against both UCB and haplodonor were present before transplantation in this case. A second patient entering transplantation with active leukemia (5% BM blasts) progressed with acute leukemia immediately after transplantation and died on day 32 without recovery of neutrophils. There were 2 cases of secondary graft failure. One occurred on day 180 after treatment with rituximab for EBV viremia. This patient died while undergoing a second allogeneic transplantation. Another patient developed secondary graft failure on day 210 and was retransplanted using the same haploidentical donor but different UCB. The patient is currently doing well 60 days after second transplantation. A third patient, transplanted for therapy-related MDS lost donor chimerism by day 210 but recovered host hematopoiesis and remains in a morphologic and cytogenetic remission 8 months after transplantation, and 1 patient transplanted for hypoplastic MDS initially recovered granulocytes, but subsequently developed graft failure manifested by pancytopenia despite 100% donor UCB chimerism.
GVHD
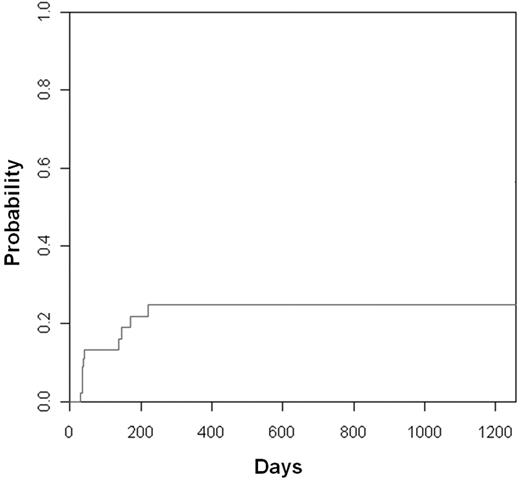
The cumulative incidence of acute GVHD (grade II-IV) was 25% (95% CI, 11-39; Figure 5). Most cases were grade II, and only 2 cases of grade 3 acute GVHD occurred. There were only 2 patients with chronic GVHD for a cumulative incidence of 6% at 1 year. One case of cGVHD treated with steroids was complicated with a fatal opportunistic infection. The latter patient had a concomitant diagnosis of porphyria cutanea tarda with chronic skin changes. This complicated the assessment of treatment response.
Survival, PFS, relapse, TRM, and causes of death
Cumulative incidence of treatment-related mortality was 9% (95% CI, 1-17) at day 100 and 28% (95% CI, 13-43) at 1 year. Cumulative incidence of disease recurrence was 11% (95% CI 3-19) at day 100 and 30% (95% CI 14-44) at 1 year (Figure 6A). With a median follow-up for survivors of 330 days (range 64-1259), estimated 1-year survival was 55% (95% CI, 39-71; Figure 6B) and PFS was 42% (95% CI, 25-79). Not surprisingly, active disease at the time of haplo-cord SCT tended to impair PFS, but this difference did not reach statistical significance (P = .06; Figure 6C). No other predictors of outcome could be identified.
Transplant outcomes. (A) Cumulative incidence of relapse and TRM. (B) Progression-free survival by disease status at transplantation. (C) Overall survival.
Transplant outcomes. (A) Cumulative incidence of relapse and TRM. (B) Progression-free survival by disease status at transplantation. (C) Overall survival.
Viral reactivation, PTLD, and fungal infections
The cumulative incidence of CMV viremia was 42% (95% CI, 26-58), but only 4 cases of CMV disease occurred for a cumulative incidence of 12% (95% CI, 0%-18%). One case occurred in a patient with refractory ALL who was receiving treatment for CMV retinitis before conditioning therapy.
The ninth patient on protocol died of fulminant EBV-related PTLD on day 74 after transplantation. After this occurrence, routine screening for EBV viremia was instituted. The cumulative incidence of EBV viremia was 42% (95% CI, 26%-58%), but most cases were self-limited or transient and only 7 of 16 patients with EBV reactivation received rituximab treatment. Five patients developed biopsy or radiologic proven PTLD, for a cumulative incidence of 11% (95% CI, 0-22). Delay in diagnosis in one case because of lack of surveillance and in another case because of patient's failure to return to clinic was probably responsible for the fatal course of 2 cases, including the one described in this section. Three other patients responded promptly to rituximab and have remained in remission for over a year after their diagnosis. One patient received another course of rituximab for EBV reactivation approximately 10 months after the first rituximab treatment. Only one case of adenovirus infection occurred and there were no cases of toxoplasmosis. Fungal infection was the cause of death in 3 patients. One of them had primary graft failure, a second one had delayed engraftment because of failure of the haplograft. The third patient was on chronic steroid therapy for asthma and had a history of fungal pneumonia preceding transplantation.
Discussion
Allogeneic HSCT using matched related donor or HLA-matched URDs is the treatment of choice for many patients with high-risk or recurrent hematologic malignancies.25,26 But only approximately 70% of white patients have an available fully HLA-matched unrelated donor; and for blacks, this probability is only 20%.27,28 As a result, blacks with a transplantation indication are only half as likely to receive an unrelated donor SCT as whites.29
Approaches for those lacking matched family donors or well-matched URDs include HLA-mismatched unrelated UCB, haploidentical (haplo) family donor SCT (Haplo SCT) or partially matched adult URDs. UCB SCT has garnered considerable interest because of its ready availability, low incidence of aGVHD and cGVHD, low incidence of disease recurrence, and relatively robust immune reconstitution. But the erratic and delayed recovery of neutrophils and platelets is its Achilles heel and is responsible for the high early transplantation-related mortality.2-5
Myeloablative cord SCT with third-party donor support was pioneered by the group from the Universidad Autónoma de Madrid led by Dr M. Fernandez. Their recently updated data demonstrated rapid neutrophil and platelet recovery.30,31 Initial engraftment by the third-party donor was ultimately superseded by permanent UCB engraftment. Their long-term outcome data were encouraging, but, with a median age of 34 years, their study was restricted to relatively young patients. Our pilot study demonstrates that the concept of haplo-cord SCT can be extended to older and less well patients by using reduced intensity conditioning. Using a widely used reduced-intensity conditioning (RIC) regimen, we observed rates of neutrophil and platelet recovery that parallel those observed with transplantation from adult HLA-matched peripheral blood stem cell allografts. The median time to neutrophil engraftment was shorter than that observed in most other UCB studies (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Time to neutrophil engraftment was more predictable with 75% of patients recovering their neutrophil counts by 15 days after transplantation.5,32-35 By contrast, in a study of nonmyeloablative conditioning and double UCB transplantation, 30% of patient required > 21 days until neutrophil recovery.31 The median duration of thrombocytopenia was 19 days, compared with a range of 41 to 62 days in reports of double UCB-SCT from other groups5,33-35 (supplemental Table 1). As a result, our patients received an average of 12 platelet transfusions after transplantation compared with 25 after double UCB transplantation with nonmyeloablative conditioning.5 The median length of hospital stay, one of the major drivers of cost of transplantation, was only 26 days compared with 38 days after RIC and double UCB-SCT.3
Over time UCB hematopoiesis predominated in the large majority of patients, but we also regularly observed prolonged mixed chimerism, with minor but durable contributions from the haplograft and from the host. Residual host chimerism was even more prominent in CD3 cells. It is tempting to speculate that this persistent host lymphoid chimerism, which is protective of chronic GVHD in transplantation from HLA-identical donors, may have contributed to the low incidence of acute and particularly of chronic GVHD in our study.18,36
Common practice for UCB unit selection focuses on cell dose at the expense of matching. We reversed the priority scheme. We hypothesized that the haploidentical peripheral blood stem cell donor would achieve reliable early engraftment, safely allowing delayed cord blood recovery. The results of our trial support our hypothesis; UCB cell dose had no measurable impact on outcomes. Thus, cell dose no longer constitutes a limitation in UCB-graft identification and as a result, suitable donors for a much larger proportion of patients could be identified.37 For example, we transplanted 6 patients weighing over 100 kg. Obesity has become epidemic in the United States and other countries and is even more frequent in blacks who also tend to lack adult HLA-matched donors.38 Because cell dose no longer plays a role, we were also able to focus on identifying units that are more closely HLA-matched. Only 20% of our UCB were more than one Ag-mismatched, compared with percentages ranging from 50% to 90% in many other studies (supplemental Table 1). Better matching is likely to favorably affect long term outcomes39,40
Graft failure, of both grafts was unusual in our series, but several cases of failure of either the haplograft or the UCB graft were observed. In one case of primary graft failure and in one case of UCB failure with persistence of the haplograft, it was related to the presence of donor specific Abs. We therefore support the recommendation of others to avoid UCB or any grafts targeted by donor-specific Abs10,11 In most other cases, the etiology of graft failure remains unexplained. Further study of larger numbers of patients may help to elucidate this. Postthaw UCB viability may play a role in some cases of UCB graft failure.40 And in one case of hypocellular MDS, we suspect that failure of functional graft recovery was because of stromal damage. Of note, the one patient with a maternal donor had prompt engraftment, contrary to the findings of group from Spain.
The group from Spain reported a high incidence of CMV disease and other opportunistic infections including hepatitis B reactivation and toxoplasmosis. In our experience, the incidence of CMV disease is quite low, and there was only one fatal case in a patient with preexisting CMV retinitis. Differences in CMV prophylaxis and the avoidance of high-dose steroids likely explain this difference. EBV reactivation and PTLD were common in both studies as they are in many studies using ATG,41 but were manageable once we implemented aggressive EBV screening and rapid intervention. Life-threatening fungal infections were rare, except in patients with failed or delayed neutrophil recovery.
Competition between grafts of various sources is commonly observed after double UCB transplantation and, as a rule, leads to elimination of one of the grafts over time. It is usually attributed to immunologic graft-versus-graft effects, and there is preliminary evidence supporting this concept.40,42 Others have posited that order of infusion may be important and that the unit first infused tends to survive better.34 Lastly, a recent study suggests that cord blood viability is important to assure unit dominance.40 We observe a similar competition phenomenon after haplo-cord transplantation, with routine long-term predominance of the UCB graft, despite infusion of the UCB graft after the haplograft. Immunologic mechanisms likely play a role in the UCB cord blood dominance and in this regard the T-cell depletion by CD34 selection of the haplograft may be essential. It is also possible that, in addition to immunologic mechanisms, differences in proliferative potential of the UCB stem cells confer an ultimate advantage, as was shown previously in a murine model,43 and as suggested by a recent study of cord blood viability and dominance.40
The rate of elimination of the haplograft was highly variable and in some cases the haplograft persisted permanently at the expense of the UCB graft. We were unable to identify UCB characteristics that conferred an advantage but did find that the haplo-CD34 content (rather than its CD3 content) was closely related to the percentage haplochimerism on day 100 (Figure 3). This may be because of hematopoietic competition between UCB and haplo-stem cells, or to a veto effect of large numbers of CD34 cells that hinder their elimination, a mechanism originally studied by Reisner et al.44
While it is clear that the haplo-cord approach results in much reduced time and variability of neutrophil and platelet engraftment, our study was not designed to address long-term efficacy. Our clinical outcomes relating to survival and disease control are therefore to be considered preliminary and hypothesis generating and their interpretation should take into account the high median age, the advanced disease status, the frequent comorbidities, and the ethnic diversity of our patient population. Given these adverse characteristics, the estimated 1-year survival that exceeds 50% and a PFS exceeding 40% compare favorably with most reported studies. Our outcomes are equivalent to those reported by the Bone and Marrow Transplant Clinical Trials Network (BMT-CTN) using double UCB nonmyeloablative SCT. But eligibility for the CTN trials was restricted to patients in remission.45 For the 19 participants on our trial who were in complete remission (CR) at the time of transplantation, 1-year PFS was 58% compared with 46% to 48% in the CTN trial. In addition, approximately 40% of patients with refractory disease, mostly AML, remained in remission. Our approach therefore may have considerable promise for patients with refractory myeloid malignancies who are at high risk for treatment-related mortality with double UCB and at extreme risk for disease recurrence with haplo-SCT.46
In summary, our pilot study of haplo-cord transplantation revealed reliable and fast engraftment of neutrophils and platelets, low incidences of acute and chronic GVHD, low frequency of delayed opportunistic infections, reduced transfusion requirements, shortened length of stay, and promising long-term outcomes for patients lacking suitable matched donors. UCB cell dose has no impact on time to hematopoietic recovery. Therefore, UCB selection can prioritize matching and better matched donors can be identified rapidly for most patients. We believe our results strongly suggest that haplo-cord transplantation improves short-term outcomes of UCB transplantation by hastening hematopoietic recovery. It is likely that as a result of faster recovery and of use of better matched UCB units, long-term outcome will also be improved compared with single or double UCB transplantation. Confirmation of this hypothesis will require a prospective study comparing double UCB transplantation to haplo-cord. Finally, the further clinical and laboratory investigation of the mixed adult UCB concept may provide interesting clues on hematopoietic stem cell biology and behavior.
The online version of this article contains a data supplement.
Presented in part at the 37th annual meeting of the European Group for Blood and Marrow Transplantation, Paris, France, April 4, 2011. Presented in part at the 2011 ASCO annual meeting, Chicago, IL, June 3, 2011.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the nurses and staff of the transplantation unit for their dedicated care and invaluable contributions to making this work possible; Guadalupe Martinez, Lauren Frehr, Timothy Pohner, and Elizabeth Lindeman of the Clinical Cell Processing Laboratory for their technical assistance; Miltenyi Pharmaceuticals for IND support; and they extend their gratitude and respect to all patients who agreed to participate in these studies.
This work was supported in part by an unrestricted grant from Genzyme Pharmaceuticals. K.v.B. is supported by National Cancer Institute grant K24 CA 116471.
National Institutes of Health
Authorship
Contribution: H.L. analyzed data and wrote the manuscript; E.S.R. designed the study, enrolled patients, and contributed to analysis and writing; L.G., O.O., J.K., V.N., J.C., R.A.L., W.S., and A.S.A. enrolled patients, and contributed to analysis and writing; L.J. performed chimerism analysis and contributed to the manuscript; S.M. selected donors, analyzed data, and contributed to the manuscript; P.d.C., L.S., and L.P. enrolled patients, assured study conduct, and contributed to analysis and writing; A.W. provided scientific and technical input in preparation of cellular products; and K.v.B. designed and supervised the study, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: K.v.B. and W.S. received research support from Genzyme. K.v.B., O.O., and J.K. served on Genzyme advisory boards. The remaining authors declare no competing financial interests.
Correspondence: Koen van Besien, Weill Cornell Medical College, 520 East 70th St, ST-341, New York, NY 10021; e-mail: kov9001@med.cornell.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal