Abstract
Extracorporeal photopheresis (ECP) is an important therapeutic option in steroid-refractory chronic graft-versus-host disease (cGVHD). Few biomarkers predicting response exist. We measured serum B-cell activating factor (BAFF) in 46 cGVHD patients receiving ECP before and during treatment course. BAFF level at 1 month of ECP predicted 3- and 6-month skin disease response, with BAFF less than 4 ng/mL associated with significant skin improvement and complete resolution in 11 of 20 patients. High BAFF at 1-month ECP associated with a worsening median 6-month skin score and resolution in 1 of 10 patients. BAFF level at 3 months also predicted the likelihood of maintaining skin disease improvement at 6 months. BAFF level was not correlated directly with extracutaneous cGVHD response, although full cutaneous responders exhibited improved extracutaneous organ response rates compared with skin nonresponders (65% vs 35%). This study suggests that early BAFF measurement during ECP for cGVHD represents a potentially useful biomarker in prediction of treatment outcome.
Introduction
Distorted B-cell homeostasis is evident during chronic graft-versus-host disease (cGVHD).1,2 Particularly, the disease is characterized by a deficiency of memory CD27+ B cells and relative elevation of CD21− transitional/immature B-cell numbers.2,3 B-cell activating factor (BAFF) has described roles in immature B-cell survival and promotes production of autoantibodies.4-6 Excess BAFF may contribute to cGVHD maintenance by protecting alloreactive and autoreactive clones from apoptosis that would, under normal physiologic B-cell/BAFF ratios, be deleted.7 Elevated BAFF levels reportedly correlate with cGVHD activity.8 A recent study also indicates that genetic variation in BAFF modulates GVHD phenotype.9
Extracorporeal photopheresis (ECP) represents an important second-line therapeutic intervention in steroid-refractory cGVHD with recognized efficacy as a steroid-sparing agent.10,11 Interest in reliable biomarkers predicting ECP response has recently focused on the B-cell compartment with reported association between relative amounts of immature CD21− B lymphocytes and predictive power for response.12 We report the use of a serum biomarker (BAFF) as a predictor of ECP treatment outcome.
Methods
We evaluated 46 adult patients undergoing ECP for steroid-refractory, -resistant, or -intolerant active cGVHD. Organ involvement is detailed in Table 1. ECP was performed using the Therakos XTS or Cellex devices. ECP treatment schedule was paired treatments every 2 weeks performed for an initial 3 months, then reduced to monthly paired treatments for a further 3 months and then reassessment. The study was approved by the Research and Development department of the Rotherham NHS Foundation Trust and York Research Ethics Committee.
Response to ECP was assessed for each affected organ at 3 and 6 months of therapy; cutaneous disease response was assessed using the modified Rodnan skin scoring system reflecting body surface area involvement and severity.13 Reduction in total skin score (TSS) between 50% and 99% was regarded as partial response (PR) and full skin resolution as complete response (CR). For ocular disease, PR was defined as reduction in dryness/artificial tear use and restoration of normal function, CR. Lung disease was scored according to National Institutes of Health classification,14 with score reduction representing PR and forced expiratory volume in 1 second return to normal range, CR. Liver cGVHD involvement was defined as hyperbilirubinemia or liver enzymes more than 2 times the upper limit of normal range. PR was classified as National Institutes of Health score reduction, CR as normalization of bilirubin level and liver enzyme. For gut, mucous membrane, and genital involvement, symptomatic reduction in score was regarded as PR, normalization as CR. Steroid taper was initiated when feasible after at least 4 ECP treatments at 1 month of ECP.
Soluble BAFF in patient serum samples was measured before ECP and at 1, 3, and 6 months of therapy using a commercially available ELISA (R&D Systems) according to the manufacturer's instructions.
Results and discussion
Clinical response to ECP at 3 and 6 months is summarized in Table 1. The overall organ response rate (CR + PR) at 6 months was 52%. Median interval between transplantation and cGVHD onset was 3.8 months (range, 2-98 months) and between cGVHD onset and ECP 13 months (range, 1-93 months). A total of 38 of 46 patients were receiving steroids at ECP start, and 38 of 46 were receiving cyclosporine or tacrolimus (Table 2).
Characteristics of cGVHD patients and response to ECP therapy
| Organ affected by cGVHD at ECP start . | Pre-ECP, (n = 46) . | Response after 3 mo of ECP, % (n = 46) . | Response after 6 mo of ECP,* % (n = 39) . | ||||
|---|---|---|---|---|---|---|---|
| CR . | PR . | Nonresponse . | CR . | PR . | Nonresponse . | ||
| Skin, n (%) | 35 (76) | 12 (34) | 13 (37) | 10 (29) | 12 (42) | 3 (10) | 14 (48) |
| Liver, n (%) | 24 (52) | 2 (8) | 9 (38) | 13 (54) | 2 (10) | 7 (36) | 11 (55) |
| Ocular, n (%) | 23 (50) | 6 (26) | 5 (22) | 12 (52) | 4 (21) | 6 (32) | 9 (47) |
| Gut, n (%) | 12 (26) | 5 (42) | 5 (42) | 2 (17) | 6 (60) | 1 (10) | 3 (30) |
| Mucous membrane, n (%) | 11 (23) | 3 (27) | 4 (36) | 4 (36) | 6 (60) | 1 (10) | 3 (30) |
| Lungs, n (%) † | 10 (21) | 1 (10) | 1 (10) | 6 (60) | 1 (10) | 0 (0) | 6 (60) |
| Genital, n (%) | 3 (6) | 0 (0) | 3 (100) | 0 (0) | 0 (0) | 2 (100) | 0 (0) |
| Organ affected by cGVHD at ECP start . | Pre-ECP, (n = 46) . | Response after 3 mo of ECP, % (n = 46) . | Response after 6 mo of ECP,* % (n = 39) . | ||||
|---|---|---|---|---|---|---|---|
| CR . | PR . | Nonresponse . | CR . | PR . | Nonresponse . | ||
| Skin, n (%) | 35 (76) | 12 (34) | 13 (37) | 10 (29) | 12 (42) | 3 (10) | 14 (48) |
| Liver, n (%) | 24 (52) | 2 (8) | 9 (38) | 13 (54) | 2 (10) | 7 (36) | 11 (55) |
| Ocular, n (%) | 23 (50) | 6 (26) | 5 (22) | 12 (52) | 4 (21) | 6 (32) | 9 (47) |
| Gut, n (%) | 12 (26) | 5 (42) | 5 (42) | 2 (17) | 6 (60) | 1 (10) | 3 (30) |
| Mucous membrane, n (%) | 11 (23) | 3 (27) | 4 (36) | 4 (36) | 6 (60) | 1 (10) | 3 (30) |
| Lungs, n (%) † | 10 (21) | 1 (10) | 1 (10) | 6 (60) | 1 (10) | 0 (0) | 6 (60) |
| Genital, n (%) | 3 (6) | 0 (0) | 3 (100) | 0 (0) | 0 (0) | 2 (100) | 0 (0) |
Seven patients halted treatment between 3 and 6 months: 3 died of progressive disease and concurrent infection and were regarded as nonresponders, 1 missed treatments because of infection, 2 had disease relapse, and 1 patient lost venous access. A total of 37 of 39 patients continued ECP therapy beyond 6 months.
Two patients had missing lung function data at 3 and 6 months.
Steroid dose reduction during 6 months of ECP treatment
| . | . | Reduction in steroid dose after 3 mo of ECP, no. (%) of patients on steroids . | Reduction in steroid dose after 6 mo of ECP, no. (%) of patients on steroids . | ||
|---|---|---|---|---|---|
| Reduced by at least 50% . | < 50% . | Reduced by at least 50% . | < 50% . | ||
| Receiving steroids pre-ECP, no. (%) of total | 38 (83) | 20 (53) | 18 (47) | 20 (74) | 10 (26) |
| Steroid dose, mg/day, pre-ECP, mean (range) | 26.2 (5-120) | 13.1 (0-40) | 20.9 (7-120) | 7.3 (0-15) | 15.5 (7-40) |
| . | . | Reduction in steroid dose after 3 mo of ECP, no. (%) of patients on steroids . | Reduction in steroid dose after 6 mo of ECP, no. (%) of patients on steroids . | ||
|---|---|---|---|---|---|
| Reduced by at least 50% . | < 50% . | Reduced by at least 50% . | < 50% . | ||
| Receiving steroids pre-ECP, no. (%) of total | 38 (83) | 20 (53) | 18 (47) | 20 (74) | 10 (26) |
| Steroid dose, mg/day, pre-ECP, mean (range) | 26.2 (5-120) | 13.1 (0-40) | 20.9 (7-120) | 7.3 (0-15) | 15.5 (7-40) |
Serum BAFF levels in cGVHD patients before ECP were significantly higher than healthy controls in agreement with other studies3,8 (n = 30, data not shown; mean, 3.3 ng/mL; range, 0.60-10.2 ng/mL vs mean, 0.58 ng/mL; range, 0.35-0.9 ng/mL; P < .001). Prior studies suggest that BAFF level is modulated by steroid dose8 ; and although we could not identify consistent steroid dose-BAFF correlation at ECP start (data not shown), the timing of BAFF measurement at 1 month of ECP before steroid dose taper alleviated the potential confounding influence of steroid dose changes on BAFF level.
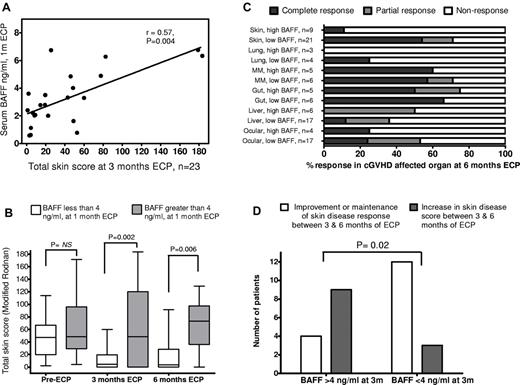
BAFF level exhibited weak correlation with cutaneous cGVHD severity before ECP start (r = 0.24, P = .16; data not shown) but a stronger, significant correlation between BAFF level after paired photopheresis treatments (at 1 month of ECP) and skin disease scores (> 0) at 3 months (Figure 1A; r = 0.57, P = .004, Spearman rank). After 1 month of ECP, BAFF level was predictive of skin disease response, with serum BAFF less than 4 ng/mL associated with significant skin improvement (Figure 1B; median TSS, 47 before ECP reducing to 4.5 after 3 months) in contrast to patients with BAFF more than 4 ng/mL (median TSS before ECP, 48.5; median TSS 3 months ECP, 46). Lower BAFF level at 1 month was also associated with continuing improvement after 6 months ECP (median TSS, 3.5) and complete skin resolution in 11 of 20 (55%) patients, whereas higher 1-month circulating BAFF was associated with a worsening median skin score (median TSS, 76) and complete skin resolution in 1 of 10 (10%) patients.
Relationship between BAFF and ECP treatment outcome for cGVHD. (A) Correlation between serum BAFF level after 2 paired photopheresis treatments (at 1 month of ECP) and total skin disease scores (> 0) at 3 months (r = 0.57, P = .004, Spearman rank). BAFF level showed weaker correlation with cutaneous cGVHD severity before ECP start (r = 0.24, P = .16, data not shown). (B) Serum BAFF level in the early stages of ECP treatment predicted cGVHD skin disease response. Patients grouped into serum BAFF level either less than 4 ng/mL (white bars, n = 24) or more than 4 ng/mL (gray bars, n = 11) after 2 paired treatments (1 month) of ECP had similar TSSs (median, 47 vs 48.5) before starting ECP. After 3 months of ECP, patients with early lower BAFF level demonstrated significant improvement in skin disease compared with those with higher BAFF 2 months earlier (median TSS, 4.5 vs 46, P = .002). The disparity in disease improvement between the low and high serum BAFF groups at 3 months was still evident after 6 months of ECP (median TSS, 3.5 vs 76, P = .006). (C) Lower circulating BAFF level at 1 month of ECP is associated with superior cutaneous disease outcome at 6 months (P < .01). Combined response rate (CR + PR, dark and light gray bars) for low BAFF level patients (< 4 ng/mL) was also higher in lung, mucous membrane, and ocular GVHD, but individual organ response rates were not significantly different for these less common manifestations (P > .05). Patients who achieved full or partial cutaneous response at 6 months of ECP did exhibit contrasting rates of extracutaneous organ response at the same time point (65% and 50%, respectively) compared with cutaneous nonresponders (35%, P = .06, not significant, Fisher exact). (D) Serum BAFF level after 3 months of ECP predicted durability of skin disease response at 6 months. Four of 13 patients (31%) with BAFF level greater than 4 ng/mL at 3 months saw further improvement or maintenance of initial response (white bars), whereas remaining patients (69%) saw deterioration in skin disease (gray bars). In contrast, 12 of 15 (80%) patients with BAFF less than 4 ng/mL at 3-month ECP had durable responses, either maintaining full skin disease resolution or continuing improvement at 6 months (P = .02, Fisher exact). Patients with high BAFF after 3 months of ECP had lower 6-month extracutaneous organ response rate than low BAFF patients (42% vs 54%; data not shown, P = .2, not significant).
Relationship between BAFF and ECP treatment outcome for cGVHD. (A) Correlation between serum BAFF level after 2 paired photopheresis treatments (at 1 month of ECP) and total skin disease scores (> 0) at 3 months (r = 0.57, P = .004, Spearman rank). BAFF level showed weaker correlation with cutaneous cGVHD severity before ECP start (r = 0.24, P = .16, data not shown). (B) Serum BAFF level in the early stages of ECP treatment predicted cGVHD skin disease response. Patients grouped into serum BAFF level either less than 4 ng/mL (white bars, n = 24) or more than 4 ng/mL (gray bars, n = 11) after 2 paired treatments (1 month) of ECP had similar TSSs (median, 47 vs 48.5) before starting ECP. After 3 months of ECP, patients with early lower BAFF level demonstrated significant improvement in skin disease compared with those with higher BAFF 2 months earlier (median TSS, 4.5 vs 46, P = .002). The disparity in disease improvement between the low and high serum BAFF groups at 3 months was still evident after 6 months of ECP (median TSS, 3.5 vs 76, P = .006). (C) Lower circulating BAFF level at 1 month of ECP is associated with superior cutaneous disease outcome at 6 months (P < .01). Combined response rate (CR + PR, dark and light gray bars) for low BAFF level patients (< 4 ng/mL) was also higher in lung, mucous membrane, and ocular GVHD, but individual organ response rates were not significantly different for these less common manifestations (P > .05). Patients who achieved full or partial cutaneous response at 6 months of ECP did exhibit contrasting rates of extracutaneous organ response at the same time point (65% and 50%, respectively) compared with cutaneous nonresponders (35%, P = .06, not significant, Fisher exact). (D) Serum BAFF level after 3 months of ECP predicted durability of skin disease response at 6 months. Four of 13 patients (31%) with BAFF level greater than 4 ng/mL at 3 months saw further improvement or maintenance of initial response (white bars), whereas remaining patients (69%) saw deterioration in skin disease (gray bars). In contrast, 12 of 15 (80%) patients with BAFF less than 4 ng/mL at 3-month ECP had durable responses, either maintaining full skin disease resolution or continuing improvement at 6 months (P = .02, Fisher exact). Patients with high BAFF after 3 months of ECP had lower 6-month extracutaneous organ response rate than low BAFF patients (42% vs 54%; data not shown, P = .2, not significant).
BAFF level before ECP did not correlate with cGVHD severity as determined by National Institutes of Health score or other assessment in non-skin sites (data not shown). Response rates (CR + PR) in the low BAFF group were higher for ocular, lung, and mucous membrane involvement at 3 and 6 months (Figure 1C, 6-month outcomes). However, extracutaneous cGVHD manifestations were less common than skin cGVHD in the cohort, restricting correlation analysis between individual organ response outcome and BAFF level (Figure 1C). Patients who achieved full, partial, or no cutaneous response at 6 months of ECP did exhibit different rates of extracutaneous organ response at 6 months (65%, 50%, and 35% respectively; P = .06, Fisher exact), supporting the perception that skin disease resolution, significantly more common among the low BAFF cohort (55% vs 10% for high BAFF cohort at 6 months), is associated with superior visceral organ outcome. Patients with BAFF levels less than 4 ng/mL at 1 month were more likely to achieve at least a 50% steroid dose reduction between 1 and 6 months of ECP than high BAFF patients (14 of 20, 70% vs 5 of 11, 46%), supporting linkage between lower disease activity and the ability to reduce steroid burden. It was anticipated that steroid dose changes after 1 month of ECP may confound analysis by influencing BAFF level. Nevertheless, BAFF level after 3 months of ECP predicted the durability of skin disease improvement and likelihood of deterioration at 6 months (Figure 1D), suggesting that continuing high BAFF levels may either reflect or influence uncontrolled disease activity.
The nature of B-cell–T-cell cross-talk in cGVHD is poorly understood.15 B-cell lymphopenia and relative B-cell immaturity common in cGVHD are proposed to generate an imbalance between bioavailable BAFF produced by antigen-presenting cells (APCs) and BAFF receptor-positive B cells.3,8 Continued presence of excess BAFF in patients receiving ECP may perpetuate the dysregulated B-cell homeostasis and augment T cell–associated inflammatory processes.3,8,16,17 In contrast, lower BAFF levels may encourage B-cell compartment normalization, promoting coherent negative selection.18 Leukocyte apoptosis generated by ECP is known to influence APC cytokine production in cGVHD.19,20 Modulation of APC BAFF production by ECP is plausible but untested. It is unclear why early low BAFF level is more strongly associated with subsequent skin disease improvement than in other GVHD-affected organs in our study, although the skin is the most responsive and common GVHD target for ECP in our and other studies.21,22
This initial report suggests that early measurement of circulating BAFF during ECP therapy for cGVHD represents a potentially useful and technologically undemanding biomarker in the prediction of treatment outcome. However, one of the central aims of ECP therapy, namely, tapering and ultimately cessation of steroid administration, probably limits its usefulness later in the course of treatment because of diminishing glucocorticoid influence on BAFF-producing APCs. Whether association of early lower BAFF level and clinical improvement of cGVHD is restricted to ECP remains to be determined, although rituximab treatment failure in cGVHD is reportedly associated with persisting high BAFF levels.23 Our data support further larger prospective studies to assess the potential prognostic value of early BAFF measurement in ECP therapy for cGVHD.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: R.W. designed and performed the research, analyzed the data, and wrote the manuscript; and P.C.T. performed the clinical research and wrote the manuscript.
Conflict-of-interest disclosure: P.C.T. has received honoraria for providing lectures for Therakos, suppliers of the ECP device. R.W. declares no competing financial interests.
Correspondence: Robert Whittle, Haematology Department, Rotherham General Hospital, Moorgate Road, Rotherham, South Yorkshire, United Kingdom, S60 2UD; e-mail: robert.whittle@rothgen.nhs.uk.