Abstract
Abstract 980
Lenalidomide is an immunomodulatory drug with single agent activity in untreated (Badoux × et al. 2011) and relapsed or refractory CLL (Chanan-Khan A et al. 2006; Ferrajoli A et al. 2008). Lenalidomide enhances NK-cell mediated antibody-dependent cytotoxicity of rituximab against CLL cells in-vitro (Wu L et al. 2008). Based on the potential for synergistic activity between lenalidomide and rituximab and as there are no overlapping toxicities between these two drugs, we designed this phase II trial of lenalidomide and rituximab as salvage therapy for CLL patients (pts). We present a final analysis of responses to therapy.
Between 09/2008 and 09/2009, 59 pts with relapsed or refractory CLL were enrolled. All had received prior purine analogue containing therapy, and had indications for therapy according to NCI-WG criteria. Pts were required to have an ECOG performance status ≤ 2 and adequate organ function (serum creatinine ≤ 2mg/dl, bilirubin ≤ 2mg/dl). There was no minimum requirement for neutrophil or platelet count. Pts with HIV, active hepatitis B or C, or tuberculosis within 5 years were excluded. Therapy consisted of 28-day cycles of lenalidomide 10mg p.o. daily starting from day 9 of cycle 1; rituximab 375mg/m2 i.v. was given weekly for 4 weeks starting on day 1 of cycle 1, and on day 1 of cycle 3 to 12 each. The dose of lenalidomide was reduced for grade ≥ 3 hematological toxicity (dose −1: 5mg/day, dose −2: 2.5 mg/day). Lenalidomide was administered for a total of 12 months, but could continue longer if there was benefit to the pts. Allopurinol was administered from days 1 to 14 of cycle 1. The primary endpoint was response according to the 1996 NCI-WG criteria which was determined after 3 and 6 cycles and every 6 cycles thereafter.
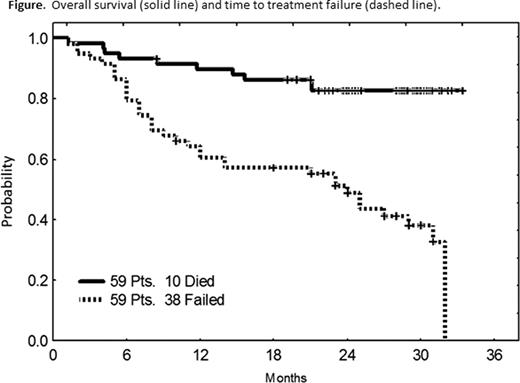
The median age was 64 years [34–82]. The median number of prior regimens was 2 [1–8]. Almost all pts had previously received rituximab (98%), and either FCR or CFAR (88%). Other pre-treatment characteristics have been previously described (Ferrajoli et al. ASH abstracts 2010). The overall response rate (ORR) is 66% including 6 complete responses (CR, 10%), 10 nodular partial responses (nPR, 17%) and 23 partial responses (PR, 39%). Of 15 pts with del(17p), 2 achieved CR (13%), 2 nPR (13%) and 4 PR (27%) for an ORR of 53%. With a median follow-up of 25 months, 15 pts (25%) remain on therapy with an estimated median time to treatment failure of 24 months. Forty-nine pts are alive with an actuarial 2-year overall survival of 83%. There have been 3 deaths on therapy: 1 stroke, 1 early death with infectious exacerbation of chronic obstructive pulmonary disease and 1 death from an unrelated cardiac arrhythmia. One pt died from pneumocystis pneumonia while receiving immunosuppression for autoimmune haemolysis and 6 pts died following subsequent therapy for progressive disease (n=2) or Richter's transformation (n=4). One patient was diagnosed with colon cancer 10 months are initiation of therapy and another patient developed a myelodysplastic syndrome after 6 months. No other second malignancies have been observed on therapy. Grade ≥3 hematological toxicity included neutropenia (40 pts, 47%), thrombocytopenia (13 pts, 22%), and anemia (6 pts, 10%). Eighteen pts (31%) experienced grade ≥3 infections. There was one episode of grade 3 tumor lysis. All tumor flare (16 pts, 27%) reactions were grade ≤2. Common grade ≤2 non-hematological toxicities included fatigue (42 pts, 71%), diarrhea (23 pts, 39%), rash (16 pts, 27%), sensory peripheral neuropathy (15 pts, 25%) and constipation (13 pts, 22%).
Off Label Use: Lenalidomide is an immune modulator being studied in the treatment of relapsed and refractory CLL. Keating:Celgene: Membership on an entity's Board of Directors or advisory committees. O'Brien:Celgene: Consultancy; GSK: Consultancy. Wierda:Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees. Lerner:Celgene: Membership on an entity's Board of Directors or advisory committees. Ferrajoli:Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal