Abstract
Abstract 945
The mTORC1 signaling pathway is activated in nearly 100% of AML and is one of the key pathway of blast proliferation. RAD001 (Everolimus) is a specific mTORC1 inhibitor with demonstrated clinical activity in solid tumor. We present here a clinico-biological phase I trial combining RAD001 and chemotherapy in younger AML patients (NCT 01074086).
Patients (pts) less than 65 years old with relapsing AML at least one year after CR following prior chemotherapy +/− auto or alloSCT were recruited from the GOELAMS centers from March 2008 to July 2011. The treatment regimen consisted of escalating doses of oral RAD001 at D1 and D7 + daunorubicin 60mg/m2 D1-3 and cytarabine CIV 200 mg/m2/d D1-7. In the absence of dose-limiting toxicity (DLT) after the inclusion of 3 patients, the RAD001 doses were escalated by 10mg over 7 dose levels (DL) from 10mg to 70mg. If there were more than 5% of bone marrow blasts on D15, a second course of induction chemotherapy was administered with daunorubicin 35mg/m2 on D17-D18 and cytarabine 1000mg/m2 BID on D17-D20. A plasma inhibitory activity (PIA) test reflecting the % of inhibition of p70S6K phosphorylation (a direct substrate of mTORC1) in MOLM-14 cells by the serum of the patients was performed. H0, H4, H24 and D7 serum samples were obtained for the DL 10 mg to 40mg and supplementary D3 and D5 samples were collected for the DL 50 and 60mg.
Twenty one pts have been included in this trial. Their median age was 50.3y (range 20–65); AML FAB subtypes were as follows: AML0=1, AML1=4, AML2=4, AML4=4, AML5=5, secondary AML=3. Cytogenetics evidenced 1 inv(16), 2 pts with abnormal intermediate risk, 14 normal, 3 poor risk cytogenetics while 1 patient had no available karyotypic information. Documented infections occurred in 16 subjects with grade 3 or more infections in 3 cases. Liver and lung mucormycosis along with appendicitis mass and a septic shock was observed in one patient at 10mg of RAD001. A Fournier gangrene occurred in another one at D40 at DL 50mg. Finally, for another pt, a Candida parapsilosis sepsis occurred on D20 of treatment on a central catheter at DL 50. Two grade 3 oral stomatites at the DL of 20mg and 30mg were observed, as well as 1 grade 3 digestive toxicity at DL 10mg, 2 grade 3 hemorragic events at DL 20mg and 30mg, 2 grade 3 total body erythema at DL 10 and 20mg, 2 grade 3 hepatic cytotoxicity at DL 20mg and 50mg, resolving in less than 14 days and 2 grade 2 renal toxicity at DL 20 and 60mg. None of these effects were considered to be directly related with RAD001. For each DL from 10mg to 40mg and 60mg, 3 pts were included according to the classical 3+3 inclusion schema. For DL 50mg, 6 pts were included as 2 grade>3 SAE have been observed at this level (hepatic dysfunction and fungal sepsis which resolved within 28 days of induction). Hematological toxicities duration were reported as usual (mean neutropenia duration 38 days (range 20–45), mean thrombocytopenia duration 40 days (range 30–65d)). The MTD was not attained at 60mg and accrual is ongoing at 70mg. Four deaths occurred in the study after D40 of induction but none in relation to RAD001 administration.
Overall, 15/21 pts achieved CR (71%), among which 9 pts required a second induction course at D15. Four pts were allo-transplanted in CR. Clinical responses occurred across all dose levels but 8/9 pts obtained CR for DL 50mg and 60mg.
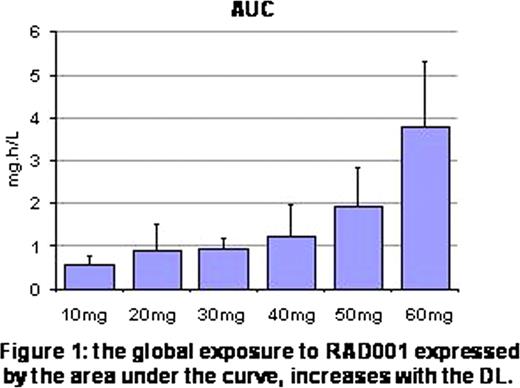
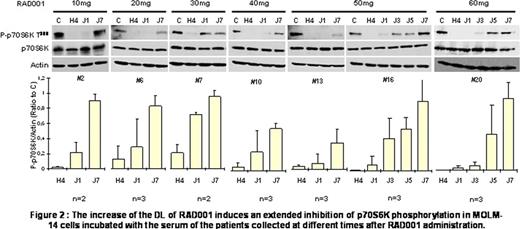
As shown in Figure 1, the global exposure to RAD001 (as expressed by the area under the curve, AUC) increased with the dose. Using a PIA test in MOLM-14 cells (Figure 2), we observed that there was an excellent inhibition of p70S6K phosphorylation in almost all samples 4 hours after administration of RAD001 whatever the DL (mean decrease of 93%). However, this strong inhibition seems to be maintained until D1 only with 50mg and 60mg of RAD001 (mean decrease of 63% with 10 to 40mg of RAD001 vs 93% with 50 and 60mg). As expected, an increase of RAD001 DL induces an extended inhibition of the mTORC1 signaling pathway until D3 (decrease of 94% with 60mg vs 57% with 50mg). In contrast, at D5 and D7, phosphorylation of p70S6K is only barely affected.
In this trial testing the association of RAD001 + chemotherapy, the MTD for RAD001 was not attained at DL 60mg. The CR rate was 71% and the PIA with 60mg of RAD001 seems to be promising. We conclude that these results could be improved with an increased DL of RAD001. The trial is therefore still ongoing with a DL of 70mg and updated results will be presented at the meeting.
Park:Amgen: Honoraria; Janssen-Cilag: Honoraria; Celgene: Honoraria; Novartis: Research Funding. Off Label Use: RAD001. Merlat:Novartis: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal