Abstract
Abstract 677 FN2
FN2
It is well recognized that patients with multiple myeloma (MM) who undergo induction chemotherapy experience treatment-induced numbness and tingling, indicating the development of peripheral neuropathy (PN) that can be a dose-limiting side effect. There is need to identify which patients are more at risk for this side effect, to understand its association with the development of other symptoms, and to explore possible biomarkers associated with its development. Inflammation has been proposed as one mechanism underlying PN.
Patients with MM who were scheduled to receive bortezomib-based chemotherapy with or without lenalidomide before evaluation for autologous stem cell transplantation were enrolled on study. Enrollment generally occurred before induction therapy, but no later than two cycles of induction. Patients with pre-existing PN were excluded, but patients with diabetes were not. Patients repeatedly rated numbness/tingling, pain, and other symptoms via the M. D. Anderson Symptom Inventory (MDASI) during induction therapy (twice a week for 12 weeks, then weekly up to 16 weeks). Patients contributed a serum sample before start of every chemotherapy cycle. A panel of cytokines was examined by Luminex method. Mixed regression analysis was used to describe the trajectory of numbness/tingling over time and to examine the relationship of numbness to other symptoms during this period. Group-based trajectory modeling was used to identify a group of patients who developed more-severe numbness during induction, to determine baseline risk factors for numbness development, and to compare serum cytokine profiles between high-numbness and low-numbness groups.
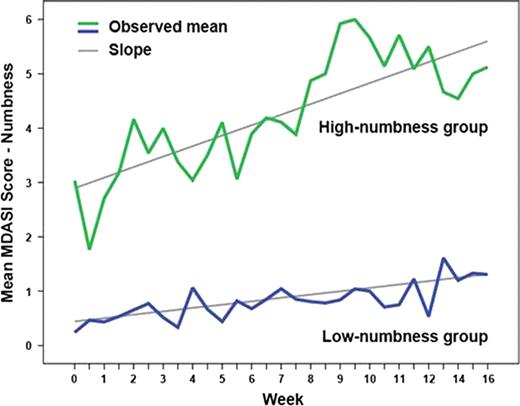
Sixty-four patients were enrolled. During induction, numbness/tingling persistently worsened from baseline (P=.011). A diagnosis of diabetes without PN (10/64) was the only baseline clinical predictor of high numbness development (P=.01). Increasing numbness over time was significantly associated with pain (P=.005), disturbed sleep (P<.0001), and muscle weakness (P=.0001). In group-based trajectory modeling, 42% (27/64) of patients were in the high-numbness group and 58% (37/64) were in the low-numbness group (Figure 1). Both groups showed a linear increase in numbness over time (both P<.05). Those patients with higher moderate to severe baseline ratings of symptoms other than numbness, including fatigue, pain, disturbed sleep, drowsiness, and muscle weakness, were at significantly higher risk to be in the high-numbness group (OR=3.35, 95% CI=1.15–9.72, P=.02). Patients in the high-numbness group were also more likely to develop more-severe pain over time (P=.010). Levels of circulating cytokines (IL-1R1, IL-6R, sTNF-R1, sTNF-RII, and TNF-alpha) were significantly different between the two numbness groups (each P<.01).
This prospective, longitudinal study evidenced that in patients with newly diagnosed MM receiving induction therapy, 42% suffer from a rapid increase in numbness/tingling. Diabetes and high symptom levels at baseline were predictive of membership in the high-numbness group. An increase in numbness was associated with increases in other symptoms, including pain, disturbed sleep, and muscle weakness. We also observed a proinflammatory response (IL-1, IL-6, and TNF-alpha) during induction therapy that was associated with the development of more-severe numbness, warranting further translational studies to confirm the status of these cytokines as biomarker(s) related to PN and, ultimately, to develop mechanism-driven, effective patient care during standard induction therapy.
This study was funded by NIH grant P01 CA124787
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal