Abstract
Abstract 634
Pts with relapsed/refractory multiple myeloma (RRMM), resistant to LEN and BORT, have poor outcomes. POM (2 or 4 mg/d for 28 d of each 28-d cycle) is an immunomodulatory compound with activity in pts refractory to both LEN and BORT (Lacy MQ et al. Blood 2011;doi:10.1182). This multicenter phase 1/2 study investigated the safety and efficacy of POM alone or in combination with LoDex (POM+LoDex) for treatment of pts with RRMM who had received both BORT and LEN. Phase 1 identified 4 mg/d of POM as the recommended dose for phase 2 (Richardson PG et al. Blood 2010;116:Abs 864). Phase 2 results are presented.
Pts with RRMM after ≥ 2 prior regimens, including ≥ 2 cycles of LEN and BORT separately or in combination, were eligible. Pts had to have progressed ≤ 60 d of their last treatment prior to study entry (refractory disease). This analysis evaluated the efficacy and safety of POM+LoDex (POM 4 mg/d 1–21 d of each 28-d cycle; Dex 40 mg/wk) and POM alone in a randomized open-label study. Results presented here were based on investigator assessed responses for the intent-to-treat population. Responses were independently adjudicated. The primary endpoint was progression-free survival (PFS). Secondary endpoints were objective response (partial response [PR] or ≥ PR), duration of response (DOR), overall survival (OS), and safety. All pts received daily low-dose aspirin thromboprophylaxis.
A total of 221 pts were enrolled in phase 2 (POM+LoDex n = 113; POM n = 108); 219 received ≥ 1 cycle of study treatment and 191 pts were evaluable for response. Baseline characteristics were comparable between the two arms with a median of 5 (range 2–13) prior therapies in both arms; 74% of pts in POM+LoDex and 76% of pts in POM alone had prior autologous stem-cell transplantation (ASCT). The remaining pts were elderly (aged > 75 yrs) or ineligible for ASCT; all pts were exposed to corticosteroids and 84% in the POM+LoDex and 95% in POM alone arms were exposed to alkylators. Pts were refractory to LEN (POM+LoDex 77% and POM alone 79%), BORT (73% and 69%), or both drugs (61% and 59%). Among pts who were randomized to receive POM alone, 61 (56%) subsequently went on to receive POM+LoDex due to progressive disease (PD) per protocol.
A median of 5 (range 1–17) treatment cycles were received by pts in both arms. Median treatment duration was 5.0 mos.
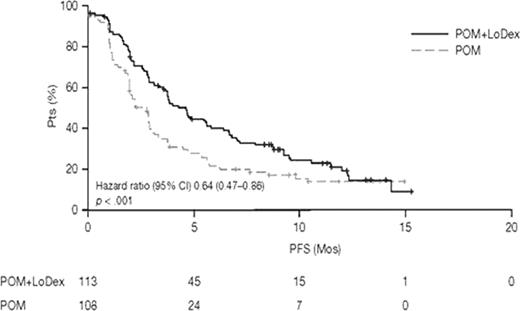
Response of ≥ PR was seen in 34% of pts in the POM+LoDex arm and 13% in the POM alone arm, including 1% complete response (CR) in each arm; ≥ minor response (MR) was 45% vs 29%, respectively. Median DOR was 7.7 mos with POM+LoDex and 8.3 mos with POM alone, and median PFS was 4.6 mos and 2.6 mos, respectively (Fig 1). Median OS was comparable for both arms (14.4 and 13.6 mos). Results from independent adjudication were similar, with ≥ PR in 30% of pts in the POM+LoDex arm and 9% in the POM alone arm, including 1% and 0% CR, respectively, in each arm. ≥ MR was achieved with POM+LoDex in 45% and with POM alone in 25%; PFS was 3.8 mos and 2.5 mos, respectively.
In the subgroup of pts refractory to both LEN and BORT, 30% and 16% of pts treated with POM+LoDex or POM alone, respectively, achieved ≥ PR; ≥ MR was 45% and 30%, respectively. Median PFS was 3.8 mos for POM+LoDex and 2.0 mos for POM alone; median OS showed a similar trend (13.5 and 10.8 mos, respectively).
The main reason for treatment discontinuation was PD in both arms (POM+LoDex 51%; POM alone 44%); discontinuations due to adverse events (AEs) were 7% and 12%, respectively. Grade 3/4 AEs in POM+LoDex vs POM alone, respectively, were: neutropenia 38% and 47%; febrile neutropenia 2% and 2%; thrombocytopenia 19% and 21%; anemia 21% and 17%; pneumonia 19% and 8%; and fatigue 10% and 8%. All grades of peripheral neuropathy, deep vein thrombosis, and renal failure occurred in 7% and 10%, 2% and 1%, and 2% and 1% of pts for POM+LoDex vs POM alone, respectively.
POM (4 mg/d 1–21 d of each 28-d cycle) with or without LoDex demonstrates clinical activity and is generally well tolerated in pts with advanced MM who have received multiple prior therapies and are refractory to both LEN and BORT. Prospective comparison indicates that POM+LoDex is associated with greater clinical benefit and no increased toxicity vs POM alone. This is supported by high response rates, long DOR, and PFS benefit achieved with POM+LoDex. The regimen is now being investigated both in phase 3 trials, and as part of combination treatment including with BORT.
Richardson:Millennium Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Celgene Corporation: Membership on an entity's Board of Directors or advisory committees; Novartis Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Johnson & Johnson: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees. Off Label Use: Pomalidomide is a new treatment for multiple myeloma. Siegel:Celgene Corporation: Honoraria, Research Funding, Speakers Bureau. Vij:Celgene: Consultancy, Research Funding, Speakers Bureau. Jagannath:PER Group: Honoraria; J & J Pharm: Membership on an entity's Board of Directors or advisory committees; Envision Communication: Honoraria, Membership on an entity's Board of Directors or advisory committees; Imedex: Honoraria; Dava Oncology: Membership on an entity's Board of Directors or advisory committees; Merck Sharp & Dohme: Investigators meeting; Celgene Corporation: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jansen Pharmaceuticals: Honoraria; Medicom Worldwide: Membership on an entity's Board of Directors or advisory committees. Chen:Celgene Corporation: Consultancy, Honoraria, Research Funding. Lonial:Celgene Corporation: Consultancy; Millennium Pharmaceuticals: Consultancy; Novartis: Consultancy; Bristol-Myers Squibb: Consultancy; Onyx: Consultancy; Merck: Membership on an entity's Board of Directors or advisory committees. Jakubowiak:Ortho Biotech: Consultancy, Honoraria, Speakers Bureau; Celgene Corporation: Consultancy, Honoraria, Speakers Bureau; Millennium Pharmaceuticals: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Onyx Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Exelixis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Bahlis:Celgene Corporation: Consultancy, Honoraria, Research Funding, Speakers Bureau. Baz:BMS: Research Funding; Millennium: Research Funding; Celgene Corporation: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Research Funding. Larkins:Celgene Corporation: Employment. Chen:Celgene Corporation: Employment. Zaki:Celgene Corporation: Employment. Anderson:Acetylon Pharmaceuticals: Consultancy, Equity Ownership; Celgene Corporation: Membership on an entity's Board of Directors or advisory committees; Novartis Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Millennium Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Onyx: Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Merck: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal