Abstract
Abstract 4701
Allogeneic hematopoietic stem cell (HSC) transplant can cure patients with high risk and relapsed acute leukemia via a graft versus leukemia (GvL) effect. In our previous studies, depletion of CD11b+ cells (containing CD11b+ dendritic cells, CD11b+ NK cells, and myeloid suppressor progenitor cells) from donor bone marrow (BM) improved immune reconstitution and enhanced GvL effects without increased rates of GvHD in mice (Li, et al. BBMT 2004). We also have transplanted combinations of FACS purified HSC, donor T cells, and CD11b- dendritic cells (DC) and found similar improvement in GvL activity associated with donor T cells polarized towards a Th1 phenotype (Li, et al. JI 2009). In contrast, grafts containing FACS purified HSC, donor spleen T cells, and CD11b+ DC did not have significant GvL activity and donor T cells were polarized towards a Th2 phenotype after transplant. The objective of this study was to determine if enriching the relative numbers of donor plasmacytoid dendritic cells by selectively depleting CD11b+ DC from BM would yield similar enhancement of the GvL activity of donor T-cells as transplants of purified populations of HSC, CD11b-DC, and T cells in tumor-bearing mice.
Selective depletion of CD11b+ DC from the BM allograft was achieved by FACS sorting and selectively removing the CD11b+ DC that comprised ∼1% of the BM. The CD11b+ DC-depleted graft thus contained ∼99% of all nucleated cells in the BM. Control undepleted BM grafts were also stained and sorted using only light scatter gates. To study the immunological effects of specific CD11b+ DC depletion, lethally irradiated B10.BR or BA.B10 recipients were transplanted with 3 × 10E6 CD11b+ DC FACS-depleted or undepleted BM cells and 1×10E6 spleen T cells from C57BL/6J or BA donors. Mice received 500,000 luciferase-positive LBRM cells (a T cell lymphoma) i.v. 1 day prior to transplant. Recipient mice were monitored for survival, weight change, and GvHD score (based on weight change, activity, posture, fur texture, and skin condition) throughout the duration of the experiment and for donor cell engraftment at days 30, 60, and 100 post transplant. Donor T cells were recovered from transplant recipients on days 3 and 10 post transplant and were examined for proliferation, Th1/Th2 polarization by flow cytometry, and ELISA measured serum cytokines.
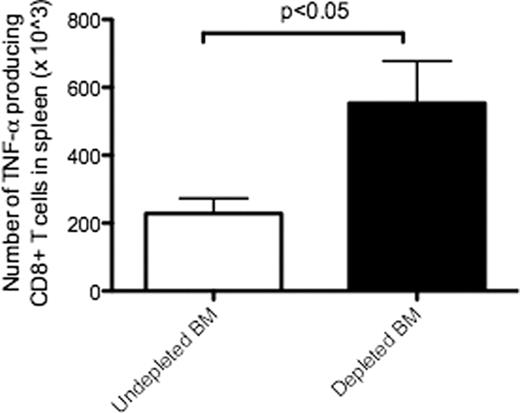
BMT recipients of CD11b+ DC-depleted BM had higher survival than recipients of undepleted BM in tumor-bearing mice (p<0.05) (Figure 1). T cell chimerism at day 100 was > 95% for all mice, regardless of CD11b+ DC-depletion from the BM allograft. The level of GvHD in recipient mice did not differ significantly between groups. Donor T cell proliferation was increased on day 3 post transplant in recipients of CD11b+ DC-depleted BM (p< 0.05). On day 10 post transplant, serum IFN-g levels were increased in recipients of CD11b+ DC-depleted BM compared with undepleted BM(p<0.05). On day 10 post transplant, recipients of CD11b+ DC-depleted BM had significantly higher numbers of TNF-alpha producing donor CD8 T cells compared with donor CD8 T cells from recipients of undepleted BM (p<0.05) (Figure 2).
Depletion of CD11b+ DC from donor BM allografts results in improved survival of recipient mice in a tumor model
Depletion of CD11b+ DC from donor BM allografts results in improved survival of recipient mice in a tumor model
Recipients of CD11b+DC-depleted BM had significantly higher numbers of TNF-alpha producing donor CD8 T cells.
Recipients of CD11b+DC-depleted BM had significantly higher numbers of TNF-alpha producing donor CD8 T cells.
Transplantation of BM allografts enriched for plasmacytoid DC by selective depletion of CD11b+ DC increased GvL activity of donor T cells without increasing GvHD. These data suggest that donor BM CD11b+ DC inhibit donor T-cell proliferation, Th1 polarization, and limit GvL activity.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal