Abstract
Abstract 3410
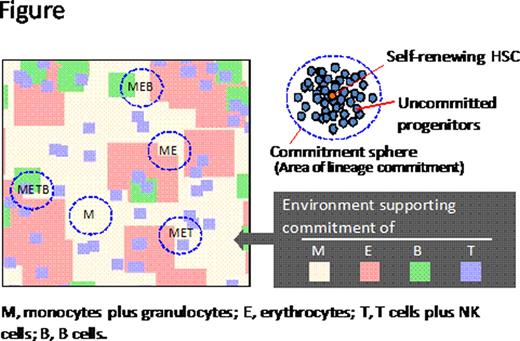
To sustain hematopoiesis, hematopoietic stem cells (HSCs) must, on the one hand, replenish themselves by self-renewal and on the other hand produce differentiating progenitor cells. However, it has been difficult to address the actual dynamics of hematopoiesis due to the lack of appropriate experimental systems, regardless of animal species. In the case of humans, however, we were lead to consider one “experiment of nature” that makes it possible to track the progeny of a HSC; i.e. by detecting blood cells deficient in glycosylphosphatidylinositol-anchored proteins (GPI-APs) using flow cytometry. These cells are known to be derived from HSCs with a mutation in the phosphatidylinositol N-acetylglucosaminyltransferase subunit A (PIG-A) gene, which have properties similar to normal HSCs. We recently found that GPI-APs− cells in patients with aplastic anemia (AA) and myelodysplastic syndrome (MDS) frequently show various patterns in the proportion of granulocytes and erythrocytes and that the individual patterns of the two lineage combinations can persist for many years. GPI-APs− cells were rather frequently found in patients with less severe bone marrow (BM) failure. We thus reasoned that the dynamics of HSCs visualized in these cases might reflect those occurring during normal hematopoiesis. Objectives/Methods: We determined the proportion of GPI-APs− cells in six major lineages, namely granulocytes (G), monocytes (M), erythrocytes (E), T cells (T), NK cells (NK) and B cells (B), in peripheral blood cells from 601 BM failure patients using a highly sensitivity flow cytometric analysis and classified the lineage combinations of GPI-APs− cells in patients possessing GPI-APs− cells. We have attempted to test our idea of hematopoiesis by modeling and simulation. Results: Of 601 patients with BM failure, GPI-APs− cells cells were detected in at least one lineage of cells of 250 patients (42%) and the lineage combinations of GPI-APs− cells were classified into 16 different patterns such as GEM, GEMTBNK and GE. Of special interest was our finding that in all 250 cases, the same combinations of lineages were detected regardless of the interval between the first and second analysis and there was a clear trend toward the pattern of the higher the percentage of GPI-APs− granulocytes in patients, the greater the number of GPI-APs− cell lineages. It was clear from our studies that most of the small clones contain only limited lineage cells and even in the case of larger clones, e.g. clones of 1–3% that might be maintained with more than two HSCs, the majority (81%) were non-full-lineage clones. Based on these results indicating that most individual human HSCs only give rise to a limited range of hematopoietic progeny, we proposed a new idea that the observed patterns could be explained by assuming the presence of mosaic-like hematopoietic environments that could simultaneously support the “commitment” of early multipotent progenitors to a certain lineage. We have attempted to test this idea by modeling and simulation and assumed that a self-renewing HSC is located in a particular location in BM and that uncommitted progenitors derived from this HSC can reach to a certain defined area (Figure), which we termed the “commitment sphere”. If the BM microenvironment is mosaic in terms of function in the commitment of progenitors towards a certain lineage, and the size of such mosaic is as large as commitment sphere, then production of progenitors of limited lineages can occur. Indeed, by simulation of many virtual HSCs in a certain type of mosaic environment, formation of similar clone types was recapitulated. We also performed simulations of many virtual HSCs in the same mosaic environment while changing clone size (i.e. changing the size of the commitment sphere). The relationship between clone size and number of lineages in each clone in vivo was quite similar to that of the simulation model. Thus, these simulations provide a reasonable explanation for the observed in vivo findings. Conclusions: Individual HSCs in humans produce only restricted lineages of blood cells. The paradoxical phenomenon can be explained by a model in which the BM microenvironment is mosaic in supporting commitment of progenitors towards distinct lineages.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal