Abstract
DDAVP is the treatment of choice for individuals with type 1 von Willebrand disease (VWD), although 20% are unresponsive, and of the 80% who do respond, the VWF increase is transient, as endothelial stores are depleted after 3 days. Further, administration requires a 30- minute intravenous infusion in a medical facility. Plasma-derived concentrates may be used in these settings, but are more costly and have potential risk of transmissible infection. We recently demonstrated that recombinant human IL-11 (rhIL-11, Neumega®), a gp-130 signaling cytokine with hematopoietic and anti-inflammatory activity, increases VWF activity up to 2-fold when given daily by subcutaneous injection, with levels persisting each day it is given, and reduces menstrual and postoperative bleeding. The effects of rhIL-11 in individuals with VWD unresponsive or allergic to DDAVP, or hemophilia A, however, have not been evaluated.
We conducted a phase II trial to evaluate the safety and biologic effects of rhIL-11 in VWD patients unresponsive or allergic to DDAVP (VWD-Un) or mild hemophilia A (HemA). rhIL-11 was given subcutaneously at 25 μg/kg daily for 4 days in the non-bleeding state, followed on day 4, 30 minutes after rhIL-11, by one dose of DDAVP intravenously, 0.3 μg/kg, if not contraindicated (pt. 2). Fluid restriction was recommended. Fluid status was assessed by height, weight, and exam. Pre- and post-dosing laboratory assays included the VWD profile, VWF multimers by SDS gel electrophoresis, and platelet VWF mRNA by qPCR.
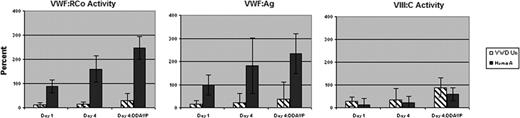
The results of the first six subjects, including three with VWD (one type IIB and two type 1 VWD), VWF:RCo 0.10–0.20 U/ml, and three with mild hemophilia A, F.VIII 0.08–0.12 U/ml, are presented. All subjects were healthy, with no hypertension or cardiac disease, and all had normal physical exams and normal EKGs. By day 4, among VWD-Un subjects, there was a 1.2-fold increase in VWF:RCo (15±3% vs. 12±0%); a 1.6-fold increase in VWF:Ag (22±8% vs.14±6%); and a 1.3-fold increase in VIII:C (34±36% vs. 27±10%), as compared with pre-rhIL-11 levels (Figure). Following DDAVP (except pt. 2), there was an additional 2.0-fold, 1.7-fold, and 2.6-fold increase in VWF:RCo, VWF:Ag, and VIII:C, respectively. Among HemA subjects, by day 4, there was a 1.8-fold increase in VWF:RCo (160±25% vs. 88±12%); a 1.8-fold increase in VWF:Ag (182±28% vs.99±18%), p<0.01; and a 1.5-fold increase in VIII:C (21±8% vs. 14±5%), as compared with pre-rhIL-11 levels. Following DDAVP, there was an additional 1.5-fold (p<0.01), 1.7-fold, and 2.8-fold (p<0.05) increase in VWF:RCo, VWF:Ag, and VIII:C, respectively. The drug was well tolerated well with less than grade 1 mild conjunctival erythema, local erythema and tenderness at the injection site; in one subject transient hyponatremia, Na 129 meq/L, occurred after excess oral fluid intake for diabetic hyperglycemia, which resolved with fluid restriction.
These data suggest that rhIL-11 increases VWF and VIII levels modestly in VWD patients unresponsive/allergic to DDAVP, and in mild hemophilia A, suggesting the potential use in treatment of clinical bleeding in these disorders.
| Subjects . | Diagnosis . | VWF:RCo(%) . | VWF:Ag(%) . | VIII:C(%) . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Pre . | Day4 . | DDAVP . | Pre . | Day4 . | DDAVP . | Pre . | Day4 . | DDAVP . |
| 1. 25/F | I-VWD | 12±0 | 21±9 | 45±26 | 12±5 | 25±6* | 49±16 | 37±18 | 10±6 | 125±36* |
| 2. 22/F | IIBVWD | 12±0 | 12±0 | ND | 25±3 | 35±18 | ND | 37±12 | 86±13† | ND |
| 3. 25/F | I-VWD | 12±0 | 12±0 | 32±0* | 4±0 | 6±0* | 26±15 | 7±0 | 5±0* | 54±34 |
| 4. 25/M | HemA | 78±5 | 121±25 | 227±11* | 81±7 | 139±3* | 207±6* | 10±0 | 19±0* | 78±12* |
| 5. 31/M | HemA | 73±3 | 151±43 | 191±25 | 80±6 | 171±9* | 222±20* | 25±1 | 37±1 | 74±4* |
| 6. 51/M | HemA | 112±42 | 208±0* | 324±60 | 135±3 | 235±13* | 273±44* | 7±0 | 8±0* | 24±8* |
| Subjects . | Diagnosis . | VWF:RCo(%) . | VWF:Ag(%) . | VIII:C(%) . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Pre . | Day4 . | DDAVP . | Pre . | Day4 . | DDAVP . | Pre . | Day4 . | DDAVP . |
| 1. 25/F | I-VWD | 12±0 | 21±9 | 45±26 | 12±5 | 25±6* | 49±16 | 37±18 | 10±6 | 125±36* |
| 2. 22/F | IIBVWD | 12±0 | 12±0 | ND | 25±3 | 35±18 | ND | 37±12 | 86±13† | ND |
| 3. 25/F | I-VWD | 12±0 | 12±0 | 32±0* | 4±0 | 6±0* | 26±15 | 7±0 | 5±0* | 54±34 |
| 4. 25/M | HemA | 78±5 | 121±25 | 227±11* | 81±7 | 139±3* | 207±6* | 10±0 | 19±0* | 78±12* |
| 5. 31/M | HemA | 73±3 | 151±43 | 191±25 | 80±6 | 171±9* | 222±20* | 25±1 | 37±1 | 74±4* |
| 6. 51/M | HemA | 112±42 | 208±0* | 324±60 | 135±3 | 235±13* | 273±44* | 7±0 | 8±0* | 24±8* |
p< 0.001;
p< 0.01.
No relevant conflicts of interest to declare.

This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal