Abstract
Abstract 2580
Challenges in developing new agents for infant MLL leukemia are compounded by rarity of cases, inherent drug resistance and unique vulnerabilities of infants to treatment-related morbidity and mortality. An additional limitation is the extent to which preclinical data and adult clinical experience can predict clinical dose-exposure and exposure-response relationships and guide dosing strategies (dose, duration, combination, schedule) and study designs (sample size, sampling scheme). Pharmacometric approaches are increasingly being used to quantify drug action and clinical performance. These require a continuum of models with hypotheses and assumptions and pharmacologic rationale to be challenged by targeted experimentation and subsequent model refinement. Key assumptions and experimental conditions are reassessed to optimize experimental designs and test evolving understanding about drug behavior and disease biology. Here we establish “proof-of-concept” quantitative pharmacology relationships between data derived from in vitro obatoclax activity in cell lines and patient samples, historical adult experience and a preclinical diseased mouse model to optimize strategies to treat infant leukemia.
Obatoclax in vitro activity was quantified by MTT assays in MLL-rearranged cell lines and primary infant ALL and bilineal MLL leukemia samples. Obatoclax biodistribution was characterized in MLL leukemia-bearing mice to evaluate its potential to achieve target exposures in infants with leukemia by the following steps: 1) A nonlinear mixed-effect model based on existing obatoclax toxicokinetic data in healthy Balb/C mice guided design of a pilot study in diseased NOD-scid-IL-2Rgnull (NSG) mice where target exposure requirements were based on the AUC24h associated with peak oligonucleosomal DNA release as an apoptosis biomarker in an adult Phase I CLL trial (O'Brien 2008) and single-agent MTT assays of obatoclax in MLL cell lines and primary infant leukemia samples. 2) NSG MLL-ENL bilineal leukemia bearing mice with established xenografts received a single IV bolus dose of obatoclax (1.2 or 4.8 mg/kg; 100% or 400% of predicted target exposure). 3) Obatoclax was measured in the plasma, spleen, liver, kidney and brain for 24 h after drug administration (courtesy GeminX). A physiologically-based pharmacokinetic model (PBPK) model was developed to predict exposures in mouse target tissues based on obatoclax physicochemical properties and then scaled to a human infant.
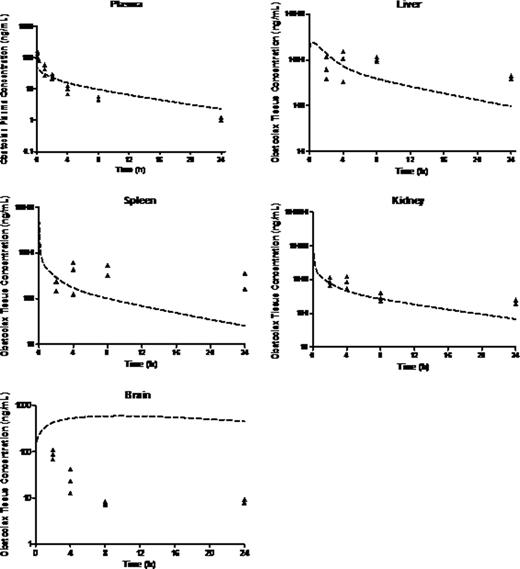
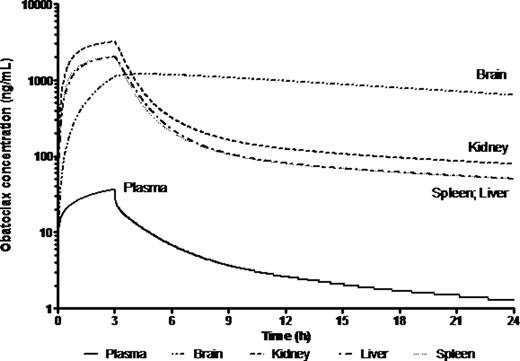
Preclinical in vitro experiments suggested IC50 values between 13 and 918 nM in primary MLL leukemia cells. Population mean (% RSE) parameter estimates for plasma clearance (CL), intercompartmental clearance (Q), plasma distribution volume (Vd) and peripheral distribution volume (V2) were 5.1 (10.1) L/h/kg, 1.78 (37) L/h/kg, 9.15 (10.2) L/kg and 17.3 (23.8) L/kg, respectively. PK parameters were close to those predicted from healthy Balb/C mice except that Cmax was lower, possibly suggesting a larger Vd in mice with disease. The half-life was almost 4-fold higher suggesting slower elimination due to mouse strain or disease burden. There was sustained drug retention in spleen, liver, kidney and brain. Brain:plasma were ∼2-10:1, higher than the <1:1 typical of most drugs, indicating excellent CNS penetration. The PBPK model for obatoclax in the mouse compared well with observed biodistribution in diseased NSG mouse tissues (Fig 1). Though brain penetration was good, deviations between observed and PBPK-simulated exposure values may suggest involvement of transporters. PBPK simulation of obatoclax administration to a 1 yr, 10 kg human infant suggested that adequate drug exposure to target organs should be achievable at clinically relevant doses (Fig 2).
PBPK simulations relative to observed obatoclax biodistribution in the NSG Mouse
PBPK-simulated obatoclax plasma and tissue exposure in a 1 year old receiving 20 mg/m2 infusion over 3 h
PBPK-simulated obatoclax plasma and tissue exposure in a 1 year old receiving 20 mg/m2 infusion over 3 h
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal