Abstract
Abstract 2578
Prognosis for patients (pts) with Ph+ ALL has improved significantly with the introduction of tyrosine kinase inhibitors (TKI). Dasatinib is ∼325 times more potent than imatinib, and has shown activity in pts with imatinib resistance or intolerance, both in CML and Ph+ ALL.
From 9/2006, pts with relapsed Ph+ ALL or CML-LB received dasatinib 100 mg po daily for the first 14 days of each of 8 cycles of alternating hyperCVAD, and high-dose cytarabine with methotrexate. Patients in complete remission (CR) continued to receive maintenance with dasatinib 100 mg po daily, with vincristine and prednisone monthly for 2 years, followed by dasatinib indefinitely. All patients proceeded to an allogeneic stem cell transplant as soon as feasible. From 8/2009, dasatinib dose was modified to 100mg po daily for the first 14 days of the first cycle, then at 70mg po daily continuously. Pts also received Rituximab on Days 1 and 11 of each of the first 4 cycles of therapy.
A total of 32 pts with relapsed Ph+ ALL (n=18) or CML-LB (n=14) have received a median of 3 cycles (range=1–8 cycles). Twenty-three pts were treated on the initial regimen and 9 pts on the modified version. Median age was 50 yrs (range 21–77). Median number of prior regimens was 1 (range=1–2): hyperCVAD plus imatinib (n=10, 3 had transplant in first CR), other combination chemotherapy (n=12), monotherapy with TKI other than dasatinib (n=8), and investigational agents (n=2). Median WBC at start of treatment was 9.8 × 109/L (range=0.3–295.5 × 109/L). Median bone marrow blast percentage was 72% (range 0–97%; 1 pt had solitary CNS relapse). Eight (25%) patients had CNS involvement. Pre-treatment ABL mutations noted in 9 pts included: T315I(n=4), Y253F(n=1), Y253H(n=4), F359V(n=1), E459K(n=1), E255K(n=1), F317L(n=3), M351T(n=1).
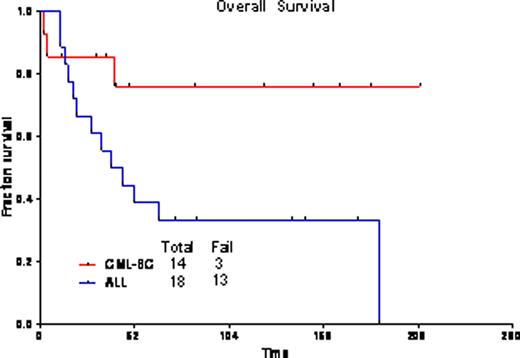
The overall response rate was 94%, with 23 pts (72%) achieving CR, and 7(22%) CR with incomplete platelet recovery (CRp). One pt died during induction. One pt had progressive disease. Twenty-five pts (83%) achieved complete cytogenetic remission after one cycle of therapy. Overall, 13 pts (43%) achieved complete molecular response, and 10 pts (33%) major molecular response (i.e., BCR-ABL/ABL<0.1%). Nine patients proceeded to allogeneic transplantation (ALL n=2, CML-LB n=7); one previously transplanted patient with ALL received donor lymphocyte infusion. Grade 3/4 toxicities included bleeding (GI, GU, and subdural hematomas), pleural effusions, pericardial effusions, infections, diarrhea, hypophosphatemia, hypocalcemia, elevated transaminases, and hyperbilirubinemia. The median follow-up for pts with CML-LB is 85 wks (range=12–209 wks); 3-yr OS is 76% (median not reached); and, 82% remain in CR at 3 yrs [median CR duration (CRD) not reached]. For ALL pts, median follow-up was 139 wks (range=74–175 wks); 3-yr OS is 33% (median=42 wks); and, 30% remain in CR at 3 yrs (median CRD=38 wks). The outcomes were the same for pts with CML-LB who did or did not receive a transplant (3-yr OS 83% for both cohorts). Among pts with ALL, outcome was better for those who underwent transplant (2 of 2 alive at 3 yrs as opposed to 4 of 16 without transplant).
The combination of HyperCVAD regimen with dasatinib is effective in patients with relapsed Ph+ ALL and CML-LB.
Cortes:Novartis: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Ariad: Consultancy, Research Funding. Jabbour:Novartis: Honoraria; Pfizer: Honoraria; BMS: Honoraria. Kantarjian:BMS: Research Funding. Ravandi:Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal