Abstract
Abstract 2145
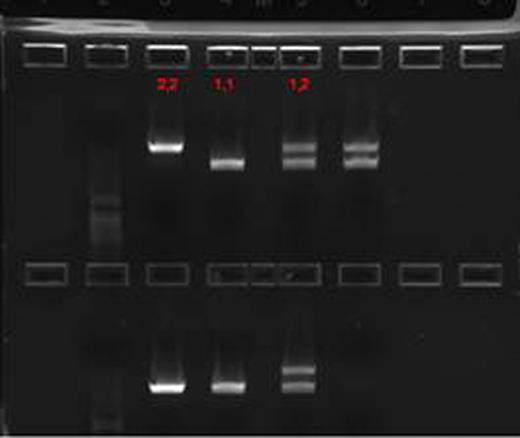
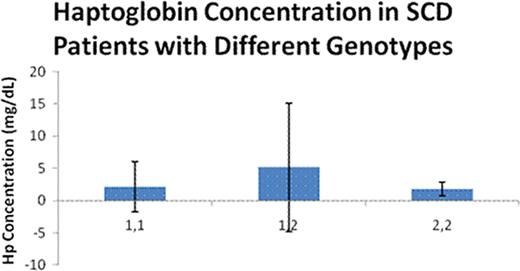
Patients with sickle cell disease (SCD) have both extravascular and intravascular hemolysis; it is estimated that 1/3 of the hemolytic process in SCD is intravascular. Hemoglobin (Hb) released from hemolyzed red blood cells is bound by haptoglobin (Hp) in a 1:1 stochiometric complex, which is then taken up by macrophages through the scavenger receptor, CD163. When the binding capacity of haptoglobin is exceeded, concentration of cell free Hb in plasma increases, excess cell free Hb is a nitric oxide (NO) scavenger and leads to a NO depletion state. This, in turn, leads to endothelial dysfunction with increased expression of adhesion molecules and inflammatory markers, platelet activation, and an inflammatory state. This process has been incriminated in the pathogenesis of certain complications of SCD and other chronic hemolytic states with a significant intravascular hemolytic component. These include increased pulmonary artery pressure (PASP), priapism, leg ulcers, and renal dysfunction. In addition to these deleterious effects of excess cell free Hb, the fate of Hb—Hp complexes may play a significant role in the pathogenesis of the inflammatory state. Hp is a polymorphic protein with two well characterized alleles, Hp-1 and Hp-2. A large body of evidence suggests that Hp-2 allele si a major susceptibility gene for the development of vascular complications (coronary artery restenosisand cardiovascular disease). This has been attributed to the generation of a more potent inflammatory response after the binding of the larger Hp-2/Hb complexes to the CD163 receptor. We previously reported on the distribution of Hp genotypes in our pediatric and adult populations [Hughes et al, Blood 106(11), 3805 30(b), December 2005] and more recently have shown that binding of Hp-2-Hb complexes to mononuclear cell cultures from SCD patients resulted in a 3–12 fold higher inflammatory cytokine release (GM-CSF, IL1, IL-10, and TNF) compared to that observed with Hb-Hp1 complexes [Bora et al, Blood 112(11):513(1441), 2008]. We studied the haptoglobin levels in the plasma of SCD patients on and off hydroxyurea (HU) and in relation to their Hp genotype. Since clinical assays for haptoglobin are rather insensitive below 6 mg/dl, we used a sensitive ELISA (Genway Biotech) technique to quantify Hp. Hp genotypes are determined by a previously published PCR procedure, which resulted in a 3245 bp product for Hp-1 and 4945 bp for Hp-2 (Fig. 1).
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal