Abstract
Abstract 2048
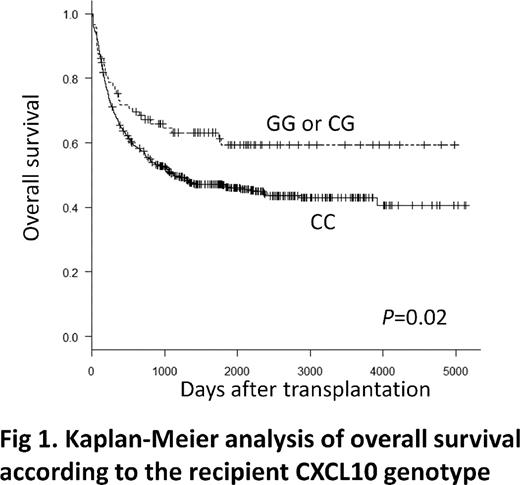
CXCL10, also known as interferon gamma inducible protein 10, is a chemokine secreted by several cell types including monocytes, endothelial cells and fibroblasts that plays important roles in the stimulation and migration of natural killer cells and T cells. The single nucleotide polymorphism (SNP) rs3921 located in the 3'-untranslated region of the CXCL10 gene has been reportedly associated with acute GVHD and with invasive aspergillosis after allogenic stem-cell transplantation. In this study, we analyzed the impact of rs3921 polymorphism on transplant outcomes in patients undergoing unrelated HLA-matched myeloablative bone marrow transplantation (BMT) through the Japan Marrow Donation Program. The CXCL10 genotypes were retrospectively analyzed in a cohort of 652 pairs of patients with hematologic malignancies and their unrelated donors. The genotype frequencies of GG, CG and CC were 1%, 13% and 86% in recipients and 1%, 12% and 87% in donors (P=0.62). The recipient GG or CG genotype was associated with significantly better overall survival (OS; P=0.02; Fig1). No difference was noted in transplant-related mortality, disease relapse or graft-versus-host disease in relation to the recipients' polymorphism. The multivariate analysis showed that in patients with low risk disease, the recipient GG or GC genotype remained statistically significant for better OS (hazard ratio [HR] 0.55; 95% confidence interval [CI], 0.30 to 1.01; P=0.05). The donor CXCL10 genotypes did not significantly influence the transplant outcomes. These results suggest an association of the recipient GG or GC genotypes with better transplant outcomes after unrelated HLA-matched BMT. These findings could therefore be useful in creating therapeutic strategies such as risk-adapted management of transplanted patients.
Disclosures:
No relevant conflicts of interest to declare.
Author notes
*
Asterisk with author names denotes non-ASH members.
© 2011 by The American Society of Hematology
2011

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal