Abstract
Abstract 1959
Reduced intensity conditioning (RIC) prior to allogeneic transplantation allows donor cell engraftment with the maintenance of a graft versus malignancy effect. A recent CIBMTR registry study suggested worse outcomes for patients receiving anti-thymocyte globulin (ATG) as part of the conditioning regimen. To further study the role of ATG when added to a fludarabine and melphalan RIC regimen in the unrelated donor (URD) setting, we have retrospectively studied 76 consecutive patients with advanced hematologic malignancies who underwent URD transplantation. Comparisons were made between cohorts that did or did not receive ATG (given primarily for HLA disparity) for pretransplant graft vs. host disease (GvHD) prophylaxis.
Seventy six patients with advanced hematologic malignancies (43% CIBMTR high-risk) received fludarabine and melphalan (FluMel) conditioning with or without pre-transplant rabbit ATG 6 mg/kg as part of GvHD prophylaxis. Twenty five patients who received ATG (24 of whom received grafts from donors mismatched at 1 or 2 HLA loci) were compared with 51 patients who did not receive ATG (45 of whom received grafts from HLA matched donors with six donors mismatched at a single locus). The majority of patients received post-transplant tacrolimus with either methotrexate (MTX) or mycophenolate mofetil for GvHD prophylaxis. With the exception of HLA disparity, pre-transplant patient characteristics were similar between groups (Figure 1).
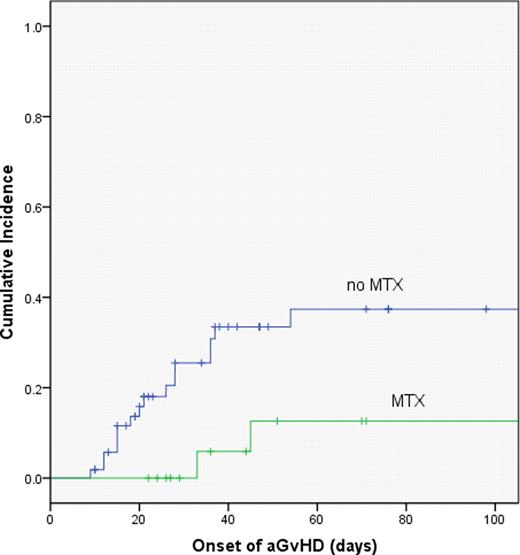
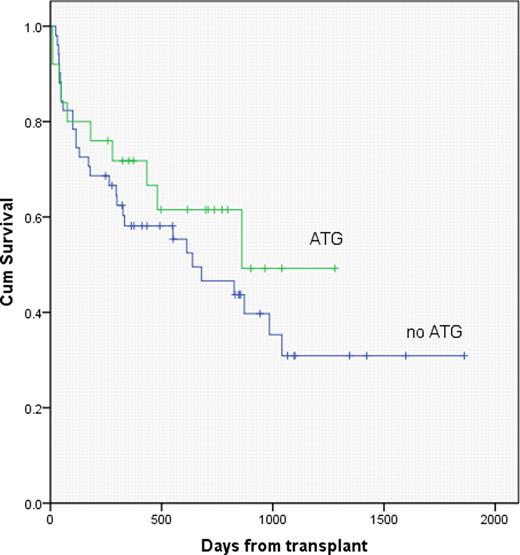
With median follow up of 481 days and 376 days for the ATG and no ATG cohorts, respectively, K-M overall survival estimates were similar, with 2 year OS of 62 vs. 47 percent, respectively, for the ATG vs no ATG groups (p=.34). On multivariate analysis, the inclusion of MTX in GvHD prophylaxis was favorably associated with overall survival (p=.004), and was associated with a significant reduction in the incidence of severe (gr 3–4) acute GvHD (p=.03). There were no significant differences in pre-transplant creatinine, age, or comorbidity index scores between patients who did or did not receive MTX.
Fludarabine and melphalan RIC, with or without pre-transplant ATG, produced similar outcomes following URD transplantation, despite the fact that patients receiving ATG in this series were more likely to have received a HLA mismatched graft. The inclusion of MTX for GVHD prophylaxis resulted in less severe acute GVHD and better survival when compared with tacrolimus/MMF.
– Patient Characteristics
| Characteristic . | Flu/Mel . | Flu/Mel/ATG . | P . |
|---|---|---|---|
| No. of patients | 51 | 25 | |
| Median age (range) | 50 (21–71) | 53 (23–65) | NS |
| Male sex (%) | 27 (53) | 15 (60) | NS |
| Disease status* | |||
| IR & HR (%) | 38 (75) | 18 (69) | NS |
| HR (%) | 23 (45) | 10 (38) | NS |
| CMV positive | |||
| Recip (%) | 41 (80) | 20 (77) | NS |
| Donor(%) | 24 (47) | 15 (58) | NS |
| Donor Type | |||
| HLA-identical | 45 | 1 | <0.001 |
| HLA-mismatched | 6 | 24 | |
| Graft Type | |||
| BM | 7 | 4 | NS |
| PBSC | 44 | 21 | |
| Median follow up (days) | 376 | 481 |
| Characteristic . | Flu/Mel . | Flu/Mel/ATG . | P . |
|---|---|---|---|
| No. of patients | 51 | 25 | |
| Median age (range) | 50 (21–71) | 53 (23–65) | NS |
| Male sex (%) | 27 (53) | 15 (60) | NS |
| Disease status* | |||
| IR & HR (%) | 38 (75) | 18 (69) | NS |
| HR (%) | 23 (45) | 10 (38) | NS |
| CMV positive | |||
| Recip (%) | 41 (80) | 20 (77) | NS |
| Donor(%) | 24 (47) | 15 (58) | NS |
| Donor Type | |||
| HLA-identical | 45 | 1 | <0.001 |
| HLA-mismatched | 6 | 24 | |
| Graft Type | |||
| BM | 7 | 4 | NS |
| PBSC | 44 | 21 | |
| Median follow up (days) | 376 | 481 |
* CIBMTR criteria
– Patient Characteristics
| Characteristic . | Flu/Mel . | Flu/Mel/ATG . | P . |
|---|---|---|---|
| No. of patients | 51 | 25 | |
| Median age (range) | 50 (21–71) | 53 (23–65) | NS |
| Male sex (%) | 27 (53) | 15 (60) | NS |
| Disease status* | |||
| IR & HR (%) | 38 (75) | 18 (69) | NS |
| HR (%) | 23 (45) | 10 (38) | NS |
| CMV positive | |||
| Recip (%) | 41 (80) | 20 (77) | NS |
| Donor(%) | 24 (47) | 15 (58) | NS |
| Donor Type | |||
| HLA-identical | 45 | 1 | <0.001 |
| HLA-mismatched | 6 | 24 | |
| Graft Type | |||
| BM | 7 | 4 | NS |
| PBSC | 44 | 21 | |
| Median follow up (days) | 376 | 481 |
| Characteristic . | Flu/Mel . | Flu/Mel/ATG . | P . |
|---|---|---|---|
| No. of patients | 51 | 25 | |
| Median age (range) | 50 (21–71) | 53 (23–65) | NS |
| Male sex (%) | 27 (53) | 15 (60) | NS |
| Disease status* | |||
| IR & HR (%) | 38 (75) | 18 (69) | NS |
| HR (%) | 23 (45) | 10 (38) | NS |
| CMV positive | |||
| Recip (%) | 41 (80) | 20 (77) | NS |
| Donor(%) | 24 (47) | 15 (58) | NS |
| Donor Type | |||
| HLA-identical | 45 | 1 | <0.001 |
| HLA-mismatched | 6 | 24 | |
| Graft Type | |||
| BM | 7 | 4 | NS |
| PBSC | 44 | 21 | |
| Median follow up (days) | 376 | 481 |
* CIBMTR criteria
Flowers:Genentech/Roche (unpaid): Consultancy; Celgene: Consultancy; Millennium/Takeda: Research Funding; Wyeth: Research Funding; Novartis: Research Funding. Kaufman:Merck: Research Funding; Keryx: Consultancy; Novartis: Consultancy; Onyx Pharmaceuticals: Consultancy; Millenium: Consultancy; Celgene: Research Funding. Lonial:Millennium Pharmaceuticals, Inc.: Consultancy; Celgene: Consultancy; Novartis: Consultancy; Bristol-Myers Squibb: Consultancy; Onyx: Consultancy; Merck: Consultancy. Sinha:Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal