Abstract
Abstract 1842
Multiple myeloma (MM) remains incurable despite improved response rates and improved progression free survival in the era of therapy with novel agents, including bortezomib, thalidomide, and lenalidomide. Disease persistence is presumably due to residual malignant plasma cell clones that evade or develop resistance to available therapies. The efficacy of radioimmunotherapy (RIT) in the treatment of hematologic malignancies is well established and the radiosensitivity of malignant plasma cells has been demonstrated in both preclinical and clinical settings. The ectoenzyme receptor CD38 is a plasma cell antigen that exhibits relatively specific, stable and uniform expression (95–100%) at a high epitope density on myeloma cells, making it an attractive target for antibody based therapies, including RIT. Pretargeted RIT (PRIT), using a multi-step streptavidin (SA)-biotin targeting system enhances the therapeutic index of delivered radiation. We have generated an anti-CD38 antibody (Ab)-SA synthetic chemical conjugate (OKT10-SA). The OKT10-SA construct binds with high avidity to myeloma cells while retaining full biotin-binding capability for radiolabeled DOTA-biotin.
Blood, tumor and nonspecific organ uptakes of OKT10-SA were directly measured in biodistribution experiments involving athymic nude mice bearing human MM xenograft tumors. Groups of 5 mice with s.c. L363 human MM (IgG) xenograft tumors received 1.4 nmol (300 μg) of either OKT10-SA (anti-CD38 SA) or an IgG1 isotype matched control Ab BHV1-SA (bovine herpes virus-1) 22 hrs prior to synthetic biotin-acetyl-galactosamine clearing agent (CA; 5.8 nmol [50 μg]) and 24 hrs prior to trace labeled 111In-DOTA-biotin (1 μg). The CA removed >95% of both unbound OKT10-SA and BHV1-SA from the mouse circulation within 30 minutes of administration. Animals were euthanized and comprehensive tissue biodistributions were assessed 2, 24, 48 and 96 hrs after 111In-DOTA-biotin injection. Tumors excised from mice pretargeted with OKT10-SA contained 13.1 ± 1.9 % of the injected dose of 111In-DOTA-biotin per gram (% ID/g) after 2 hrs and 8.8 ± 2.8 % ID/g after 24 hrs compared to 2.4 ± 0.6 % ID/g after 2 hrs and 0.9 ± 0.4 % ID/g after 24 hrs in tumors excised from control mice pretargeted with BHV1-SA. Tumor-to-normal organ ratios of absorbed radioactivity were 8:1; 10:1; 8:1; and 6:1 respectively for blood, lung, liver and kidney in mice pretargeted with OKT10-SA; compared to 0.6:1; 0.9:1; 0.8:1 and 0.4:1 respectively, in control mice pretargeted with BHV1-SA.
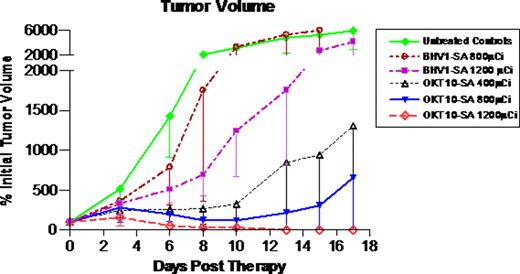
Therapy studies were then performed in athymic nude mice (n=9-10/group) bearing s.c. L363 human MM xenograft tumors. Reagent concentrations and time-points for administration of OKT10-SA, BHV1-SA and CA were identical to those reported for the biodistribution studies. The high energy beta particle emitter 90Yttrium (t1/2 = 64 hrs) was used as the therapeutic radionuclide. 90Y-DOTA-biotin (2 μg) was labeled with 400 μCi, 800 μCi, or 1200 μCi per mouse in 3 OKT10-SA groups and 3 control groups (untreated control; 800 μCi or 1200 μCi 90Y-DOTA-biotin following BHV1-SA). All mice in the untreated control and BHV1-SA control groups experienced exponential MM tumor growth and 78% of the untreated control animals required euthanasia within 17 days. All mice pretargeted with OKT10-SA demonstrated tumor shrinkage by day 6 at all dose levels (see figure). After 17 days, 90% of the OKT10-SA treated animals in the 400 μCi and 1200 μCi groups and 100% of the animals in the 800 μCi remained alive. One animal treated with 1200 μCi was euthanized on day 10 due to weight loss, however the remaining 9 animals from that group were 106 ±9% of initial body weight on day 17. Objective remissions were observed within 6 days in 100% of the mice treated with OKT10-SA followed by 1200 μCi of 90Y-DOTA-biotin, including 100% complete remissions (no detectable tumor in OKT10-SA treated mice compared to tumors that were 5240 ± 2495% of initial tumor volume in untreated control animals) by day 17.
Gopal:Millenium. Wood:BD Biosciences: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal