Abstract
Abstract 1688
Allogeneic stem cell transplantation (allo-SCT) remains an important option for patients with chronic myeloid leukemia (CML) who failed tyrosine kinase inhibitors (TKI). Data on CML patients after allo-SCT in the TKI era are rare. Meanwhile, patients are more often submitted to allo-SCT beyond first chronic phase (CP) which leads to poorer outcome (Sauβele et al. Blood 2010, BMT 2011). Therefore the aim of this study was to evaluate the outcome of CML patients after allo-SCT within the EUTOS (European Treatment and Outcome Study) for CML registry.
The “In-study” registry section collects data of patients recruited to trials of national study groups (France, Germany, Italy, Spain, The Netherlands, Nordic European Countries, UK). The “Out-Study” registry section collects data of patients within national registries (Czech Republic, Poland, Romania, Russia, Spain, UK). Inclusion criteria were Philadelphia chromosome or BCR-ABL positive CML diagnosed between 2002–2006, treatment with imatinib within 6 months after diagnosis and age ≥ 18 years.
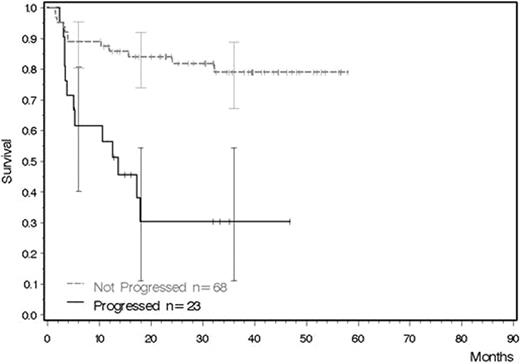
“In-study” registry section: 2060 patients were registered. 556 patients from France were excluded since no data on allo-SCT were available. 1504 patients were evaluable. Allo-SCT was reported in 91 patients (6%). Of these, 52 were from Germany, 16 from Italy, 4 from the Nordic countries (Sweden, Norway, Finland and Island) and 19 from the Netherlands. The transplantation rate varied between countries: Germany 52 out of 699 (7%), Italy 16 out of 546 (3%), Nordic countries 4 out of 140 (3%) and The Netherlands 19 out of 119 (16%). Characteristics of the 91 patients were: 65% were men (compared to 59% in the total cohort), median age was 42 years (range 18–65; compared to 52 years in the total cohort), median therapy duration before allo-SCT was 16.1 months (range 3.5–55.9 months), median follow-up after transplantation was 51.5 months (range 10.9–71.9). 68 patients were transplanted in chronic phase, 23 after progression, i.e. beyond first CP. Two year OS was 84.1% for chronic phase patients and 30.4% for patients in advanced phases. “Out-study” section: 1536 patients were registered and evaluable. Allo-SCT was reported in 60 patients (4%), 1 from Romania (1 of 13, 8%), 20 from Czech Republic (20 of 333, 6%), 20 from Poland (20 of 283, 7%), 3 from Spain (3 of 184, 2%), 5 from Russia (5 of 606, 1%) and 11 from UK (11 of 117, 9%). Patients' characteristics were: 59% were male (compared to 52% in the total cohort), median age was 34 years (range 18–60 years, total cohort 48 years), median time to transplantation 12 months (range 3 to 64 months). Outcome data in this section will be updated at the ASH meeting.
Combining the “in-study” and “out-study” registries, overall 151 out of 3040 evaluable patients (5%) have been submitted to allo-SCT.
Transplantation rates between countries participating in the EUTOS for CML registry vary substantially. Survival data from the “in-study” registry show that allo-SCT remains an attractive and important rescue therapy for CML patients. This survey deals with patient diagnosed between 2002–2006; an epidemiological EUTOS registry, open to all patients diagnosed since mid 2009 will investigate the rate of allo-SCT after the implementation of second generation TKI in CML treatment Europe-wide.
Overall survival after transplantation of patients from the EUTOS-“in-study” registry
Overall survival after transplantation of patients from the EUTOS-“in-study” registry
Saussele:EUTOS for CML: Research Funding, is a cooperation as per contract between ELN and Novartis. Piccolo:Novartis: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal