Abstract
Abstract 1431
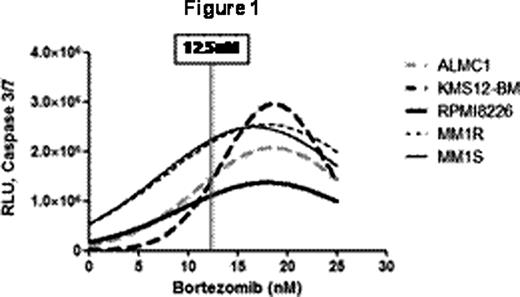
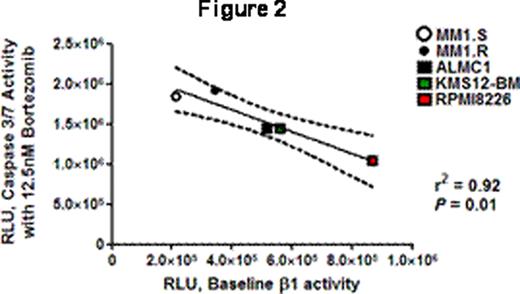
An irony of the era of increased knowledge of molecular and genetic aspects of MM, and of improved MM patient survival with proteasome inhibitor therapy (PSI), is that the sensitivity of MM cells to PSI is not related to specific addictive oncogenic abnormalities but rather to generic plasma-cell dependence on the ubiquitin-proteasome system. MM patients develop resistance to PSI, however, and a better understanding of factors that modulate sensitivity and resistance to PSI is needed to improve therapies. We hypothesized that in MM cells the differences in baseline levels of proteasome activity inversely correlated with activation of apoptosis in response to PSI, as has been suggested by qualitative experimental evidence (Blood 2009;113: 3040). A corollary of this hypothesis is that proteasome activity in MM patient cells may provide a biomarker allowing customized therapy. We characterized the chymotrypsin-like (β5), trypsin-like (β2) and caspase-like (β1) activity of five MM cell lines (MM1S, MM1R, ALMC1, KMS12-BM and RPMI 8226) using the site-specific peptide substrates Suc-LLVY-aminoluciferin (AML) (β5), Z-LRR-AML (β2) and Z-nLPnLD -AML (β1) in buffers optimized for cell permeabilization and for proteasome and luciferase activity (Promega) following manufacturer's instructions with read-out in relative luminescent units (RLU) (minus control). We also characterized intracellular proteasome-directed and signaling-related ubiquitinated proteins in these cell lines by flow cytometry with anti-ubiquitin lysine48- and lysine63-specific monoclonal antibodies (Millipore). Read-out was mean fluorescent intensity minus isotype control (MFI). Cells were cultured for 24 hours with increasing doses of BTZ or EPX (0, 3.125, 6.25, 12.5 and 25nM) and evaluated for caspase 3/7 activation in a cell-based luminescence assay (Promega). Read-out was the mean RLU of each dose level minus the mean baseline RLU. We used 104 cells per well in all luminescence assays and all experiments were performed in triplicate for each situation on 3 different days. Statistical analyses were performed with GraphPad PRISM. The differences in baseline β1, β2 and β5 activity among these cell lines were significant by unpaired t test (P <0.05) in 52/60 comparisons and trended to significance in 4/60 (P ≤0.10). RPMI8226 cells had the highest and MM1S/R the lowest levels of overall activity while KMS12-BM and ALMC1 were intermediate. The coefficients of variation for each assay were 0.10+/−0.02 (β1), 0.2+/−0.05 (β2) and 0.17+/−0.04 (β5) (means+/−SE). The lys48 MFI was highest for MM1S/R and lowest for KMS12-BM and RPMI 8226 while lys63 MFI was highest for KMS12-BM and ALMC1 and lowest for MM1S/R. The caspase 3/7 dose-response curves with BTZ are shown in Figure 1 and were comparable for EPX. For BTZ and EPX the 12.5nM dose level was the point at which slopes were maximal. We used linear regression calculating caspase 3/7 activity with 12.5nM BTZ or EPX as a function of baseline β1, β2, β5 and both MFI, and obtained non-significant results for β2 and both MFI but significant results for β1 (r2 0.92, 0.82; P <0.01, 0.03) and β5 activity (r2 0.76, 0.64; P 0.05, 0.11). Figure 2 shows the typical inverse relationship with 95% CI. We used multivariate regression analysis evaluating baseline β1, β2, β5 and both MFI as predictors of caspase 3/7 activity at 12.5nM BTZ or EPX and identified baseline β1 activity as the only significant predictor of outcome. We conclude from these results that in MM baseline β1 and β5 activity affect the level of apoptotic signaling in response to PSI. Of note, these results highlight the importance of β1 activity in modulating apoptotic signaling, downplay the significance of proteasome load, and also, given the CV measures, identify a need for further refinement of these assays, perhaps with the inclusion of proteasome 20S controls, in order to apply them with high reliability to the assessment of CD138+ patient MM samples.
Disclosures:
No relevant conflicts of interest to declare.
Author notes
*
Asterisk with author names denotes non-ASH members.
© 2011 by The American Society of Hematology
2011

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal