Abstract
Abstract 142
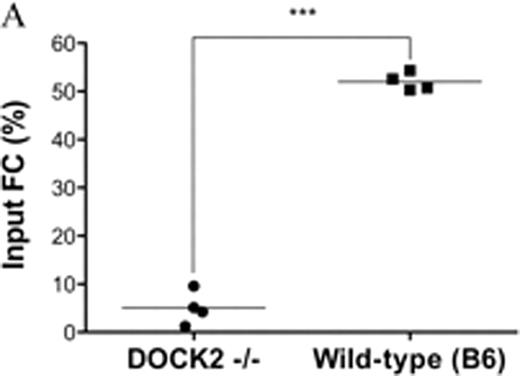
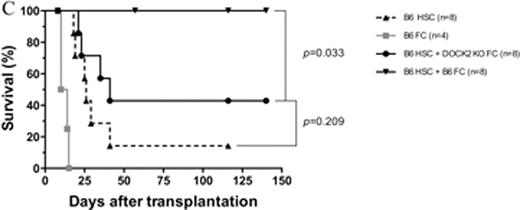
CD8+/TCR− graft facilitating cells (FC) have a potent capability to facilitate relatively small numbers of highly purified hematopoietic stem cells (HSC) in both allogeneic and syngeneic recipients. The mechanisms by which FC promote HSC engraftment have not been fully elucidated. We previously found that FC from non-obese diabetic mice (NOD) were compromised in enhancing engraftment of syngeneic and allogeneic HSC compared with FC from diabetes free congenic NOR mice. We therefore compared gene expression profiling between NOD FC and control NOR FC by microarray analysis. Among 18 most significant differentially expressed genes revealed from 45101 genes by false discovery rate control analysis with a cut-off value at level 0.05, dedicator of cytokinesis 2 (DOCK2) was identified as the gene with the most significant difference (P = 1.07 × 10−7). DOCK2 is a hematopoietic cell-specific member of the Caenorhabditis elegans Ced-5, mammalian DOCK180 and Drosophila melanogaster myoblast city (CDM) family of guanine nucleotide exchange factors. DOCK2 activates the small GTPase Rac and is indispensable for migration of plasmacytoid dendritic cells (pDC). FC is a heterogeneous cell population, with a predominant subpopulation resembling plasmacytoid precursor DC (p-preDC FC). In vitro studies demonstrated that FC increased clonogenicity of HSC and improved the ability of the impaired stroma to support late cobblestone area formation by HSC, which suggests that FC homing to hematopoietic niche as a potential component might be a perquisite for FC to enhance HSC engraftment. Therefore, we hypothesized that signaling pathways controlling cell migration via DOCK2 are critical for FC to enhance HSC engraftment. To test our hypothesis, DOCK2 expression data from microarray analysis was further confirmed using relative quantitative real-time PCR and high content image analysis. The expression level of DOCK2 in FC was significantly lower in NOD mice compared with NOR mice (p < 0.01 vs. NOR FC). Functional phenotypes of DOCK2-deficient FC were determined by Transwell migration assay and colony-forming cell assay in vitro, and by co-transplantation of HSC with FC in vivo as well as enumeration of CellTrack Green labeled FC by flow cytometry in spleen, thymus, and bone marrow of femurs and tibias 18 hours post-transplantation. Deficiency of DOCK2 in FC did not affect the ability of FC in promoting HSC colony formation when the two were cultured together. However, DOCK2-deficient FC were compromised in migration to the α-cheemokine, stromal derived factor-1 (SDF-1) at dose 200 ng/ml (Fig. A, P < 0.01 vs. wild-type FC). Homing of FC to spleen and bone marrow of femurs and tibias was also significantly impaired in DOCK2-deficient FC. Moreover, deficiency of DOCK2 in FC abrogated enhancement of HSC engraftment by FC in the syngeneic and allogeneic in vivo assays (Fig. B, syngeneic model: 500 B6 HSC plus 30K B6 or DOCK2−/−FC into lethally irradiated B6 recipients; Fig. C, allogeneic model: 10K B6 HSC plus 30K B6 or DOCK2−/−FC into lethally irradiated B10.BR recipients, P < 0.05 vs. B6 HSC plus wild-type FC). Taken together, our results indicate that deficiency of DOCK2 in FC leads to the dysfunction in migration, and suggest that the signaling pathways involved in FC migration are crucial for FC to enhance HSC engraftment.
Disclosures:
Ildstad:Regenerex LLC: Equity Ownership.
Author notes
*
Asterisk with author names denotes non-ASH members.
© 2011 by The American Society of Hematology
2011


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal