Abstract
This study was conducted to elucidate the influence of immunosuppressive treatment (IST) and GVHD on risk of recurrent malignancy after allogeneic hematopoietic cell transplantation (HCT). The study cohort included 2656 patients who received allogeneic HCT after high-intensity conditioning regimens for treatment of hematologic malignancies. Rates and hazard ratios of relapse and mortality were analyzed according to GVHD and IST as time-varying covariates. Adjusted Cox analyses showed that acute and chronic GVHD were both associated with statistically similar reductions in risk of relapse beyond 18 months after HCT but not during the first 18 months. In patients with GVHD, resolution of GVHD followed by withdrawal of IST was not associated with a subsequent increase in risk of relapse. In patients without GVHD, withdrawal of IST was associated with a reduced risk of relapse during the first 18 months, but the risk of subsequent relapse remained considerably higher than in patients with GVHD. In summary, the association of GVHD with risk of relapse changes over time after HCT. In patients without GVHD, early withdrawal of IST might help to prevent relapse during the first 18 months, but other interventions would be needed to prevent relapse at later time points.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) has been established to cure various hematologic malignancies.1 With the dramatic decrease in early nonrelapse mortality after HCT during the past 20 years,2 recurrent malignancy has become the leading cause of death.3,4 As a result, the National Cancer Institute recently sponsored an international workshop to promote a coordinated research effort addressing relapse after HCT.5-7
Both the pretransplantation conditioning regimen and GVL effects mediated by donor cells contribute to the curative potential of allogeneic HCT. GVL effects in humans have been documented in observational studies by showing a reduced risk of recurrent malignancy associated with acute and chronic GVHD.8-12 Subsequent studies showed that infusion of donor lymphocytes could induce remission in patients who relapsed after allogeneic HCT, thereby providing direct evidence for the potency of GVL effects.13,14
GVL effects are mediated primarily by T cells and natural killer cells.15,16 The immune milieu in the recipient changes dramatically across time after HCT,17 and is affected by GVHD and systemic immunosuppressive treatment (IST). Previous studies have evaluated the relative contributions of historically defined acute and chronic GVHD in mediating GVL effects,8-11 but this question has not been examined with GVHD defined according to National Institutes of Health (NIH) consensus criteria.18
The influence of IST on risk of relapse after HCT has been examined in several studies. The combination of methotrexate and cyclosporine compared with either methotrexate or cyclosporine alone19 and prophylactic regimens using high doses of cyclosporine20,21 have been associated with an increased risk of relapse in certain groups of patients. Likewise, mycophenolate mofetil added to initial treatment for chronic GVHD appeared to increase the risk of relapse.22 On the other hand, the risk of relapse was not increased by methotrexate added to cyclosporine and prednisone for prevention of GVHD,23 by prophylaxis or treatment with glucocorticoids24,25 or by azathioprine added to initial treatment for chronic GVHD.26 The current retrospective study was intended to provide a broad overview of associations of acute GVHD, NIH chronic GVHD, and IST with risks of recurrent malignancy and mortality after allogeneic HCT. Our results provide new information about the effects of withdrawing IST both in patients with no history of GVHD and in those with a history of GVHD that has resolved.
Methods
Patients and data collection
This retrospective study included 2656 consecutive patients with acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), chronic myeloid leukemia (CML), myelodysplastic syndrome (MDS), or myeloproliferative neoplasms (MPN) who had a first allogeneic HCT after high-intensity (ie, myeloablative) conditioning between January 1992 and December 2005 at our institution. Patients considered to be at low risk of relapse included CML in chronic phase (CP), acute leukemia in first remission, and MDS without excess blasts. Patients in all other diseases and stages were considered to be at high risk of relapse. Patients had given written consent allowing the use of medical records for research, in accordance with the Declaration of Helsinki, and the Institutional Review Board approved the study.
Follow-up clinical information was available from medical records submitted by referring physicians and from documentation generated by our Long Term Follow Up clinical program. Acute GVHD was graded according to previously described criteria.27 Chronic GVHD was diagnosed according to NIH criteria,18 and the onset was defined at the beginning of systemic treatment.28 Relapse was defined by hematologic criteria. In addition, any unplanned intervention to prevent progression of malignancy in patients with molecular, cytogenetic or flow cytometric evidence of malignant disease persisting or recurring after HCT was defined as relapse. Immunosuppressive treatment was generally withdrawn at ∼ 6 months in patients with no history of GVHD and in those with acute GVHD that had previously resolved, and was continued beyond 6 months in patients with persistent acute GVHD and in those with chronic GVHD.
Statistical analysis
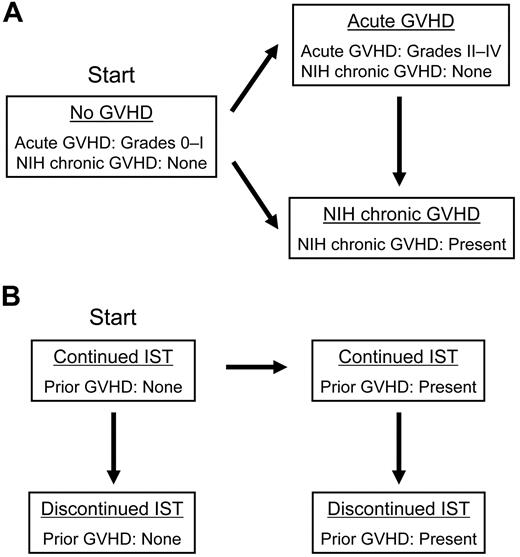
GVHD and IST were analyzed as time-varying covariates. Patients at risk of relapse or mortality were classified in 3 mutually exclusive conditions based on their histories of acute GVHD or NIH chronic GVHD requiring systemic treatment (Figure 1A): grades 0-I acute GVHD without NIH chronic GVHD (hereafter designated “no GVHD”), grades II-IV acute GVHD without NIH chronic GVHD (hereafter designated “acute GVHD”), or NIH chronic GVHD with or without acute GVHD (hereafter designated “NIH chronic GVHD”). In this analysis, no distinction was made between the chronic GVHD subcategories of “classic” chronic GVHD and “overlap syndrome.”18 All patients started with “no GVHD” and were classified in that condition until the onset of acute GVHD or NIH chronic GVHD. Patients with “acute GVHD” were classified in that condition regardless of whether acute GVHD had resolved, until the onset of NIH chronic GVHD, if it occurred. Patients with “NIH chronic GVHD” were classified in that condition thereafter, regardless of whether chronic GVHD had resolved.
GVHD and immunosuppressive treatment conditions used for the time-varying analysis. Patients transit from one condition to another in the direction of an arrow at the onset of acute or NIH chronic GVHD, and at the discontinuation of immunosuppressive treatment. (A) GVHD conditions. (B) GVHD and IST conditions.
GVHD and immunosuppressive treatment conditions used for the time-varying analysis. Patients transit from one condition to another in the direction of an arrow at the onset of acute or NIH chronic GVHD, and at the discontinuation of immunosuppressive treatment. (A) GVHD conditions. (B) GVHD and IST conditions.
To determine the effects of IST on risks of relapse, patients were classified in 4 mutually exclusive conditions (Figure 1B). All patients started with “continued IST without GVHD” and were classified in that condition until GVHD developed or all systemic IST for prophylaxis of GVHD had been permanently withdrawn. Patients classified as having “continued IST with prior GVHD” remained in that condition until all systemic IST for treatment of GVHD had been permanently withdrawn with no subsequent systemic treatment for GVHD,29 whereupon they were classified as having “discontinued IST with prior GVHD.” Patients with “discontinued IST,” either with or without prior GVHD were classified in that condition thereafter. We did not attempt to account for short intervals of discontinued IST that occurred in a small number of patients before the onset of NIH chronic GVHD.
Details of methods used to illustrate relapse and mortality event rates are provided in supplemental Methods (available on Blood Web site; see the Supplemental Materials link at the top of the online article). Rates of relapse and death for patients in each given GVHD or IST condition were estimated as the number of events in sequential 90-day intervals after HCT divided by the number of person-years of observation time in each condition during each interval. Smoothed estimates of the event rates were obtained by fitting a Poisson regression model to the observed numbers of events, using cubic spline terms for time.4 Unlike Kaplan-Meier and cumulative incidence estimates, event rates among patients at risk and estimates of the underlying hazard rates are not affected by competing risks.
Time-dependent Cox proportional hazards models were used to evaluate the association of GVHD and IST conditions with risks of relapse and mortality without right truncation.9,10 Models for relapse were adjusted for disease type and risk category, and pretransplantation conditioning with or without total body irradiation, because these factors could potentially affect the risk of recurrent malignancy independently of GVHD. Models for overall mortality were adjusted for additional factors that could potentially affect the risks of acute or chronic GVHD and death, including the age of the patient at HCT, donor/recipient sex, donor type, HLA-mismatch, source of stem cells, GVHD prophylaxis, and CMV serologic status. In general, adjustments had relatively minor effects on the estimated hazard ratios, as would be expected if the GVHD and IST conditions were temporally and causally proximal to the events of interest.
Interaction was evaluated by allowing additional terms for each of the GVHD and/or IST variables in the model, depending on the presence or absence of the factor being tested. Models with and without the interaction terms were compared using a likelihood ratio test. If the overall interaction was significant at the P = .005 level, the interaction terms were tested individually, also using a likelihood ratio test. In the full dataset, tests of interaction were performed to evaluate the effects of donor type, HLA-mismatch, and diagnosis at HCT (CML-CP vs other). In patients with diseases other than CML-CP, tests of interaction were performed to evaluate diagnosis at HCT (ALL vs other), disease risk category, and the source of stem cells. A significance level of .005 for the overall interaction was chosen to compensate at least partially for the multiplicity of interaction factors and models evaluated.
Results
Patient characteristics and transplantation outcomes are summarized in Tables 1 and 2, respectively. Median follow-up of the cohort was 86 months (range, 3-178 months) among survivors. The cumulative incidence of grades II-IV GVHD was 78% (95% CI, 77%-80%) at 100 days after HCT, consistent with previous results from our center.30 The cumulative incidence of NIH chronic GVHD was 34% (95% CI, 32%-36%) at 2 years.
Patient characteristics
| Characteristic . | All patients (n = 2656) . |
|---|---|
| Median patient age, y (range) | 39 (0-71) |
| Patient sex, no. (%) | |
| Male | 1528 (58) |
| Female | 1128 (42) |
| Diagnosis at transplantation, no. (%) | |
| Acute myeloid leukemia | 848 (32) |
| Acute lymphoblastic leukemia | 412 (16) |
| Chronic myeloid leukemia | 894 (34) |
| Myelodysplastic syndrome or myeloproliferative neoplasms | 502 (19) |
| Disease risk at transplantation, no. (%)* | |
| Low | 1191 (45) |
| High | 1465 (55) |
| High-intensity conditioning regimens, no. (%) | |
| With total body irradiation | 1628 (61) |
| Without total body irradiation | 1028 (39) |
| HLA and donor type, no. (%) | |
| HLA-identical related | 1088 (41) |
| HLA-matched unrelated | 912 (34) |
| HLA antigen or allele-mismatched related | 243 (9) |
| HLA antigen or allele-mismatched unrelated | 413 (16) |
| Donor/patient sex, no. (%) | |
| Male/male | 873 (33) |
| Female/male | 655 (25) |
| Male/female | 584 (22) |
| Female/female | 544 (20) |
| Graft source, no. (%) | |
| Bone marrow | 1760 (66) |
| Mobilized blood hematopoietic stem cells | 896 (34) |
| GVHD prophylaxis, no. (%) | |
| Cyclosporine + methotrexate alone | 1885 (71) |
| Tacrolimus + methotrexate alone | 125 (5) |
| Rabbit antithymocyte globulin + other medications | 74 (3) |
| T cell partially depleted | 15 (1) |
| Other regimens | 557 (21) |
| CMV-positive patient or donor, no. (%) | 1745 (66) |
| Characteristic . | All patients (n = 2656) . |
|---|---|
| Median patient age, y (range) | 39 (0-71) |
| Patient sex, no. (%) | |
| Male | 1528 (58) |
| Female | 1128 (42) |
| Diagnosis at transplantation, no. (%) | |
| Acute myeloid leukemia | 848 (32) |
| Acute lymphoblastic leukemia | 412 (16) |
| Chronic myeloid leukemia | 894 (34) |
| Myelodysplastic syndrome or myeloproliferative neoplasms | 502 (19) |
| Disease risk at transplantation, no. (%)* | |
| Low | 1191 (45) |
| High | 1465 (55) |
| High-intensity conditioning regimens, no. (%) | |
| With total body irradiation | 1628 (61) |
| Without total body irradiation | 1028 (39) |
| HLA and donor type, no. (%) | |
| HLA-identical related | 1088 (41) |
| HLA-matched unrelated | 912 (34) |
| HLA antigen or allele-mismatched related | 243 (9) |
| HLA antigen or allele-mismatched unrelated | 413 (16) |
| Donor/patient sex, no. (%) | |
| Male/male | 873 (33) |
| Female/male | 655 (25) |
| Male/female | 584 (22) |
| Female/female | 544 (20) |
| Graft source, no. (%) | |
| Bone marrow | 1760 (66) |
| Mobilized blood hematopoietic stem cells | 896 (34) |
| GVHD prophylaxis, no. (%) | |
| Cyclosporine + methotrexate alone | 1885 (71) |
| Tacrolimus + methotrexate alone | 125 (5) |
| Rabbit antithymocyte globulin + other medications | 74 (3) |
| T cell partially depleted | 15 (1) |
| Other regimens | 557 (21) |
| CMV-positive patient or donor, no. (%) | 1745 (66) |
HLA indicates human leukocyte antigen; GVHD, graft-versus-host disease; and CMV, cytomegalovirus.
The low-risk category included chronic myeloid leukemia in chronic phase, acute leukemia in first remission, and myelodysplastic syndrome without excess blasts. The high-risk category included all other diseases and stages.
Transplantation outcome
| Characteristic . | All patients (n = 2656) . |
|---|---|
| Cumulative incidence of recurrent malignancy at 5 y, % | |
| Low-risk disease | 18 (95% CI, 16-20) |
| High-risk disease | 34 (95% CI, 32-37) |
| Cumulative incidence of nonrelapse mortality at 5 y, % | |
| Low-risk disease | 24 (95% CI, 21-26) |
| High-risk disease | 37 (95% CI, 34-39) |
| Overall survival rates at 5 y, % | |
| Low-risk disease | 66 (95% CI, 64-69) |
| High-risk disease | 32 (95% CI, 30-34) |
| Acute GVHD | |
| Days from transplantation to onset of grades II-IV GVHD, median (range) | 19 (4-302) |
| Cumulative incidence of grades II-IV GVHD at 100 d, % | 78 (95% CI, 77-80) |
| Peak grade, no. (%) | |
| Grade 0 | 465 (18) |
| Grade I | 93 (4) |
| Grade II | 1399 (53) |
| Grades III-IV | 699 (26) |
| NIH chronic GVHD (n = 932) | |
| Days from transplantation to onset, median (range) | 161 (66-1615) |
| Cumulative incidence of chronic GVHD at 2 y, % | 34 (95% CI, 32-36) |
| Type of onset, no. (%) | |
| De novo | 133 (14) |
| Quiescent or interrupted | 682 (73) |
| Progressive | 117 (13) |
| Characteristic . | All patients (n = 2656) . |
|---|---|
| Cumulative incidence of recurrent malignancy at 5 y, % | |
| Low-risk disease | 18 (95% CI, 16-20) |
| High-risk disease | 34 (95% CI, 32-37) |
| Cumulative incidence of nonrelapse mortality at 5 y, % | |
| Low-risk disease | 24 (95% CI, 21-26) |
| High-risk disease | 37 (95% CI, 34-39) |
| Overall survival rates at 5 y, % | |
| Low-risk disease | 66 (95% CI, 64-69) |
| High-risk disease | 32 (95% CI, 30-34) |
| Acute GVHD | |
| Days from transplantation to onset of grades II-IV GVHD, median (range) | 19 (4-302) |
| Cumulative incidence of grades II-IV GVHD at 100 d, % | 78 (95% CI, 77-80) |
| Peak grade, no. (%) | |
| Grade 0 | 465 (18) |
| Grade I | 93 (4) |
| Grade II | 1399 (53) |
| Grades III-IV | 699 (26) |
| NIH chronic GVHD (n = 932) | |
| Days from transplantation to onset, median (range) | 161 (66-1615) |
| Cumulative incidence of chronic GVHD at 2 y, % | 34 (95% CI, 32-36) |
| Type of onset, no. (%) | |
| De novo | 133 (14) |
| Quiescent or interrupted | 682 (73) |
| Progressive | 117 (13) |
CI indicates confidence interval; and GVHD, graft-versus-host disease.
Recurrent malignancy according to GVHD
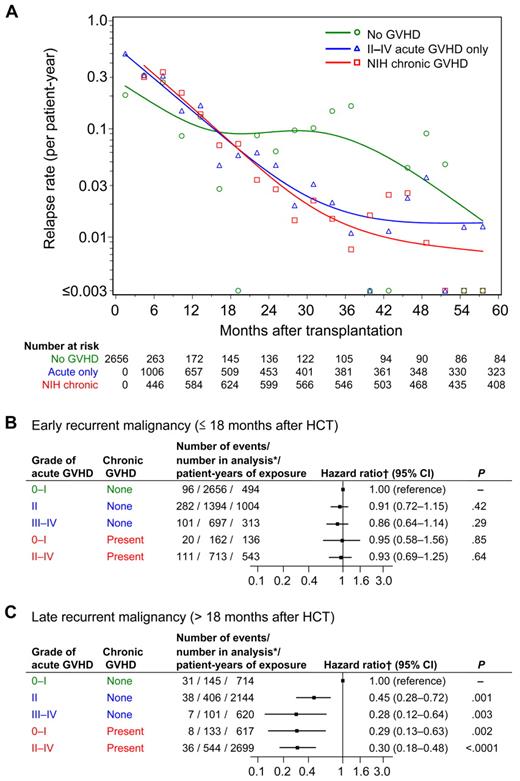
Figure 2A illustrates the change of relapse rates over time according to GVHD condition. The smoothed relapse rate per patient-year at any given time in this figure approximates the hazard of relapse associated with each condition. Relapse rates declined gradually until at least 36 months after HCT in patients with a history of either acute or chronic GVHD. In contrast, in patients without GVHD, relapse rates decreased during the first 15 to 18 months, but showed no decline between 18 and 30 months, and then decreased again, reaching rates similar to those in patients with a history of GVHD at ∼ 60 months after HCT. The curves crossed between 15 and 18 months after HCT. Patterns were similar in patients with low- and high-risk diseases (data not shown).
Rates of recurrent malignancy, and risk of early and late recurrent malignancy according to GVHD condition. (A) Relapse rates were calculated within sequential 90-day intervals for patients without GVHD shown in green, for patients with grades II-IV GVHD without chronic GVHD shown in blue, and for patients with NIH chronic GVHD shown in red. Small symbols represent the actual relapse rates for each 90-day interval. The smoothed rates were plotted as curves for each condition. Low event rates account for large variations between sequential intervals after 36 months. (B-C) Risk of early and late recurrent malignancy according to GVHD condition. *Number of patients at risk in the condition at any time during the period of analysis. †Hazard ratios were adjusted for disease, risk category, and total body irradiation.
Rates of recurrent malignancy, and risk of early and late recurrent malignancy according to GVHD condition. (A) Relapse rates were calculated within sequential 90-day intervals for patients without GVHD shown in green, for patients with grades II-IV GVHD without chronic GVHD shown in blue, and for patients with NIH chronic GVHD shown in red. Small symbols represent the actual relapse rates for each 90-day interval. The smoothed rates were plotted as curves for each condition. Low event rates account for large variations between sequential intervals after 36 months. (B-C) Risk of early and late recurrent malignancy according to GVHD condition. *Number of patients at risk in the condition at any time during the period of analysis. †Hazard ratios were adjusted for disease, risk category, and total body irradiation.
Because the results in Figure 2A showed that the hazards of relapse were not proportional before and after 18 months, separate Cox models were used to evaluate the relative risks of “early” relapse (before 18 months) and “late” relapse (after 18 months) in patients with a history of acute or chronic GVHD, compared with those without GVHD (Figure 2B-C). Neither acute nor chronic GVHD was associated with a statistically significant reduction in risk of early relapse (Figure 2B). Grade II GVHD, grades III-IV GVHD, and chronic GVHD were each associated with similar statistically significant reductions in risk of late relapse (Figure 2C). Among patients with grades II-IV acute GVHD, the subsequent development of chronic GVHD did not provide a statistically significant incremental reduction in risk of late relapse (HR, 0.73; 95% CI, 0.47-1.13; P = .16).
We evaluated the risk of relapse separately in patients with CML-CP and in those with other diseases, because CML-CP may be particularly sensitive to GVL effects (supplemental Table 1). The overall conclusions did not change. Although the hazard ratios suggested that the association of chronic GVHD with reduced risk of late relapse may be somewhat stronger in patients with CML-CP than in those with other diseases, the test of interaction did not meet our threshold of statistical significance.
We found no interaction of donor type, HLA-mismatching, disease risk category, or stem cell source with GVHD in the analysis of relapse. Grades III-IV GVHD without chronic GVHD, however, was associated with a decreased risk of early recurrent malignancy in patients with ALL (HR, 0.31; 95% CI, 0.15-0.62) but not in those with other diseases (HR, 1.08; 95% CI, 0.77-1.50; P = .001 for interaction).
Overall mortality according to GVHD
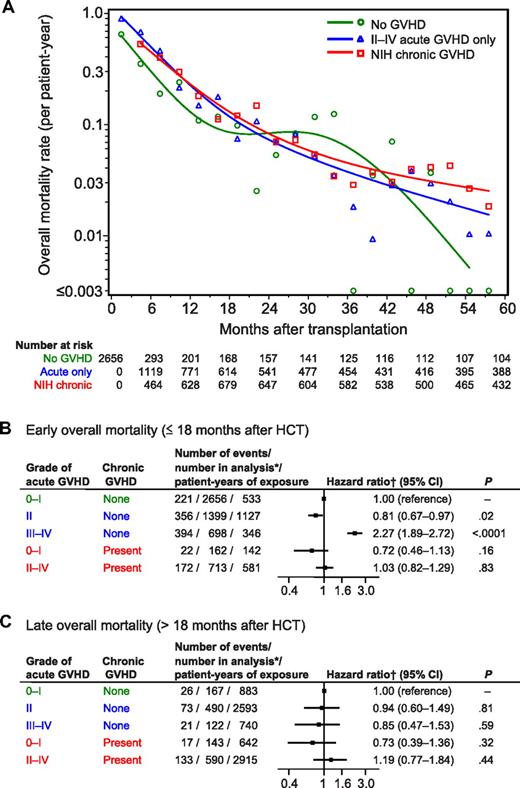
As shown in Figure 3A, overall mortality rates across time did not show striking differences according to the presence or absence of GVHD. As expected, grades III-IV GVHD was associated with an increased risk of early mortality (Figure 3B). Unexpectedly, we observed that grade II GVHD was associated with a decreased risk of early mortality (Figure 3B). Chronic GVHD did not show a statistically significant association with early mortality. Neither acute nor chronic GVHD showed a statistically significant association with late mortality (Figure 3C).
Rates of overall mortality and risk of early and late overall mortality according to GVHD condition. (A) Mortality rates were calculated within sequential 90-day intervals for patients without GVHD shown in green, for patients with grades II-IV GVHD without chronic GVHD shown in blue, and for patients with NIH chronic GVHD shown in red. Small symbols represent the actual mortality rates for each 90-day interval. The smoothed rates were plotted as curves for each condition. (B-C) Risk of early and late overall mortality according to GVHD condition. *Number of patients at risk in the condition at any time during the period of analysis. †Hazard ratios were adjusted for disease, risk category, total body irradiation, age of the patient at HCT, donor/recipient sex, donor type, HLA-mismatch, source of stem cells, GVHD prophylaxis, and CMV serologic status.
Rates of overall mortality and risk of early and late overall mortality according to GVHD condition. (A) Mortality rates were calculated within sequential 90-day intervals for patients without GVHD shown in green, for patients with grades II-IV GVHD without chronic GVHD shown in blue, and for patients with NIH chronic GVHD shown in red. Small symbols represent the actual mortality rates for each 90-day interval. The smoothed rates were plotted as curves for each condition. (B-C) Risk of early and late overall mortality according to GVHD condition. *Number of patients at risk in the condition at any time during the period of analysis. †Hazard ratios were adjusted for disease, risk category, total body irradiation, age of the patient at HCT, donor/recipient sex, donor type, HLA-mismatch, source of stem cells, GVHD prophylaxis, and CMV serologic status.
Associations of GVHD with increased risk of early mortality were stronger for patients with CML-CP than for those with other diseases (supplemental Table 2). This observation applied for the effects of grades III-IV GVHD without subsequent chronic GVHD (P = .0002 for interaction) and for grades II-IV GVHD with subsequent chronic GVHD (P = .002 for interaction).
We found 2 other interactions in the analysis of mortality. Grades III-IV GVHD without chronic GVHD was associated with an increased risk of early mortality in patients with diseases other than ALL (HR, 2.48; 95% CI, 2.01-3.06) but not in those with ALL (HR, 0.91; 95% CI, 0.61-1.35; P < .0001 for interaction). Further, Grade II GVHD without chronic GVHD was associated with a lower risk of early mortality in patients with ALL (HR, 0.45; 95% CI, 0.31-0.65) but not in those with other diseases (HR, 0.91; 95% CI, 0.73-1.12; P = .0008 for interaction).
Influence of immunosuppressive treatment on recurrent malignancy
Figure 4A illustrates relapse rates across time when both GVHD and IST are considered. Effects of discontinued IST were analyzed without distinguishing between acute and chronic GVHD, because they had similar effects on risk of relapse. In patients with prior GVHD, relapse rates declined gradually until at least 36 months after HCT, both during IST and after withdrawal of IST. In the absence of GVHD, relapse rates during IST peaked between 6 and 9 months after HCT. After 12 months, the number of patients in this condition was too small to illustrate. During the first 12 months, relapse rates in patients without GVHD who discontinued IST were lower than in those continuing IST. After 24 months, however, patients without GVHD who discontinued IST (red curve) had much higher relapse rates than those with prior GVHD (blue and purple curves).
Rates of recurrent malignancy and risk of early and late recurrent malignancy according to GVHD and immunosuppressive treatment conditions. (A) Results are shown as rates per patient-year during successive 90-day intervals after transplantation. Small symbols represent the actual relapse rates for each 90-day interval. The smoothed rates were plotted as curves for each condition defined by the history of GVHD and continuation or discontinuation of IST. Plots were not illustrated for patients without GVHD who continued IST beyond 12 months because of insufficient sample size. (B-C) Risk of early and late recurrent malignancy according to GVHD and immunosuppressive treatment conditions. *Number of patients at risk in the condition at any time during the period of analysis. †Hazard ratios were adjusted for disease, risk category, and total body irradiation. ‡Effects of discontinued IST were not analyzed beyond 18 months in patients without GVHD, because the reference group was too small.
Rates of recurrent malignancy and risk of early and late recurrent malignancy according to GVHD and immunosuppressive treatment conditions. (A) Results are shown as rates per patient-year during successive 90-day intervals after transplantation. Small symbols represent the actual relapse rates for each 90-day interval. The smoothed rates were plotted as curves for each condition defined by the history of GVHD and continuation or discontinuation of IST. Plots were not illustrated for patients without GVHD who continued IST beyond 12 months because of insufficient sample size. (B-C) Risk of early and late recurrent malignancy according to GVHD and immunosuppressive treatment conditions. *Number of patients at risk in the condition at any time during the period of analysis. †Hazard ratios were adjusted for disease, risk category, and total body irradiation. ‡Effects of discontinued IST were not analyzed beyond 18 months in patients without GVHD, because the reference group was too small.
Withdrawal of IST was associated with a decreased risk of early relapse in patients without GVHD (HR, 0.41; 95% CI, 0.22-0.77; P = .006, Figure 4B). A trend suggesting a reduced risk of early relapse was also observed after withdrawal of IST among patients with prior GVHD (HR, 0.74; 95% CI, 0.53-1.04; P = .08). Withdrawal of IST was not associated with a statistically significant decrease in risk of late relapse in patients with prior GVHD (HR, 0.98; 95% CI, 0.63-1.53; P = .93, Figure 4C). The HR of late relapse in patients without GVHD after withdrawal of IST was 2.95 (95% CI, 1.94-4.48; P < .0001) compared with those with prior GVHD.
We found no statistical interactions in the analysis of relapse with GVHD and IST conditions. Withdrawal of IST after resolution of GVHD was associated with a reduced risk of early relapse in patients with CML-CP but not in those with other diseases (supplemental Table 3), although the test of interaction did not meet our threshold of statistical significance.
Discussion
Our results expand on previous analyses of GVL effects after allogeneic HCT in 2 major ways. First, we demonstrate that the association of GVHD with risk of relapse changes dramatically over time after HCT. Second, we evaluated the effects of discontinued IST both in patients with and without GVHD. In patients without GVHD, withdrawal of IST was associated with reduced risk of relapse during the first 18 months, but not thereafter. In patients with prior GVHD, resolution of GVHD followed by withdrawal of IST did not increase the risk of subsequent relapse.
Gratwohl et al11 previously showed differences in the association of acute GVHD with risk of relapse across time after HCT in patients with CML-CP. Grades II-IV GVHD was associated with a decrease in risk of relapse from day 100 until 3 years after HCT but not during the first 100 days or beyond 3 years. In our study, GVHD was associated with a lower risk of relapse beyond 18 months but not with a lower risk of relapse before 18 months in patients with CML-CP. Differences in the time frames analyzed might account for the discordant observations in the 2 studies.
The difference between the continued risk of relapse among patients without GVHD and the constantly declining risk among patients with prior GVHD suggests a change in GVL effects at ∼ 15-18 months after HCT in the absence of GVHD. Mechanisms of GVL activity in patients with and without GVHD could be similarly effective during the first 18 months but might persist for longer periods of time in patients with overt GVHD. Alternatively, mechanisms of GVL activity associated with overt GVHD during the first 18 months might produce more profound reductions in the numbers of malignant stem cells,31 yielding more prolonged protection against relapse than in patients without GVHD. This change in risk of relapse could reflect the biology of the diseases being studied rather than a change in GVL effects. In fact, however, the associations of GVHD with risk of relapse before and after 18 months showed more similarities than differences when we compared CML-CP versus other diseases.
To the best of our knowledge, effects of discontinued IST on transplantation outcomes according to the history of GVHD and time after HCT have not been evaluated in previous studies. Our current results indicate that the risk of relapse is decreased by withdrawal of IST in patients without GVHD, suggesting that calcineurin inhibitors dampen GVL effects in patients without GVHD. In contrast, withdrawal of IST had minimal effects on the risk of early or late relapse among patients with prior GVHD, except during the first 18 months in patients with CML-CP. In theory, one might expect resolution of GVHD to be associated with a reduction in GVL effects, and withdrawal of IST to be associated with an increase in GVL effects. If so, withdrawal of IST after resolution of GVHD would be expected to have little net overall effect on the risk of recurrent malignancy, as observed for patients with diseases other than CML-CP in the current study. In patients with CML-CP, withdrawal of IST after resolution of GVHD was associated with a reduced risk of early relapse. By extrapolating from these results, it might be reasonable to use the least intensive, yet effective regimen of IST for GVHD in patients with CML-CP during the first 18 months in hopes of preserving optimal GVL effects. Our results, however, do not support efforts to minimize the intensity of IST for GVHD to preserve optimal GVL effects in patients with other malignancies.
Our results shed light on several questions that have been addressed in previous studies, including the relative strength of GVL effects associated with acute and chronic GVHD, variation in the potency of GVL effects against different diseases, the tradeoff between GVL effects and nonrelapse mortality, and GVL effects after HCT with reduced-intensity conditioning regimens compared with high-intensity conditioning regimens. Previous studies using the historical definition of chronic GVHD have produced conflicting conclusions regarding the relative strength of GVL effects associated with acute and chronic GVHD. Horowitz et al10 found that GVL effects associated with chronic GVHD were stronger than those observed with acute GVHD, while Ishiyama et al32 found that GVL effects were associated with acute GVHD but not with chronic GVHD. The similarity of GVL effects associated with acute and NIH chronic GVHD in the current study is consistent with findings from an earlier study at our center.9 Our current results suggest that GVL effects attributable to acute and NIH chronic GVHD are similarly potent.
Previous studies have highlighted disease and stage-specific differences in the association of acute GVHD with risk of relapse after allogeneic HCT. For example, Sullivan et al9 showed that acute GVHD was associated with a decreased risk of relapse in patient with ALL at any stage of the disease, and in patients with AML beyond first remission but not in those with AML in first remission. Horowitz et al10 also showed that acute GVHD was associated with a reduced risk of relapse in patients with ALL in first remission but not in those with AML in first remission. In the present study, we extend the results of earlier studies by showing that grades III-IV acute GVHD is associated with a decreased risk of early relapse in patients with ALL but not in those with other diseases. In addition, we found that grade II GVHD was associated with a decreased risk of early mortality in patients with ALL, while grades III-IV GVHD was not associated with an increased risk of early mortality in these patients. The mechanisms that account for these differences between ALL and other diseases during the first 18 months after HCT remain to be defined.
Consistent with results of previous studies,8,11,33 our analysis of early overall mortality demonstrates that the tradeoff between GVL effects and nonrelapse mortality is clearly unfavorable for CML-CP patients with grades III-IV GVHD and for those with chronic GVHD requiring systemic treatment. The clinical implication is that survival after HCT with high-intensity conditioning regimens in patients with CML-CP could be improved by approaches that prevent grades III-IV GVHD and chronic GVHD, without decreasing the risk of grade II GVHD and its associated GVL effects. Such approaches might offer a somewhat smaller survival benefit in patients with diseases other than CML-CP, because our results showed that chronic GVHD was not associated with an increased risk of early or late mortality in these patients. Prevention of chronic GVHD would certainly improve quality of life for these patients, even if survival might not be improved.
Baron et al34 and Thepot et al35 found that the risk of relapse was reduced by chronic GVHD but not by acute GVHD after HCT with reduced-intensity conditioning regimens. These results contrast with those of the current study showing a reduced risk of relapse with both acute and chronic GVHD after HCT with high-intensity conditioning regimens. This discordance could reflect differences in patient age, the types of diseases treated, the agents used for pretransplantation conditioning, the intensity of the conditioning regimen, the types of cells used for HCT, the agents used for posttransplantation immunosuppression, and the timing of recurrent malignancy. Further studies will be needed to identify reasons for differences in the association of GVL effects with acute GVHD after high-intensity and reduced-intensity conditioning regimens.
Our study has several limitations. We could not perform meaningful analyses of certain small subsets, such as patients with grade I acute GVHD. We were not able to analyze outcomes as related to the severity of chronic GVHD, because global severity scores according to NIH criteria could not be determined from information available for this retrospective study. Our study was limited to patients receiving T cell–replete HCT with high-intensity conditioning regimens. Finally, in using allogeneic transplantation recipients without GVHD as the reference group, our results reflect only the incremental GVL effects associated with clinically evident GVHD. GVL effects in the absence of overt GVHD have been demonstrated previously using syngeneic recipients as the reference group.10 The number of syngeneic transplantation recipients at our center was too small to use as a reference group.
In summary, both acute GVHD and chronic GVHD were associated with similar GVL effects after HCT with high-intensity conditioning regimens. Specific interventions could be tested as strategies to prevent relapse in patients who do not develop GVHD. Early withdrawal of IST36 might help to prevent relapse during the first 18 months in patients without GVHD, but the risk of subsequent relapse remains considerably higher than in patients with GVHD. Other interventions would be needed to prevent late relapse in patients who have not had GVHD. Strategies that could be used for this purpose include prophylactic donor lymphocyte infusion, immunotherapy, or maintenance therapy with novel disease-specific drugs such as hypomethylating agents, histone deacetylase inhibitors, or FLT3 inhibitors for myeloid malignancy, tyrosine kinase inhibitor for Philadelphia chromosome positive leukemia, and JAK2 inhibitor for MPN.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the patients who participated. We thank members of the research and clinical staff and referring physicians for their dedication and for their many years of service contributing to the long-term care of our patients after hematopoietic cell transplantation.
This research was supported by grants (CA18029 and CA15704) from the National Cancer Institute and a grant (HL36444) from the National Heart, Lung, and Blood Institute. Y.I. is a recipient of the Banyu Fellowship Program from the Banyu Life Science Foundation International, and the JSPS Postdoctoral Fellowships for Research Abroad.
National Institutes of Health
Authorship
Contribution: Y.I., M.E.D.F., and P.J.M. designed the study, collected and analyzed data, and wrote the paper. B.E.S. performed the statistical analysis and wrote the paper; S.J.L., P.A.C., E.H.W., and H.J.D. collected data and wrote the paper; and R.F.S. and F.R.A. provided administrative support and funding through program project grants. All authors critically revised the manuscript for important intellectual content and approved the manuscript to be published.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paul J. Martin, MD, Fred Hutchinson Cancer Research Center D2-100, PO Box 19024, Seattle, WA 98109-1024; e-mail: pmartin@fhcrc.org.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal