Abstract
Platelets are highly specialized blood cells critically involved in hemostasis and thrombosis. Members of the protein kinase C (PKC) family have established roles in regulating platelet function and thrombosis, but the molecular mechanisms are not clearly understood. In particular, the conventional PKC isoform, PKCα, is a major regulator of platelet granule secretion, but the molecular pathway from PKCα to secretion is not defined. Protein kinase D (PKD) is a family of 3 kinases activated by PKC, which may represent a step in the PKC signaling pathway to secretion. In the present study, we show that PKD2 is the sole PKD member regulated downstream of PKC in platelets, and that the conventional, but not novel, PKC isoforms provide the upstream signal. Platelets from a gene knock-in mouse in which 2 key phosphorylation sites in PKD2 have been mutated (Ser707Ala/Ser711Ala) show a significant reduction in agonist-induced dense granule secretion, but not in α-granule secretion. This deficiency in dense granule release was responsible for a reduced platelet aggregation and a marked reduction in thrombus formation. Our results show that in the molecular pathway to secretion, PKD2 is a key component of the PKC-mediated pathway to platelet activation and thrombus formation through its selective regulation of dense granule secretion.
Introduction
Platelet activation underlies the arterial thrombosis that causes the acute severe symptoms of heart disease and thrombotic stroke,1 and it is therefore important to determine the molecular mechanisms regulating platelet activity. We and others have shown that protein kinase C (PKC) isoforms regulate all of the essential functions of platelets, including actin rearrangements, adhesion through integrins, and secretion of granule contents.2-6 Of the isoforms of PKC expressed in platelets, the conventional PKCs, PKCα and PKCβ, have clear positive signaling roles, and mouse platelets lacking expression of PKCα show marked attenuation of responses and thrombus formation.2,7 The critical function regulated by PKCα is the secretion of dense granule content, which is rescued by the addition of exogenous ADP.2 Therefore, we sought to identify proteins that lie downstream of PKC in the pathway to regulation of dense-granule secretion to investigate the molecular regulation of this essential function.
The protein kinase D (PKD) family of Ser/Thr kinases consists of 3 members, PKD1 (also known as PKCμ), PKD2 and PKD3.8 PKDs contain a tandem repeat of zinc finger–like cysteine-rich motifs at their N-termini, highly homologous to domains found in diacylglycerol (DAG)/phorbol ester–sensitive PKCs and other signaling proteins regulated by DAG. However, unlike PKCs, PKDs lack the C2 domain responsible for the Ca2+ sensitivity of conventional PKCs, whereas they possess an autoinhibitory PH domain. Further, the catalytic domain of PKD has low homology with the conserved kinase domain of the PKCs. These differences make the PKD family a distinct set of kinases.
We were interested in PKDs because they had been shown to be activated in a PKC-dependent manner in a range of cells in response to a variety of stimuli.8-10 It has been proposed that DAG, which is generated by phospholipase C activation, binds to zinc finger 2 of the PKD N-terminus and facilitates the recruitment of the kinase to the plasma membrane, where it may be phosphorylated by PKCs. Residues Ser744 and Ser748 in the activation loop of PKD1 (conserved in PKD2/3) have been shown to be the critical sites for PKC-dependent PKD activation, leading to subsequent autophosphorylation of other residues such as Ser916.8 PKD2 may be activated by PKCα through the cholecystokinin b/gastrin receptor in human gastric carcinoma cells11 and during the process of angiotensin-induced endothelial cell exocytosis.12
A study by Stafford et al13 revealed the expression of PKD in platelets, and pharmacologic evidence suggested that PKD may be activated in a PKC-dependent manner. However, the high degree of homology between PKD1 and PKD2 means that the antibody used in this study was not able to distinguish between these 2 isoforms, and furthermore the expression of PKD3 was not addressed. The function of PKD in platelets was also not investigated in that study. However, with the generation of novel mutant mice this has now become possible. According to the comprehensive dataset for gene expression in blood cells, HaemAtlas, mRNA for PKD1 is absent from the human platelet precursor megakaryocytes, but PKD2 and PKD3 are present at the transcript level.14 To evaluate isoform-specific involvement of PKD in the regulation of essential platelet functions, we used PKD2 gene-trapped knockout mice described recently,9 and were able to confirm the expression of PKD2 and PKD3 and the absence of PKD1 in mouse platelets. Using mice generated with mutations in the PKC-dependent phosphorylation sites in PKD2 (Ser707/Ser711), PKD2SSAA/SSAA knock-in mice, we show that PKD2 is the exclusive PKD isoform that fulfils the role of PKC substrate in platelets, whereas PKD3 is not regulated downstream of PKC. We also elucidate the cellular function of PKD2 downstream of PKC activation using platelets from the PKD2SSAA/SSAA mouse. Using this approach, we show that PKD2 has a distinct role in selectively regulating dense-granule secretion, but not α-granule secretion, and in turn regulates thrombosis. We have therefore demonstrated PKD2 to be a major effector of PKC, particularly of PKCα, mediating its dense granule secretory function and subsequently thrombus formation.
Methods
Materials
The glycoprotein VI (GPVI)–specific agonist collagen-related peptide (CRP) was provided by Richard Farndale (University of Cambridge, Cambridge, United Kingdom). Thrombin, BSA, ADP, protein G-Sepharose, GF109203X, fibrinogen, sodium orthovanadate, 2-glycerophosphate, PMSF, and sodium fluoride were purchased from Sigma-Aldrich. Complete Mini Protease Inhibitor tablets were from Roche Applied Science. Secondary horseradish peroxidase-conjugated anti–rabbit antibody was obtained from Amersham. JON/A (anti-αIIbβ3), WugE9 (anti–P-selectin), and JAQ-1 (anti-GPVI) antibodies were from Emfret Analytics. Phycoerythrin-conjugated anti-CD41 antibody and its isotype-matched control, PE-conjugated rat IgG1, were from AbD Serotec. PKCα rabbit polyclonal antibody was BD Biosciences. Alexa Fluor 647, Annexin A5 and CountBright counting beads were from Invitrogen. PKCβII rabbit, PKD2 goat, and PKCμ polyclonal rabbit antibodies were from Santa Cruz Biotechnologies. P-PKD (Ser 916) and P-PKD (Ser916) rabbit polyclonal antibodies were from Cell Signaling Technologies. PKD3 rabbit polyclonal was an in-house antibody. LY333531 was supplied by A.G. Scientific. CHRONO-LUME reagent came from Chrono-log (Labmedics).
Mouse platelet preparation
Gene-trapped PKD2−/− and knockin PKD2SSAA/SSAA mice were generated as described previously.9 Mice were bred and maintained in the University of Bristol animal facility in accordance with United Kingdom Home Office regulations. All procedures were undertaken with United Kingdom Home Office approval in accordance with the Animals (Scientific Procedures) Act of 1986 (project license numbers: 40/2212, 40/2749, and 30/2386). Blood was drawn by cardiac puncture under terminal anesthesia into acid citrate dextrose (20mM citric acid, 110mM sodium citrate, and 5mM glucose) at a 1:7 ratio (vol/vol). Platelets were prepared as described previously.15 In brief, blood was diluted with 250 μL of modified Tyrode HEPES buffer (134mM NaCl, 0.34mM Na2HPO4, 2.9mM KCl, 12mM NaHCO3, 20mM HEPES, 5mM glucose, and 1mM MgCl2, pH 7.3) and centrifuged at 180g for 6 minutes at room temperature. Platelet-rich plasma was removed and platelets were isolated by centrifugation at 550g for 6 minutes in the presence of PGE1 (140nM) and indomethacin (10μM). Pelleted platelets were resuspended to the required density in modified Tyrode HEPES buffer, and rested for 30 minutes at 37°C in the presence of 10μM indomethacin before stimulation.
Human platelet preparation
Blood was obtained from healthy drug-free volunteers in accordance with approved guidelines from the Local Research Ethics Committee of the University of Bristol. Informed consent was obtained in accordance with the Declaration of Helsinki. Indomethacin (10μM) was added to PRP and throughout subsequent preparation steps.
Platelet aggregation
Platelets were resuspended in Tyrode HEPES buffer at a final concentration of 2 × 108/mL. Aggregation of agonist-stimulated platelets was monitored in an optical aggregometer (Chrono-log; Labmedics) at 37°C, with continuous stirring at 800 revolutions per minute.
Flow cytometry
Two-color analysis of mouse platelet activation was conducted using PE-conjugated JON/A, an antibody that preferentially binds to the active form of αIIbβ3 integrin, and with a FITC-conjugated antibody specific for CD62P (P-selectin). Twenty-five microliters of washed platelets (4 × 107/mL in Tyrode HEPES buffer) was mixed with 10 μL of antibody and subsequently stimulated either with CRP (5 μg/mL) or thrombin (1 unit/mL) for 15 minutes at room temperature. The reaction was stopped by the addition of 400 μL ice-cold PBS, and samples were analyzed within 30 minutes. For estimation of surface expression of αIIbβ3, platelets were stained with PE-conjugated anti-CD41. Annexin V-FITC was used to detect surface phosphatidylserine exposure. Platelets (5 × 107/mL) were stimulated in the presence of CaCl2 (2mM) for 10 minutes. For flow cytometry platelets were gated by their forward and side scatter profile. Flow cytometry was performed on a FACSCalibur flow cytometer (BD Biosciences), using CellQuest software Version 3.1f (BD Biosciences), and a total of 20 000 events per sample were collected. Data were analyzed using WinMDI Version 2.8.
Measurement of ATP secretion
ATP secretion was measured using CHRONO-LUME reagent according to the manufacturer's protocol. Five microliters of luciferase-luciferin was added directly to the platelets, which were being continuously stirred (1000 revolutions per minute), and CRP or thrombin at various concentrations were added to activate platelets for 1 minute. The luminescence intensity was measured at a setting of 0.05×.
Platelet static adhesion assay
Coverslips were coated with either fibrinogen (0.1 mg/mL) or CRP (5 μg/mL) and left overnight, followed by nonspecific blocking with 2% BSA for 1 hour. For spreading experiments, coverslips were coated with CRP (5 μg/mL) or mouse fibrinogen (100 μg/mL) overnight at 4°C. After twice washing with PBS, coverslips were blocked with 1% fatty acid–free BSA in PBS for 2 hours at room temperature and then rinsed with PBS. BSA-coated coverslips were used a negative control. Washed murine platelets (107/mL) were plated on coverslips for 20 minutes at room temperature. After washing of unbound platelets, the adherent platelets were fixed with 4% paraformaldehyde for 20 minutes at 4°C. After the final wash, platelets were visualized with a Leica DMIRBE inverted microscope. Fifteen to 20 images in randomly chosen areas of the coverslip were captured using OpenLab 4.03 (Improvision) software, and the number of adherent platelets was estimated using ImageJ software (http://rsbweb.nih.gov/ij/).
Immunoblotting
For stimulated samples, 2 × 108/mL of washed platelets were preincubated with different inhibitors or vehicle solution (0.1% DMSO final concentration) for 10 minutes at 37°C and subsequently activated with CRP or thrombin at various concentrations and were solubilized in an equal volume of SDS-Laemmli sample buffer. For immunoprecipitation assays platelets were lysed into an equal volume of 2 × lysis buffer (50mM HEPES, pH 7.4, 150mM NaCl, 2% (vol/vol) Triton X-100, 1mM sodium orthovanadate, 4mM PMSF, 20mM 2-glycerophosphate, 200mM sodium fluoride, 200mM benzamidine, and complete protease inhibitors). Incubations with immunoprecipitating antibody were carried out overnight at 4°C, beads were washed 3 times in 1 × lysis buffer, and bound proteins were eluted by the addition of SDS-sample buffer. Proteins were resolved by electrophoresis in 7% SDS-PAGE. Samples were then transferred to PVDF membranes, blocked with 10% BSA, and subjected to immunoblotting with various antibodies as described in the figure legends.
Thrombus formation under flow in vitro
Flow-induced thrombus formation was assessed essentially as described previously.16 Heparin/D-phenylalanyl-prolyl-arginyl chloromethyl ketone (PPACK)–anticoagulated mouse blood was passed over immobilized collagen through a parallel plate perfusion chamber at a shear rate of 1000/s for 4 minutes. For each perfusion surface, phase-contrast images from 10 random microscopic fields were collected. Surface coverage was analyzed using Image-Pro software Version 4.1 (Media Cybernetics). The average percentage area covered by adherent platelets was measured by automated setting of masks for the ranges of gray levels corresponding to the presence of platelets and thrombi. For each image, the (blinded) observer needed to approve the mask settings. Averaged pixel fractions of the masks from 10 images were considered as a best estimate of the surface area coverage with thrombi.
Electron microscopy
Platelet samples from WT and PKD2SSAA/SSAA mice were prepared for transmission electron microscopy, as described previously.2 Numbers of dense granules and α-granules in equivalent-sized fields of view were counted using ImageJ software. For each genotype, 25-30 randomly chosen fields of view were examined. All microscopic images were taken at the same magnification (19 000×), and the number of cells per field of view between wild-type (WT) and PKD2SSAA/SSAA preparations were equivalent.
Analysis of bleeding time
Experiments were conducted on 25-35 g male and female mice. Mice were anesthetized (75 mg/kg ketamine and 1 mg/kg medetomidine intraperitoneally), and a transverse incision was made with a scalpel at a position where the diameter of the tail was 2.25-2.5 mm. The tail was immersed in normal saline (37°C) in a hand-held test tube, and the time from incision to cessation of bleeding was recorded.
Platelet count
To determine platelet count, 2-μL blood samples were taken from the tail vein and added to 43 μL of ACD/Tyrode buffer (1:5 vol/vol). The blood samples were incubated with anti–CD41:FITC antibody (1:10) for 20 minutes at room temperature in the dark. Blood samples were mixed with 50 μL of CountBright beads in 400 μL of filtered Tyrode buffer (following the manufacturer's guidelines) before analysis on a FACSCalibur flow cytometer (BD Biosciences). Anti–CD41:FITC-labeled platelet and CountBright bead populations were gated in a FL1 versus FL2 channel dot-plot. The triplicate counts were averaged and the platelet count per microliter of whole blood was calculated according to the manufacturer's instructions.
Results
Regulation of PKD by PKC isoforms in human and mouse platelets
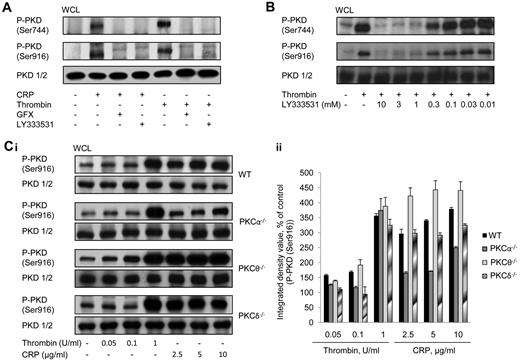
Previous studies showed that PKD activation in platelets via GPVI and PAR-receptors is dependent on PKC,4 but the identity of the PKC isoform(s) responsible has not been determined. We have addressed this using pharmacologic and genetic approaches. Figure 1A shows that the robust and rapid autophosphorylation of PKD on Ser916 and on the PKC-dependent Ser744 site by thrombin and CRP in human platelets could be completely inhibited by a broad-spectrum PKC inhibitor (GF109203X) or by the PKCβ-selective inhibitor LY333531. Although a minor and faint band appears slightly higher than the major band in pSer916 blots, this is unlikely to represent PKD because reprobing with the PKD1/2 antibody only revealed a single band. Figure 1B shows that pretreatment of platelets with LY333531 (10μM) produced a maximal inhibitory effect on thrombin-induced PKD activation. Recent evidence has suggested that PKCα may also be targeted by LY333531, in addition to PKCβ,17 so we determined the activation of PKD in platelets from PKCα-deficient mice. Within the range of agonist concentrations tested, thrombin was able to induce PKD phosphorylation on Ser916 in WT platelets, particularly at the highest concentration used (1 unit/mL; Figure 1C), although there was still a small but measurable activation of the kinase at the lower concentrations. This small degree of activation may explain the small defect in aggregation response (seen later in Figure 5) using thrombin at 0.065 units/mL. CRP induced a maximal response at 5 μg/mL, and this was the concentration subsequently used throughout the study (Figure 1C). PKCα−/− platelets showed a substantially reduced level of CRP-induced PKD phosphorylation on Ser916 compared with WT, although for thrombin there was no significant change in phosphorylation. This may indicate that, for thrombin, there is significant redundancy between PKCα and PKCβ in mediating phosphorylation of PKD, whereas for CRP there is much less redundancy, with PKCα taking the principal role. With regard to the novel family of PKC isoforms, ablation of PKCδ produced no change in agonist activation of PKD (Figure 1C), whereas ablation of PKCθ caused a small potentiation of activation of PKD activity in response to CRP. These data indicate that the classical PKC isoforms PKCα and PKCβ are likely to be positive regulators of PKD, but that the novel isoform PKCθ is a negative regulator. In addition, PKCδ has no role in regulating PKD activity, especially downstream of GPVI activation.
Agonist-induced PKD activation in platelets is dependent on classic PKC isoforms but independent of novel PKC isoforms. (A) Washed human platelets (2 × 108/mL) were incubated with GFX (10μM), LY333531 (10μM), or vehicle (DMSO) for 10 minutes and subsequently stimulated with CRP (5 μg/mL) or thrombin (1 unit/mL) for 1 minute. (B) Washed human platelets (2 × 108/mL) were incubated with various concentrations of LY333531 or vehicle (DMSO) for 10 minutes and subsequently stimulated with thrombin (1 unit/mL) for 1 minute under stirring conditions. Immunoblots were performed with P-PKD (Ser916), P-PKD (Ser744), or total PKD antibody. One representative experiment of 3 with identical results is shown. (C) Washed platelets (2 × 108/mL) from WT, PKCα−/−, PKCθ−/−, or PKCδ−/− mice, were incubated with various concentrations of CRP or thrombin for 1 minute. Immunoblots were performed with either P-PKD (Ser916) or total PKD antibody. One representative experiment (i) and densitometric analysis of 3 experiments (ii) are shown. For panel Cii, data shown are means ± SEM (n = 3).
Agonist-induced PKD activation in platelets is dependent on classic PKC isoforms but independent of novel PKC isoforms. (A) Washed human platelets (2 × 108/mL) were incubated with GFX (10μM), LY333531 (10μM), or vehicle (DMSO) for 10 minutes and subsequently stimulated with CRP (5 μg/mL) or thrombin (1 unit/mL) for 1 minute. (B) Washed human platelets (2 × 108/mL) were incubated with various concentrations of LY333531 or vehicle (DMSO) for 10 minutes and subsequently stimulated with thrombin (1 unit/mL) for 1 minute under stirring conditions. Immunoblots were performed with P-PKD (Ser916), P-PKD (Ser744), or total PKD antibody. One representative experiment of 3 with identical results is shown. (C) Washed platelets (2 × 108/mL) from WT, PKCα−/−, PKCθ−/−, or PKCδ−/− mice, were incubated with various concentrations of CRP or thrombin for 1 minute. Immunoblots were performed with either P-PKD (Ser916) or total PKD antibody. One representative experiment (i) and densitometric analysis of 3 experiments (ii) are shown. For panel Cii, data shown are means ± SEM (n = 3).
PKD expression and activation profile in mouse platelets
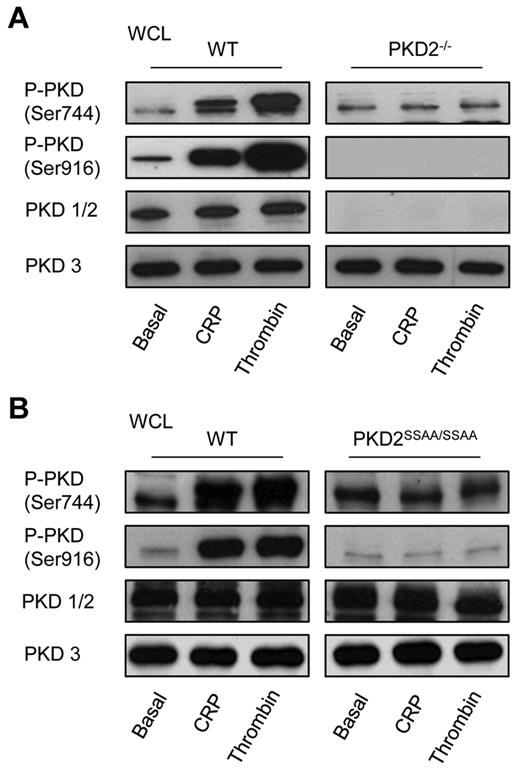
The analysis of the expression and activation profile of PKD isoforms in platelets was conducted using WT and PKD2-knockout mice. Because of the high degree of homology between PKD1 and PKD2, there is no antibody available that will distinguish between the 2, and therefore we used an antibody (PKD1/2) raised against the C-terminus of human and mouse PKD1 and PKD2. Figure 2A shows that there is no signal from PKD2−/− platelets, whereas an anti-PKD3 blot demonstrates expression of this isoform. We conclude therefore that mouse platelets express PKD2 and PKD3, but not PKD1, and that expression of PKD3 remained unaffected by gene deletion of PKD2. Figure 2A also shows that phosphorylation of the PKC sites in the PKDs (indicated as S744/S748, because the antibody relates to PKD1; however, these sites are conserved between PKD2, at S707/S711, and PKD3, at S731/S735) and Ser916 is restricted to PKD2 and not PKD3. Thus, although both PKD2 and PKD3 are expressed in platelets, it is only PKD2 that is activated upon agonist stimulation.
PKD expression and activation profile in platelets. Washed platelets from WT, PKD2−/− (A), or PKD2SSAA/SSAA (B) mice (2 × 108/mL) were incubated with CRP (5 μg/mL) or thrombin (1 unit/mL) for 1 minute under stirring conditions. Platelets were then solubilized in Laemmli sample buffer and whole cell lysates were subjected to SDS-PAGE, transferred to PVDF membrane, and immunoblotted with PKD1/2, PKD3, P-PKD (Ser916), or P-PKD (Ser744) antibodies. Data shown are representative of 3 independent experiments.
PKD expression and activation profile in platelets. Washed platelets from WT, PKD2−/− (A), or PKD2SSAA/SSAA (B) mice (2 × 108/mL) were incubated with CRP (5 μg/mL) or thrombin (1 unit/mL) for 1 minute under stirring conditions. Platelets were then solubilized in Laemmli sample buffer and whole cell lysates were subjected to SDS-PAGE, transferred to PVDF membrane, and immunoblotted with PKD1/2, PKD3, P-PKD (Ser916), or P-PKD (Ser744) antibodies. Data shown are representative of 3 independent experiments.
PKD2 activity in PKD2SSAA/SSAA mice is abolished
Phosphorylation by PKC at Ser707/Ser711 of PKD2, which may be detected using the phosphospecific antiphospho-Ser744/Ser748 antibody for PKD1, is correlated with the activation status of the kinase. Immunoblotting with antiphospho-Ser744/Ser748 demonstrated that mutation of the 2 equivalent Ser in PKD2 eliminated phosphorylation on these sites (Figure 2B). Analysis of activity of PKD2 by assessment of phospho-Ser916 showed that whereas phosphorylation of PKD2 in WT was greatly increased by agonist stimulation, no increase was seen in PKD2SSAA/SSAA platelets, indicating that PKD2 kinase activity in platelets was eliminated by these point mutations. This also indicated that agonist-induced activation of PKD2 in platelets is mediated solely by PKC. The presence of bands detected by antiphospho-Ser744/Ser748 and Ser916 antibodies in lysates of basal platelets may be a result of low-affinity cross-reactivity with nonphosphorylated PKD2 or PKD3. Alternatively, we cannot rule out the possibility that PKD3 is constitutively phosphorylated and therefore basally active, which would also explain the presence of bands under basal conditions. However, the fact that no increase in band density was seen upon stimulation would indicate that this is not a regulated activation. In addition, there was no change in the expression levels of the PKC isoforms found predominantly in mouse platelets (PKCα, PKCβ, PKCδ, and PKCθ) in PKD2SSAA/SSAA compared with WT platelets (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
PKD2 does not directly regulate integrin αIIbβ3
Our results show that the PKD2SSAA/SSAA mouse provides an ideal model for the study of the role played by PKD2 downstream of classic PKC isoforms. We showed previously that ablation of PKCα causes a significant reduction in the ability of agonists to activate integrin αIIbβ32, so we could now address whether activation of PKD2 by PKC may form the signaling pathway. However, as supplemental Figure 2 shows, the maximally effective concentrations (as determined by PKD phosphorylation on Ser916; Figure 1C) of both CRP (5 μg/mL; supplemental Figure 2Ai) and thrombin (1 unit/mL; supplemental Figure 2Aii) produced similar levels of αIIbβ3 activation in both PKD2SSAA/SSAA and WT murine platelets. As a control, supplemental Figure 3 shows no significant difference in surface expression of αIIb (CD41) or GPVI in PKD2SSAA/SSAA compared with WT. The role of PKD2 in integrin outside-in signaling was studied by assessing static adhesion to either CRP or fibrinogen. The number of platelets measured at a fixed time point demonstrated no difference between PKD2SSAA/SSAA and WT platelets (supplemental Figure 2B). There were also no differences in spreading on these surfaces between PKD2SSAA/SSAA and WT platelets (data not shown). These observations suggest that PKD2 does not directly mediate PKC-dependent signaling to αIIbβ3 activation.
PKD2 regulates dense- but not α-granule secretion
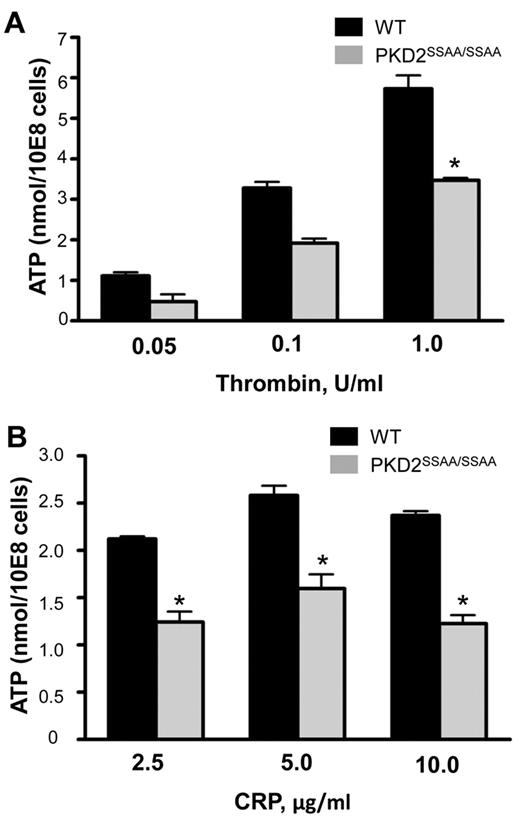
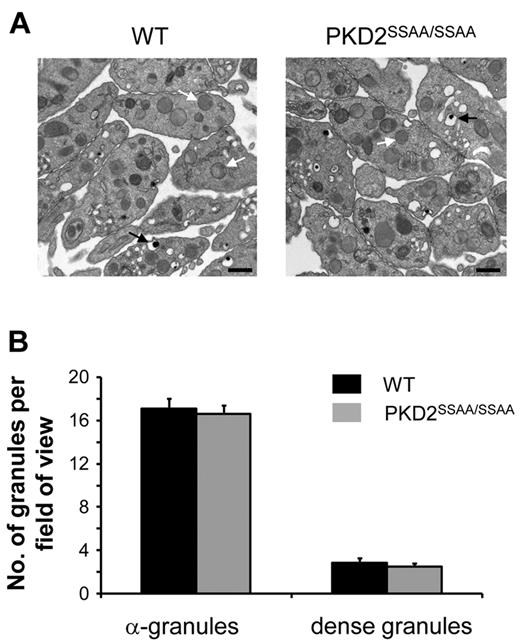
Figure 3 demonstrates that PKD2SSAA/SSAA platelets exhibit a marked reduction, although not a complete ablation, of CRP- and thrombin-induced ATP secretion relative to WT controls. At a range of agonist concentrations, a statistically significant reduction in ATP levels in PKD2SSAA/SSAA was obtained even at the highest concentrations of CRP (10 μg/mL) and thrombin (1 unit/mL). Interestingly, this defect in platelet exocytosis was restricted to dense granules, because there were no differences in the degree of P-selectin surface expression, which is used as an indicator of α-granule secretion, between WT and PKD2SSAA/SSAA platelets (supplemental Figure 4) in response to CRP (5 μg/mL) or thrombin (1 unit/mL). Quantification of the numbers of dense and α-granules by transmission electron microscopy did not reveal any significant differences between the 2 groups of platelets (Figure 4). This shows that in the absence of catalytically active PKD2, granule biogenesis is not defective and therefore cannot be the cause of the impaired dense granule secretion.
PKD2 regulates secretion of dense granules in response to CRP and thrombin in platelets. Washed platelets from WT and PKD2SSAA/SSAA mice were stimulated with various concentrations of CRP (A) or thrombin (B) as indicated, and secretion of ATP was assessed by luminometry. Error bars represent SEM, n = 3-6; *P < .05
PKD2 regulates secretion of dense granules in response to CRP and thrombin in platelets. Washed platelets from WT and PKD2SSAA/SSAA mice were stimulated with various concentrations of CRP (A) or thrombin (B) as indicated, and secretion of ATP was assessed by luminometry. Error bars represent SEM, n = 3-6; *P < .05
Biogenesis of dense and α-granules is not regulated by PKD2 activity. Washed platelets from WT or PKD2SSAA/SSAA mice were examined by transmission electron microscopy, and the number of dense granules (black arrows) and α-granules (white arrows) was quantified as described in “Electron microscopy.” (A) Images of platelets from WT (left panel) or PKD2SSAA/SSAA (right panel) mice are representative of 3 independent experiments. Original magnification was 19 000×, and the bar represents 500 nm. (B) Dense and α-granules were counted per fields of view (25-30 fields of view per section, 3 sections per preparation on average) and are shown as means ± SEM, n = 3.
Biogenesis of dense and α-granules is not regulated by PKD2 activity. Washed platelets from WT or PKD2SSAA/SSAA mice were examined by transmission electron microscopy, and the number of dense granules (black arrows) and α-granules (white arrows) was quantified as described in “Electron microscopy.” (A) Images of platelets from WT (left panel) or PKD2SSAA/SSAA (right panel) mice are representative of 3 independent experiments. Original magnification was 19 000×, and the bar represents 500 nm. (B) Dense and α-granules were counted per fields of view (25-30 fields of view per section, 3 sections per preparation on average) and are shown as means ± SEM, n = 3.
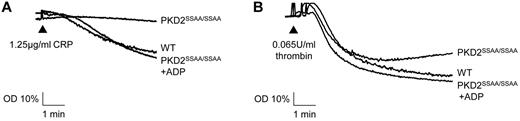
PKD2SSAA/SSAA platelets show impaired aggregation that may be rescued by exogenous ADP
We showed previously that PKCα deficiency causes a severe reduction in the ability of platelets to aggregate, principally due to a dysfunction at the level of dense-granule secretion.2 In the present study, although a significant component of dense-granule secretion remained in PKD2SSAA/SSAA mice, there was still a marked decrease in platelet aggregation in response to submaximal concentrations of CRP or thrombin (Figure 5). These responses to submaximal concentrations of agonists (1.25 μg/mL of CRP and 0.065 units/mL of thrombin) could be effectively rescued by the simultaneous addition of exogenous ADP (10μM). Higher concentrations of agonists showed no deficit in aggregation, indicating that the effect is dependent on the degree of platelet activation through secondary positive feedback mediators (data not shown).
Deficient aggregation responses in platelets from PKD2SSAA/SSAA mice are rescued by exogenously added ADP. Washed platelets from WT and PKD2SSAA/SSAA mice were stimulated with CRP (1.25 μg/mL; A) or thrombin (0.065 units/mL; B) with or without the simultaneous addition of ADP (10μM), and aggregation was assessed turbidimetrically. Aggregation traces are representative of 6 independent experiments with similar results.
Deficient aggregation responses in platelets from PKD2SSAA/SSAA mice are rescued by exogenously added ADP. Washed platelets from WT and PKD2SSAA/SSAA mice were stimulated with CRP (1.25 μg/mL; A) or thrombin (0.065 units/mL; B) with or without the simultaneous addition of ADP (10μM), and aggregation was assessed turbidimetrically. Aggregation traces are representative of 6 independent experiments with similar results.
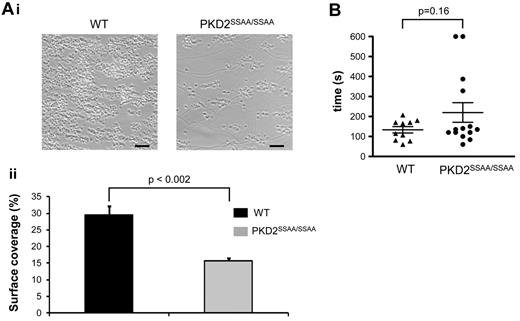
PKD2SSAA/SSAA platelets show impaired thrombus formation in vitro, but PKD2SSAA/SSAA mice retain a normal hemostatic response
Perfusion of PPACK-anticoagulated whole blood from WT mice at a high wall shear rate (1000/s) over a collagen-coated surface resulted in rapid platelet adhesion, followed by the formation of aggregates (Figure 6A) and exposure of procoagulant phosphatidylserine (supplemental Figure 5). Although the initial adhesion of PKD2SSAA/SSAA platelets remained unaffected, similar to that in static adhesion experiments to CRP or fibrinogen (supplemental Figure 2B), the subsequent thrombus formation was significantly reduced. The thrombus surface coverage for PKD2SSAA/SSAA blood, as a percentage of total area, was 15.71% ± 0.82% compared with 29.52% ± 2.71% (n = 6, P < .002) for WT (Figure 6A, i-ii). Procoagulant activity of PKD2SSAA/SSAA platelets in whole blood under flow, as determined by Annexin V binding, was unimpaired (supplemental Figure 5A); this was confirmed in platelets under static conditions by flow cytometry (supplemental Figure 5B).
PKD2SSAA/SSAA mice display reduced thrombus formation in vitro but have normal tail-bleeding responses. Heparin/PPACK-anticoagulated blood from WT or PKD2SSAA/SSAA mice was passed over collagen (shear rate 1000/s). (Ai) Representative phase-contrast images after 4 minutes of flow. Scale bars represent 20 μm. (ii) Platelet coverage of coated surfaces was quantified and the mean surface area ± standard error of the mean is shown for 3 independent experiments. *P < .05. (B) Mice were anesthetized and a transverse incision made with a scalpel at a position where the diameter of the tail was 2.25-2.5 mm. The tail was immersed in normal saline (37°C) in a hand-held test tube. The time from incision to cessation of bleeding was recorded, and mean times are shown as horizontal bars. No significant difference was seen comparing WT mice with PKD2SSAA/SSAA mice (n = 10-14).
PKD2SSAA/SSAA mice display reduced thrombus formation in vitro but have normal tail-bleeding responses. Heparin/PPACK-anticoagulated blood from WT or PKD2SSAA/SSAA mice was passed over collagen (shear rate 1000/s). (Ai) Representative phase-contrast images after 4 minutes of flow. Scale bars represent 20 μm. (ii) Platelet coverage of coated surfaces was quantified and the mean surface area ± standard error of the mean is shown for 3 independent experiments. *P < .05. (B) Mice were anesthetized and a transverse incision made with a scalpel at a position where the diameter of the tail was 2.25-2.5 mm. The tail was immersed in normal saline (37°C) in a hand-held test tube. The time from incision to cessation of bleeding was recorded, and mean times are shown as horizontal bars. No significant difference was seen comparing WT mice with PKD2SSAA/SSAA mice (n = 10-14).
PKD2SSAA/SSAA mice exhibit normal hemostatic function, as assessed by tail-vein bleeding experiments (Figure 6B). Given that these animals have normal blood platelet counts (WT 1.14 ± 0.09 × 106/μL; PKD2SSAA/SSAA 1.21 ± 0.17 × 106/μL, P > .05, n = 5), this suggests that, as demonstrated previously for PKCα−/− platelets,2 normal hemostasis may operate even under conditions in which thrombus formation is impaired, suggesting a clear distinction between the 2 processes.
Discussion
PKC plays a central role in the complex network of platelet signal transduction. It has been shown previously that several members of the family of PKC isoforms have distinct nonredundant roles in regulating essential platelet processes such as granule secretion, integrin activation, aggregation, spreading, and procoagulant activity.18 One of the major tasks now needing to be addressed in this field is to define the molecular details of the downstream signaling pathways controlled by PKCs. We adopted a candidate approach for this study that is based on the observation that one known substrate for PKC is PKD, which had been shown previously to be expressed in platelets and (using pharmacologic approaches) to be regulated downstream of PKC.9
In this study, we used 2 recently generated, genetically targeted mice to define a role for PKDs in platelets.9 First, a gene-trapped PKD2−/− mouse was used to define the expression profile of the PKDs in mouse platelets and to overcome limitations of antibodies for Western blotting. Second, a knock-in mouse replacing expression of WT PKD2 with a double point mutant, PKD2Ser707Ala/Ser711Ala (PKD2SSAA/SSAA), was used to define how PKD2 is regulated by upstream PKCs and to define the downstream functions of PKD2. The data indicate that mouse platelets express 2 isoforms of PKD, PKD2 and PKD3, but not PKD1. However, of these, PKD2 but not PKD3 activity was increased upon agonist stimulation. Activation of PKD2 was shown to be entirely downstream of PKC, because no activation was detectable in the PKD2SSAA/SSAA mutant, where PKC phosphorylation regulatory Ser have been mutated to Ala. The classical PKC isoforms, PKCα and PKCβ, but not the novel isoforms PKCδ or PKCθ, were shown to lie upstream of PKD2. Functionally, PKD2SSAA/SSAA platelets partially phenocopy the deficits seen in the PKCα−/− platelets we have described previously,2,7 suggesting that PKD2 is an important effector for some, but not all, events downstream of PKCs in platelets. In particular, this study has defined PKD2 as a major effector and mediator of dense granule secretion downstream of PKCα, and therefore as a regulator of thrombus formation.
The high degree of sequence similarity between the PKDs, and in particular between PKD1 and PKD2, has caused problems with generating reagents that specifically recognize the individual isoforms. We therefore took advantage of the recently generated PKD2−/− mouse9 to use the anti-PKD1/2 antibody to define expression of PKD1 and PKD2 in platelets. Our data clearly show the absence of expression of PKD1 and the presence of expression of PKD2 in mouse platelets, which is in agreement with a similar expression profile in other blood cell types.9,14 PKD3 is sufficiently distinct in sequence to allow the generation of a specific antibody, and blotting also showed expression of this isoform in mouse platelets (Figure 1).
Having defined the expression profile of the PKD family members in platelets, we then proceeded to define their regulation and functional role. Existing evidence from other cell types shows that the major regulatory mechanism upstream of PKD is PKC, and in lymphocytes there is clear evidence for activation by novel PKC isoforms.19-21 There is also evidence that the classical PKC isoform, PKCα, may also activate PKD, as it does in VEGF-induced endothelial cell proliferation.22 Our data show that only 1 of the 2 expressed isoforms of PKD in platelets, PKD2 but not PKD3, is responsive to thrombin or CRP. In addition, the novel isoforms of PKC expressed in platelets, PKCδ and PKCθ, do not positively regulate the activity of PKD2, although the densitometry results shown in Figure 1C reveal that the absence of PKCθ tends to increase the agonist-dependent activation of PKD2 in response to both thrombin and CRP. It is therefore possible that PKCθ is a negative regulator of agonist-induced PKD2 activity. In addition, although a possible role for the other novel isoforms, PKCϵ and PKCη, cannot be excluded, the conventional isoform, PKCα, acts as an upstream regulator of PKD2, particularly for GPVI signals. For CRP-induced activation (at low concentrations in particular), this isoform of PKC plays a substantial role; however, for thrombin, other mechanisms of PKD activation may be involved, such as redundancy with other PKC isoforms (PKCβ, PKCϵ, or PKCη).
Definitive evidence that PKCs are critical for PKD2 activation was possible because of the generation of the PKD2SSAA/SSAA knock-in mouse. The mutated sites Ser707Ala and Ser711Ala are critical for PKD2 catalytic activity downstream of PKC activation.9,11 Generation of this mouse also allowed the analysis of the role of the catalytic activity of PKD2 independently of the role of any scaffolding function that this molecule may possess. PKD2SSAA/SSAA platelets showed no activation, as judged by autophosphorylation of Ser916, in response to agonist, and the data indicate that a PKC-dependent route to PKD2 activation is the only mechanism by which PKD2 may be activated. This is largely in agreement with recent data for T lymphocytes,9 although in these cells there is still a small residual activity of PKD2SSAA/SSAA, suggesting that in lymphocytes there is additional activation of PKD through a non-PKC-dependent route. Whether the ability of PKD to translocate to the membrane is affected by mutation of the PKC-dependent phosphorylation sites is not yet known.
Defining a role for PKDs has not been possible until recently, with the emergence of novel genetically targeted mice.9,23 Pharmacologic dissection of PKD function is still problematic because of the poor selectivity of the majority of PKD kinase inhibitors.24-27 In particular, isoform-specific pharmacologic inhibition of PKD is currently not possible. Therefore, the PKD2SSAA/SSAA mouse is an essential tool for the analysis of function of PKD in platelets. The marked reduction in platelet aggregation responses to submaximal concentrations of CRP and thrombin in PKD2SSAA/SSAA platelets paralleled the impaired thrombus formation in whole blood flowed over a collagen-coated surface. The deficit in aggregation, however, could be fully recovered by the exogenous addition of ADP, suggesting that the defect in dense granule secretion of ADP was responsible for the disruption to platelet aggregation. Indeed, dense granule secretion was markedly suppressed in PKD2SSAA/SSAA platelets compared with WT platelets, with an approximately 50% reduction in ATP release. Ablation of PKD2 had an effect only on dense granule secretion, whereas release of α-granules remained unimpaired. This observation indicates that PKD2 in platelets has a highly selective regulatory role, and suggests that it is responsible only for some, not all, of the signaling downstream of conventional PKCs.
The data here show that biogenesis of both dense and α-granules remains intact in PKD2SSAA/SSAA platelets compared with WT, according to estimation of granule numbers by electron microscopy. Therefore, the defect in dense granule secretion cannot be explained by a change in granule numbers. There is already evidence from other cell types that PKD may regulate vesicular protein transport from the Golgi to the plasma membrane28 and secretion processes. Sumara et al described PKD as a pivotal regulator of insulin secretion from pancreatic β cells,29 and Ge et al found that PKCα-dependent PKD2 activation was an essential mechanism for P-selectin surface expression in endothelial cells.12
Although ablation of PKD2 activity manifested itself in a pronounced reduction in platelet dense granule secretion, integrin αIIbβ3 activation remained unaffected. There may be several explanations for this. First, PKD has been shown to regulate integrin function in other cell types, partly through a scaffolding function that is independent of its kinase activity. For example, PKD1 is known to regulate the vesicular transport and incorporation of αVβ3 into focal adhesions,30 to associate with αVβ3 to regulate fibroblast migration,31 and to positively regulate β1 function via Rap1 activation in T cells.32 In addition, platelets use PKC-independent, as well as PKC-dependent, pathways to integrin activation via CalDAGGEFI and Rap1b.33 Second, the residual release of dense granules could be sufficient to maintain full integrin activation. It is possible that the ability of platelets to exhibit normal aggregation responses to high agonist concentrations, as well as normal adhesion in static and initial adhesion in flow conditions, were the consequences of this unimpaired component of dense granule release. It is noteworthy that in the flow system of assessment of thrombus formation, the released ADP is washed away rapidly by the flowing blood, which is not the case in the static conditions used to measure the level of integrin activation by flow cytometry in a tube. The difference between these 2 approaches may explain why platelet surface coverage in the flow system was reduced in PKD2SSAA/SSAA compared with WT mice (Figure 6), whereas there was no difference in integrin activation (supplemental Figure 2A).
Some features, but not others, of PKD2SSAA/SSAA platelet phenotype mimic those found in PKCα−/− and PKCβ−/− mice. We showed previously that PKCα−/− mice were characterized by ablation of secretion of both α- and dense granules, which ultimately translated into impairment of in vitro and in vivo thrombus formation.2 Similarly, PKCβ-deficient mice also demonstrate a reduction in thrombus formation under flow conditions in vitro.7 PKD2 therefore represents a downstream effector of the classical PKC isoforms in platelets, in particular PKCα, for signals downstream of GPVI.
In summary, we have shown that PKD2 plays a major role in the selective regulation of platelet dense granule secretion, aggregation, and in vitro thrombus formation, thus representing a major component of the pathway occurring downstream of PKCα. The central findings reveal a high degree of specialization of PKD2, which regulates dense granule but not α-granule platelet secretion. PKD is implicated in a variety of disease-related states. Increasing evidence points to PKD in the regulation of signaling pathways in the cardiovascular system, particularly in the processes of myocardial contraction, hypertrophy, and remodeling.34 PKD is responsive to physiologically important stimuli in endothelial cells, vascular smooth muscle cells, and platelets.13,22,35 PKD activity in general and PKD2 in particular appear to guide the processes commonly associated with atherosclerosis, such as endothelial cell migration, proliferation, induction of proinflammatory cytokines, and monocyte adhesion.36-38 Many of these cellular actions of PKD2 have also been identified as critical points of control by PKCα,39 making it plausible that the fundamentally important pathophysiologic functions of PKCα, including its atherosclerosis-related prothrombotic characteristics,40-42 are executed at least partially by PKD2.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Elizabeth Aitken and Elizabeth Emslie for their expert technical assistance supporting this work. We are grateful to Dr Paul Verkade for his assistance with electron microscopy.
The work was supported by grants from the British Heart Foundation (RG/05/015 and PG/07/118/24152 to A.W.P.).
Authorship
Contribution: O.K. designed and performed experiments, analyzed data, and wrote the manuscript; S.A.M., M.N.N., and D.C. provided essential reagents and designed experiments; M.T.H., K.G., J.M.E.M.C., C.M.W., and J.W.M.H. performed experiments and analyzed data; D.A.C. performed experiments; and A.W.P. designed experiments and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alastair W. Poole, School of Physiology & Pharmacology, Bristol Heart Institute, Medical Sciences Bldg, University Walk, Bristol, BS8 1TD, United Kingdom; e-mail: a.poole@bris.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal