Abstract
Although many literature data are available on the role of Notch signaling in T-cell acute lymphoblastic leukemia (ALL) biology, the importance of this molecular pathway in the development of B-lineage ALL (B-ALL) cells in the BM microenvironment is unknown so far. In this study, we used anti-Notch molecules neutralizing Abs and γ-secretase inhibitor (GSI) XII to investigate the role of the Notch signaling pathway in the promotion of human B-ALL cell survival in presence of stromal cell support. The treatment with combinations of anti-Notch molecule neutralizing Abs resulted in the decrease of B-ALL cell survival, either cultured alone or cocultured in presence of stromal cells from normal donors and B-ALL patients. Interestingly, the inhibition of Notch-3 and -4 or Jagged-1/-2 and DLL-1 resulted in a dramatic increase of apoptotic B-ALL cells by 3 days, similar to what is obtained by blocking all Notch signaling with the GSI XII. Our data suggest that the stromal cell–mediated antiapoptotic effect on B- ALL cells is mediated by Notch-3 and -4 or Jagged-1/-2 and DLL-1 in a synergistic manner.
Introduction
B-cell development depends on the interaction with the BM microenvironment.1,2 The complex mixture of growth factors, extracellular matrix components, and stromal cells provides extrinsic signals that regulate growth, differentiation, and survival of either normal B-cell precursors and neoplastic cells of B-lineage acute lymphoblastic leukemia (ALL) B-ALL.1,2 B-ALL is characterized by the clonal expansion of CD19-positive, neoplastic B-cell precursors at different developmental stages. Integrins represent an essential component of the BM microenvironment that regulates cell survival by interacting with the extracellular matrix.3 ALL blasts grow and accumulate in close association with BM mesenchymal cells and this event is essential for the long-term survival and expansion of leukemic lymphoblasts in vitro, as shown clearly by different authors.4-9
Stromal cells may protect B-ALL cell lines from cytarabine- and etoposide-induced cell death via a VCAM-1–dependent mechanism.4 Activation of caspase-3 by Ara-C or VP-16 treatment is reduced on coculture of B lymphoblasts with stromal cell layers.10 On the other hand, the interaction between VLA-4 on leukemia blasts cells and fibronectin or VCAM-1 on stromal cells activates PI3K/Akt/Bcl-2 signaling, an important pathway that determines B-ALL chemosensitivity and contributes to the persistence of minimal residual disease in B-ALL patients.11,12 BM stromal cells derive from mesodermal precursors, named mesenchymal stromal cells (MSCs), which are multilineage nonhematopoietic progenitor cells playing a key role in supporting lymphohematopoiesis and giving rise to different stromal cell lineages, as shown in vitro and partially in vivo.13,14
BM-MSCs express Notch ligands Jagged-1/-2 and Delta ligands,15,16 and MSC-derived osteoblasts regulate the HSC niche by using Jagged-1/Notch-1 signaling.16 Interestingly, one of the redundant mechanisms involved in the immune regulatory effect of BM-MSCs is based on the interaction between Jagged-1, expressed by BM-MSCs, and Notch-1, expressed by immune effector cells.15
The Notch signaling pathway is evolutionarily conserved and plays key role in cell-fate determination and differentiation in many tissues during embryonic and postnatal development.17,18 Four mammalian Notch receptors have been identified and designated as Notch1-4.17,18 The interaction of Notch receptors with membrane-bound ligands of Delta and Jagged families, that is, Delta-like 1 (DLL-1)/-3/-4, Jagged-1 and -2, is critical for Notch signaling.17,18 Ligand binding induces γ-secretase–mediated cleavage and translocation of the Notch intracellular domain into the nucleus, where it interacts with the DNA-binding protein RBP-J to induce the expression of downstream target genes, such as Hes-1 and Deltex-1.18 Jagged-1/-2 and DLL-1, commonly named as Delta/Serrate/LAG-2 (DSL) proteins, are ligands for Notch 1-418,19 ; Delta-4 can bind and activate Notch-1 and -4 receptors,18-20 whereas Delta-3 can bind and activate Notch-1 or similar Notch receptors.18-21
The Notch system plays an essential role in the hematopoiesis and in the embryonic development.20 Disregulation of Notch signaling is associated with several human disorders, including cancer,20 and is involved in the pathogenesis of T-cell ALL with the Notch involving translocation t(7;9)(q34;q34.3).18,22 However, little is known about the role of Notch signaling in B-ALL cell development and in leukemia cell interaction with stromal cells, although it may induce growth arrest and apoptosis in a wide range of neoplastic B-cell lines deriving from B-cell lymphomas.23 In addition, culture BM-MSCs from multiple myeloma patients and normal donors may create a very efficient niche that supports the survival and proliferation of the myeloma cells.24 For this reason, we carried out an ex vivo study on the role of Notch signaling in stroma-dependent survival of human common B-ALL cells collected from patients. In particular, we assessed the rate of apoptotic B-ALL cells in presence or absence of BM-MSCs from normal donors and B-ALL patients, and the contribution to B-ALL cell survival of the different Notch molecules expressed by either B-ALL blasts or BM-MSCs.
Methods
Samples and cell cultures
Human MSCs were obtained from normal BM samples collected from healthy donors after informed consent (hBM-MSCs). B-ALL cells were obtained from BM samples of 10 patients with newly diagnosed common B-ALL after informed consent according to the institutional guidelines and showing high blast count (median: 89.5%; range: 69-96); from 5 of these B-ALL patients, BM-MSCs (hBM-MSCs*, ie, hBM-MSC from B-ALL patients) were also obtained to carry out autologous coculture experiments (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). hBM-MSCs and hBM-MSCs* were seeded at the density of 3.1 × 104 cells/cm2 and cultured in DMEM with 20% FBS, 1% l-glutamine, and penicillin-streptomycin (Sigma-Aldrich), at 37°C in 5% CO2 and humidified atmosphere, as previously described.15,25 hBM-MSCs and hBM-MSCs* at passage 3 or 4, displaying a homogeneous mesenchymal immunophenotype (including CD105, CD44, CD73, CD146 marker expression) and multipotent differentiation potential (into osteoblastic, adipocytic, and chondrocytic lineages) were used for the coculture experiments.15,25
To study the capability of hBM-MSCs to support B-ALL cell survival, B-ALL cells were cultured alone and cocultured with hBM-MSCs at a 10:1 ratio for 3, 7, and 28 days. The expression of Notch molecules on both cell types were performed at 3 and 7 days and then most of the others experiments were performed at 3 days of culture.
Experiments with or without either inhibitors or blocking Abs against Notch molecules were carried out in 96-well plates with a confluent monolayer of stromal cells: 105 B-ALL cells were cultured in 200 μL of RPMI 1640 medium supplemented with 10% FBS, 1% l-glutamine, and 1% penicillin-streptomycine, without or with 104 adherent hBM-MSCs or hBM-MSCs*.
To study the specific relative basal sensitivity of B-ALL cells to GSI XII, B-ALL cells were cultured alone for 1 and 3 days and cocultured with hBM-MSCs for 3 days in presence of increasing concentrations of GSI XII. After that, to address whether the effects of GSI XII could be detected at different ratios, we evaluated the effects of increasing concentrations of GSI XII in hBM-MSC–induced survival of B-ALL cells after 3 days of coculture at 10:1 and 1:1 ratios. The effective concentration to kill 50% of B-ALL cells (EC50) derived from the equations that best fit the linear range of the dose-response curve.
Stock solutions of hydrocortisone were diluted to appropriate concentrations with culture media to study the role of Notch in the protection of leukemic cells from chemotherapeutic agents. Cocultured B-ALL cells were separated from hBM-MSCs or hBM-MSCs* monolayer by careful pipetting with ice-cold PBS. hBM-MSCs or hBM-MSCs* were then trypsinized, stained with acridine orange, and counted in a Burker chamber using fluorescence microscopy.
In the supplemental Methods, the following methods are described in detail: flow cytometric analysis of the expression of Notch molecules, Notch inhibitors and neutralizing Notch molecules Ab treatments, recombinant Notch ligands and IL-6/-7 treatment, annexinV/7AAD apoptosis assay, cell-cycle analysis, measurement of caspase activity, MSC morphology assessment after culture with increasing concentrations of GSI XII, immunofluorescence staining, Western blot analysis, and quantitative RT-PCR (qRT-PCR).
Statistical analysis
Statistical analysis was performed by using the Student t test to compare 2 groups and 1-way ANOVA to compare multiple groups with the Holm-Sidak test used for internal comparison between multiple groups. P ≤ .05 were considered statistically significant. Results were expressed as the mean ± SD of 10 independent experiments from different human B-ALL donors. All statistical calculations were performed using STATA Version 10.0 (StataCorp).
Results
Expression of Notch receptors and ligands on B-ALL cells and hBM-MSCs in both culture and coculture conditions
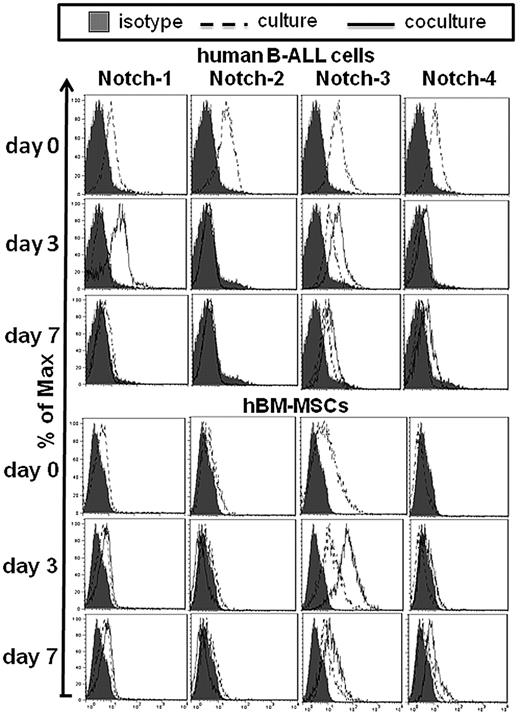
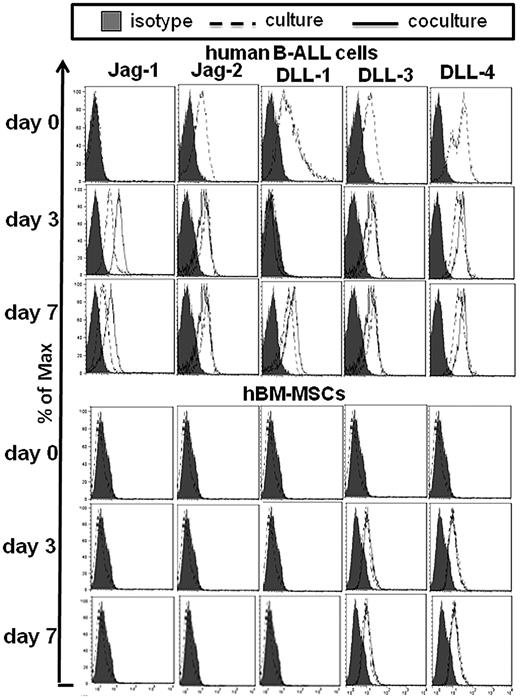
We first asked whether human common B-ALL cells (CD22+, cytoplasm-CD79a +, CD10+, CD19+, cytoplasm-μ−, IgM−) expressed Notch molecules as assessed by flow cytometry. Freshly isolated B-ALL cells (100% CD19+CD45+ cells) expressed high level of all the Notch receptors and ligands, except Jagged-1, which was present at low level. On the other hand, hBM-MSCs (100% CD73+CD45− cells) expressed Notch-1/-2 and -3 (Figure 1), and moderate levels of Notch ligands (Figure 2).
Some representative examples of flow cytometric measurements of Notch receptors expression by both B-ALL cells and hBM-MSCs. Representative cases of the expression of Notch receptors by both B-ALL cells and hBM-MSCs cultured alone and cocultured for 3 and 7 days, after electronic gating either on CD19+ B-ALL cells or CD45− hBM-MSCs in 2-color flow cytometric analysis. Filled histograms indicate the staining with control human IgG.
Some representative examples of flow cytometric measurements of Notch receptors expression by both B-ALL cells and hBM-MSCs. Representative cases of the expression of Notch receptors by both B-ALL cells and hBM-MSCs cultured alone and cocultured for 3 and 7 days, after electronic gating either on CD19+ B-ALL cells or CD45− hBM-MSCs in 2-color flow cytometric analysis. Filled histograms indicate the staining with control human IgG.
Some representative examples of flow cytometric measurements of Notch ligands expression by both B-ALL cells and hBM-MSCs. Representative cases of the expression of Notch ligands by both B-ALL cells and hBM-MSCs cultured alone and cocultured for 3 and 7 days, after electronic gating on either CD19+ B-ALL cells or CD45− hBM-MSCs in 2-color flow cytometric analysis. Filled histograms indicate the staining with control human IgG. DLL indicates Delta-like ligand; and Jag, Jagged ligand.
Some representative examples of flow cytometric measurements of Notch ligands expression by both B-ALL cells and hBM-MSCs. Representative cases of the expression of Notch ligands by both B-ALL cells and hBM-MSCs cultured alone and cocultured for 3 and 7 days, after electronic gating on either CD19+ B-ALL cells or CD45− hBM-MSCs in 2-color flow cytometric analysis. Filled histograms indicate the staining with control human IgG. DLL indicates Delta-like ligand; and Jag, Jagged ligand.
Following B-ALL cells and hBM-MSCs coculture (for 3 or 7 days), we observed Notch-1/-3 and -4 up-regulation by B-ALL cells, and up-regulation of Notch-3 and -4 by hBM-MSCs (Table 1, Figure 1). By contrast, Notch-2, although expressed at basal condition by B-ALL cells and hBM-MSCs, was down-regulated and became undetectable starting from day 3 on both cell types (Table 1, Figure 1). Notch-1 expression by hBM-MSCs in culture was similar to that observed during the coculture (Table 1, Figure 1). We then analyzed the expression of Notch ligands by both cell types after culture and coculture experiments. Jagged-1 was markedly up-regulated on B-ALL cells but not on hBM-MSCs after coculture for 3 days and 7 days (Table 1, Figure 2). We did not observe any Jagged-2 expression by hBM-MSCs during the coculture. The expression of Jagged-2, DLL-3 and -4 by B-ALL cells did not significantly change by the coculture, whereas the expression of DLL-1 was up-regulated on B-ALL cells from day 7 (Table 1, Figure 2). Jagged-1/-2 and DLL-1 expression by hBM-MSCs was below the detection limit either at basal conditions or after coculture. The expression of DLL-3 and -4 did not change in hBM-MSCs in both culture conditions (Table 1, Figure 2). Similarly to hBM-MSCs, hBM-MSCs* expressed Notch-1/-3 and -4; however, after B-ALL cells and hBM-MSCs* coculture (for 3 or 7 days), the expression of Notch-1/-3 and -4 was more dramatically up-regulated by both B-ALL cells and hBM-MSCs*, compared with what observed by using hBM-MSCs (supplemental Table 2A-B).
Expression of Notch receptors and ligands in B-ALL cells and hBM-MSCs in different culture conditions
| . | Jagged-1 . | Jagged-2 . | DLL-1 . | DLL-3 . | DLL-4 . | Notch-1 . | Notch-2 . | Notch-3 . | Notch-4 . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 3 . | Day 7 . | Day 3 . | Day 7 . | Day 3 . | Day 7 . | Day 3 . | Day 7 . | Day 3 . | Day 7 . | Day 3 . | Day 7 . | Day 3 . | Day 7 . | Day 3 . | Day 7 . | Day 3 . | Day 7 . | |
| B-ALL cells | ||||||||||||||||||
| Alone | 32.7 ± 2.2 | 19.0 ± 1.6 | 31.4 ± 9.0 | 26.6 ± 8.1 | 0.2 ± 0.0 | 26.9 ± 2.6 | 34.0 ± 5.7 | 32.1 ± 4.9 | 45.0 ± 2.0 | 47.7 ± 3.1 | 1.5 ± 0.6 | 1.9 ± 0.2 | 0.9 ± 0.1 | 0.2 ± 0.0 | 24.8 ± 0.6 | 17.5 ± 0.2 | 4.9 ± 2.1 | 1.7 ± 0.9 |
| Coculture | 52.4 ± 5.4 | 28.4 ± 3.9 | 36.0 ± 5.4 | 29.8 ± 4.0 | 0.6 ± 0.2 | 39.8 ± 4.1 | 38.4 ± 6.9 | 36.3 ± 3.8 | 49.4 ± 5.8 | 48.4 ± 1.5 | 28.3 ± 2.8 | 1.1 ± 0.4 | 0.4 ± 0.0 | 0.7 ± 0.5 | 35.3 ± 2.2 | 27.2 ± 3.3 | 9.6 ± 1.8 | 6.6 ± 2.5 |
| Student t test | P < .01 | P < .05 | NS | NS | NS | P < .01 | NS | NS | NS | NS | P < .001 | NS | NS | NS | P < .05 | P < .05 | P < .05 | P < .05 |
| hBM-MSCs | ||||||||||||||||||
| Alone | 0.7 ± 0.1 | 0.9 ± 0.0 | 1.2 ± 0.3 | 1.6 ± 1.7 | 0.3 ± 0.0 | 0.4 ± 0.0 | 31.8 ± 0.3 | 32.4 ± 0.5 | 7.1 ± 1.4 | 6.5 ± 2.3 | 7.2 ± 0.2 | 6.5 ± 0.7 | 0.7 ± 0.1 | 0.5 ± 0.1 | 30.6 ± 5.3 | 26.3 ± 1.7 | 1.6 ± 0.2 | 3.1 ± 0.7 |
| Coculture | 1.3 ± 0.4 | 1.6 ± 0.7 | 1.9 ± 0.5 | 1.1 ± 1.4 | 0.5 ± 0.0 | 0.3 ± 0.0 | 34.3 ± 0.1 | 35.6 ± 0.8 | 7.9 ± 1.9 | 8.6 ± 1.7 | 8.9 ± 0.7 | 8.1 ± 0.4 | 0.5 ± 0.0 | 0.4 ± 0.0 | 51.3 ± 2.2 | 37.2 ± 5.7 | 8.0 ± 4.1 | 9.1 ± 3.6 |
| Student t test | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | P < 0.01 | P < 0.05 | P < 0.05 | P < 0.05 |
| . | Jagged-1 . | Jagged-2 . | DLL-1 . | DLL-3 . | DLL-4 . | Notch-1 . | Notch-2 . | Notch-3 . | Notch-4 . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 3 . | Day 7 . | Day 3 . | Day 7 . | Day 3 . | Day 7 . | Day 3 . | Day 7 . | Day 3 . | Day 7 . | Day 3 . | Day 7 . | Day 3 . | Day 7 . | Day 3 . | Day 7 . | Day 3 . | Day 7 . | |
| B-ALL cells | ||||||||||||||||||
| Alone | 32.7 ± 2.2 | 19.0 ± 1.6 | 31.4 ± 9.0 | 26.6 ± 8.1 | 0.2 ± 0.0 | 26.9 ± 2.6 | 34.0 ± 5.7 | 32.1 ± 4.9 | 45.0 ± 2.0 | 47.7 ± 3.1 | 1.5 ± 0.6 | 1.9 ± 0.2 | 0.9 ± 0.1 | 0.2 ± 0.0 | 24.8 ± 0.6 | 17.5 ± 0.2 | 4.9 ± 2.1 | 1.7 ± 0.9 |
| Coculture | 52.4 ± 5.4 | 28.4 ± 3.9 | 36.0 ± 5.4 | 29.8 ± 4.0 | 0.6 ± 0.2 | 39.8 ± 4.1 | 38.4 ± 6.9 | 36.3 ± 3.8 | 49.4 ± 5.8 | 48.4 ± 1.5 | 28.3 ± 2.8 | 1.1 ± 0.4 | 0.4 ± 0.0 | 0.7 ± 0.5 | 35.3 ± 2.2 | 27.2 ± 3.3 | 9.6 ± 1.8 | 6.6 ± 2.5 |
| Student t test | P < .01 | P < .05 | NS | NS | NS | P < .01 | NS | NS | NS | NS | P < .001 | NS | NS | NS | P < .05 | P < .05 | P < .05 | P < .05 |
| hBM-MSCs | ||||||||||||||||||
| Alone | 0.7 ± 0.1 | 0.9 ± 0.0 | 1.2 ± 0.3 | 1.6 ± 1.7 | 0.3 ± 0.0 | 0.4 ± 0.0 | 31.8 ± 0.3 | 32.4 ± 0.5 | 7.1 ± 1.4 | 6.5 ± 2.3 | 7.2 ± 0.2 | 6.5 ± 0.7 | 0.7 ± 0.1 | 0.5 ± 0.1 | 30.6 ± 5.3 | 26.3 ± 1.7 | 1.6 ± 0.2 | 3.1 ± 0.7 |
| Coculture | 1.3 ± 0.4 | 1.6 ± 0.7 | 1.9 ± 0.5 | 1.1 ± 1.4 | 0.5 ± 0.0 | 0.3 ± 0.0 | 34.3 ± 0.1 | 35.6 ± 0.8 | 7.9 ± 1.9 | 8.6 ± 1.7 | 8.9 ± 0.7 | 8.1 ± 0.4 | 0.5 ± 0.0 | 0.4 ± 0.0 | 51.3 ± 2.2 | 37.2 ± 5.7 | 8.0 ± 4.1 | 9.1 ± 3.6 |
| Student t test | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | P < 0.01 | P < 0.05 | P < 0.05 | P < 0.05 |
Mean values ± SD of MFI (n = 10). The results were expressed as mean of geometric MFI values of the hBM-MSC (CD73+CD45−) or B-ALL cell (CD19+CD45+) population expressing Notch ligands and receptors.
B-ALL indicates B-lineage acute lymphoblastic leukemia; hBM-MSC, human bone marrow–mesenchymal stromal cell; DLL, Delta-like ligand; MFI, mean fluorescence intensity; and NS, nonsignificant.
Effects of Notch pathway inhibition on B-ALL cell and hBM-MSC functions
Dose-response curve showing the effects of increasing concentrations of GSI XII on B-ALL cells cultured alone for 1 day is shown in Figure 3. In our primary B-ALL samples, low percentage of spontaneous apoptosis was observed (% CD19+ annexin V+ cells: 11.6 ± 2.3% at 1 day). Thus, we calculated the percentage of GSI XII–induced apoptosis, with the following formula: (test [inhibitor induced apoptosis] − control [spontaneous apoptosis]) × 100/(100 − control).26 We found that there is a threshold concentration of GSI XII over which B-ALL cells undergo apoptosis: GSI XII dramatically decreased the survival of B-ALL cells at concentrations above 12.5μM and had no significant effect at lower concentrations; the effective concentration (EC50) was 13.9μM.
Specific relative cell viability (%) of B-ALL cells cultured alone with increasing concentrations of the GSI XII for 1 day. Percentage of live human B-ALL cells (annexin V−/7-AAD−; bottom left quadrant) was analyzed by using flow cytometric analysis. Percentage of specific apoptosis was calculated according to the following formula: (test [inhibitor-induced apoptosis] − control [spontaneous apoptosis]) × 100/(100 − control). Statistical analysis was carried out by using 1-way ANOVA, Holm-Sidak test. **P < .01. Data were represented as the mean ± SD of 10 independent experiments.
Specific relative cell viability (%) of B-ALL cells cultured alone with increasing concentrations of the GSI XII for 1 day. Percentage of live human B-ALL cells (annexin V−/7-AAD−; bottom left quadrant) was analyzed by using flow cytometric analysis. Percentage of specific apoptosis was calculated according to the following formula: (test [inhibitor-induced apoptosis] − control [spontaneous apoptosis]) × 100/(100 − control). Statistical analysis was carried out by using 1-way ANOVA, Holm-Sidak test. **P < .01. Data were represented as the mean ± SD of 10 independent experiments.
On the other hand, we observed that at up to 20.0μM concentration for 3 days, GSI XII did not induce apoptosis or favor morphologic changes of hBM-MSCs. By contrast, apoptotic cells (nuclear condensation and apoptotic bodies), as well as clear morphologic changes were observed in hBM-MSCs treated with GSI XII at 40.0μM (supplemental Figure 1).
To confirm these results, we studied the viability of hBM-MSCs by using flow cytometry with Abs directed against the active Caspase-3 after 3 days of culture in presence of increasing concentrations of GSI XII. As shown in supplemental Figure 2A and B, active Caspase-3 expression was not induced in hBM-MSCs up to GSI XII concentrations of 15.0μM. Because activation of Caspase-3 is a hallmark of death receptor-mediated apoptosis, our results support the hypothesis that inhibition of signaling pathways with small-molecule inhibitors may promote death receptor-mediated apoptosis, as has been shown with heat shock protein inhibition.27
However, GSI XII concentrations lower than 12.5μM did not affect either B-ALL cell or BM-MSC survival. In addition, we cultured hBM-MSCs with increasing concentrations of GSI XII for 3 days and we evaluated the mesenchymal marker expression by using flow cytometry. We observed that GSI XII did not affect significantly mesenchymal immunophenotype at concentrations up to 40.0μM and that hBM-MSCs, displaying active Caspase-3, normally expressed hBM-MSC markers (supplemental Figure 3).
Effects of GSI XII in hBM-MSC–induced survival of B-ALL cells
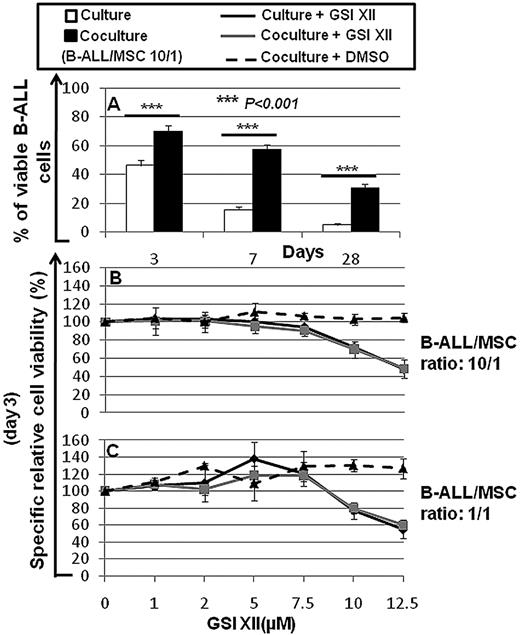
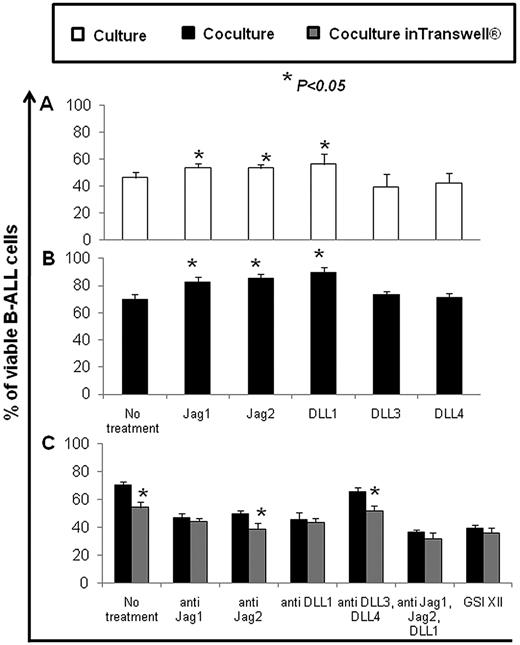
We observed that the coculture of B-ALL cells with hBM-MSCs significantly increased the number of surviving B-ALL cells, compared with that obtained by culturing B-ALL cells alone at the same conditions (Figure 4A).
Percentage of viable B-ALL cells after culture alone or coculture with hBM-MSCs and the specific relative cell viability (%) of B-ALL cells cultured alone or cocultured with hBM-MSCs in presence of increasing concentrations of GSI XII. (A) Percentage of viable B-ALL cells cultured alone or cocultured with hBM-MSCs at 10/1 ratio for 3, 7, and 28 days. (B-C) Specific relative cell viability (%) of B-ALL cells was evaluated by culturing alone or coculturing B-ALL cells with hBM-MSCs at 10:1 and 1:1 ratios in presence of increasing concentrations of GSI XII for 3 days. Statistical analysis was carried out by using the Student t test and 1-way ANOVA, Holm-Sidak test. ***P < .001. Data were represented as the mean ± SD of 10 independent experiments.
Percentage of viable B-ALL cells after culture alone or coculture with hBM-MSCs and the specific relative cell viability (%) of B-ALL cells cultured alone or cocultured with hBM-MSCs in presence of increasing concentrations of GSI XII. (A) Percentage of viable B-ALL cells cultured alone or cocultured with hBM-MSCs at 10/1 ratio for 3, 7, and 28 days. (B-C) Specific relative cell viability (%) of B-ALL cells was evaluated by culturing alone or coculturing B-ALL cells with hBM-MSCs at 10:1 and 1:1 ratios in presence of increasing concentrations of GSI XII for 3 days. Statistical analysis was carried out by using the Student t test and 1-way ANOVA, Holm-Sidak test. ***P < .001. Data were represented as the mean ± SD of 10 independent experiments.
GSI XII at low concentrations had no specific detectable cytotoxic effects on B-ALL cells either cultured alone or cocultured for 3 days with hBM-MSCs at 10:1 and 1:1 ratios (Figure 4B-C). GSI XII was capable of inhibiting specific apoptosis at concentrations below 2.0μM (10:1 ratio) and 8.8μM (1:1 ratio). However, higher concentrations of GSI XII promoted specific apoptosis of B-ALL cells in both culture conditions (Figure 4B-C). Thus, the reciprocal interactions among B-ALL cells via Notch signaling play an important role in cell survival.
Notch-3 and Notch-4 are involved in hBM-MSC–induced survival of B-ALL cells
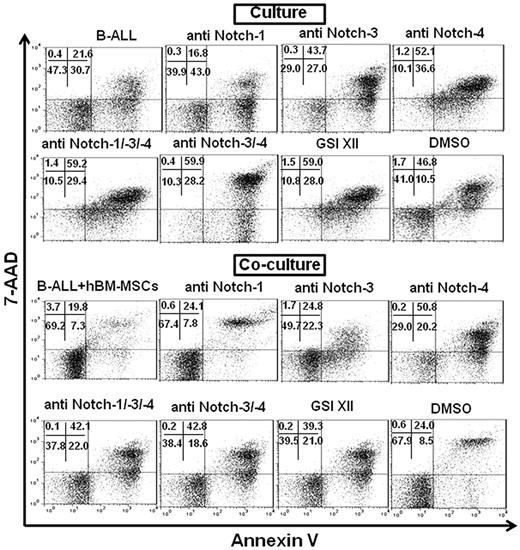
To fully describe and better understand the role of each single Notch molecule or the whole Notch pathway in the supportive/protective ability of MSCs within the B-ALL microenvironment, we first added blocking Abs against Notch molecules to B-ALL cells or MSCs (either hBM-MSCs or hBM-MSCs*) before their mixing in coculture. A dramatic increase of the apoptotic rate of B-ALL cells was achieved by blocking each single expressed Notch receptor (Notch-1, -3 and -4) in culture alone and in coculture with either hBM-MSCs or hBM-MSCs* (Table 2, Figure 5, supplemental Table 3, supplemental Figure 4). Of interest, hBM-MSCs* supported B-ALL cells survival better than hBM-MSCs after 3 days of coculture (supplemental Table 3, supplemental Figure 4). However, Notch-3 and -4 appeared to be mostly involved in both hBM-MSC– and hBM-MSC*–induced B-ALL survival, as well as in the survival of B-ALL cells cultured alone, whereas Notch-1 had little effect in the same conditions (Table 2, Figure 5, supplemental Table 3, supplemental Figure 4). We did not find a significant change in the percentage of overall live B-ALL cells at 3 days after coculture with hBM-MSCs* in presence of blocking receptor Abs, compared with that obtained with hBM-MSCs in the same conditions (supplemental Table 3, supplemental Figure 4).
Effects of Notch receptors and Ligands blockade on B-ALL cell survival
| Treatment . | B-ALL in coculture with hBM-MSCs (% of live cells) . | B-ALL in culture alone (% of live cells) . |
|---|---|---|
| No treatment | 70.2 (4.1) | 46.3 (3.9)* |
| Anti-Notch-1 | 64.6 (3.5)† | 43.0 (5.3) |
| Anti-Notch-3 | 50.9 (6.3)* | 26.0 (6.4)* |
| Anti-Notch-4 | 29.4 (13.0)* | 15.4 (5.3)* |
| Anti-Notch-1/-3/-4 | 35.1 (6.8)* | 11.8 (2.6)* |
| Anti-Notch-3/-4 | 36.0 (6.2)* | 11.3 (3.0)* |
| Anti-Jagged-1 | 46.7 (5.4)* | 26.6 (1.8)* |
| Anti-Jagged-2 | 49.7 (4.7)* | 32.5 (3.4)* |
| Anti-DLL-1 | 45.5 (8.2)* | 22.1 (4.7)* |
| Anti-DLL-3 | 65.4 (4.9) | 42.5 (2.9) |
| Anti-DLL-4 | 63.5 (6.8) | 41.1 (3.2) |
| Anti-Jagged-1/-2, DLL-1 | 36.3 (3.6)* | 12.7 (1.8)* |
| Anti-Jagged-1/-2, DLL-1/-3 | 38.2 (3.0)* | 12.2 (1.3)* |
| Anti-Jagged-1/-2, DLL-1/-4 | 34.4 (5.4)* | 12.8 (1.6)* |
| Anti-Jagged-1/-2, DLL-1/-3/-4 | 36.8 (6.4)* | 11.5 (2.1)* |
| Anti-DLL-3/-4 | 61.7 (5.1) | 43.9 (3.5) |
| GSI XII | 39.3 (4.2)* | 12.5 (5.2)* |
| DMSO | 65.9 (7.9) | 42.2 (2.7) |
| Treatment . | B-ALL in coculture with hBM-MSCs (% of live cells) . | B-ALL in culture alone (% of live cells) . |
|---|---|---|
| No treatment | 70.2 (4.1) | 46.3 (3.9)* |
| Anti-Notch-1 | 64.6 (3.5)† | 43.0 (5.3) |
| Anti-Notch-3 | 50.9 (6.3)* | 26.0 (6.4)* |
| Anti-Notch-4 | 29.4 (13.0)* | 15.4 (5.3)* |
| Anti-Notch-1/-3/-4 | 35.1 (6.8)* | 11.8 (2.6)* |
| Anti-Notch-3/-4 | 36.0 (6.2)* | 11.3 (3.0)* |
| Anti-Jagged-1 | 46.7 (5.4)* | 26.6 (1.8)* |
| Anti-Jagged-2 | 49.7 (4.7)* | 32.5 (3.4)* |
| Anti-DLL-1 | 45.5 (8.2)* | 22.1 (4.7)* |
| Anti-DLL-3 | 65.4 (4.9) | 42.5 (2.9) |
| Anti-DLL-4 | 63.5 (6.8) | 41.1 (3.2) |
| Anti-Jagged-1/-2, DLL-1 | 36.3 (3.6)* | 12.7 (1.8)* |
| Anti-Jagged-1/-2, DLL-1/-3 | 38.2 (3.0)* | 12.2 (1.3)* |
| Anti-Jagged-1/-2, DLL-1/-4 | 34.4 (5.4)* | 12.8 (1.6)* |
| Anti-Jagged-1/-2, DLL-1/-3/-4 | 36.8 (6.4)* | 11.5 (2.1)* |
| Anti-DLL-3/-4 | 61.7 (5.1) | 43.9 (3.5) |
| GSI XII | 39.3 (4.2)* | 12.5 (5.2)* |
| DMSO | 65.9 (7.9) | 42.2 (2.7) |
Values are percentages of overall live human B-ALL cells (mean ± SD of 10 independent experiments); DMSO is the vehicle control for GSI XII, 10μM.
B-ALL indicates B-lineage acute lymphoblastic leukemia; hBM-MSC, human bone marrow–mesenchymal stromal cell; GSI, γ-secretase inhibitor; Anti-, blocking Ab; and DLL, Delta-like ligand.
P < .001 (statistical analysis by 1-way ANOVA, Holm-Sidak test).
P < .05 (statistical analysis by 1-way ANOVA, Holm-Sidak test).
P < .01 (statistical analysis by 1-way ANOVA, Holm-Sidak test).
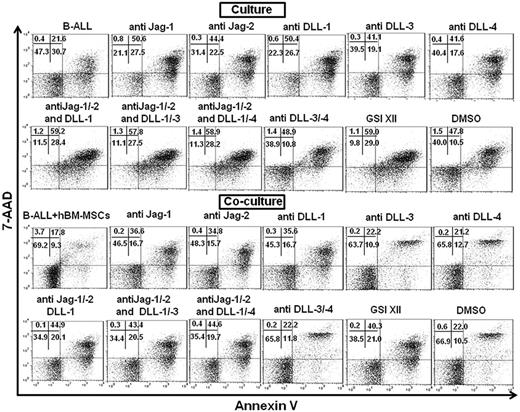
Examples of flow cytometric measurements showing the percentage of viable B-ALL cells cultured alone and cocultured with hBM-MSCs in absence or presence of neutralizing Notch receptor Abs and GSI XII. B-ALL cells were cultured alone or cocultured with hBM-MSCs (10:1 ratio) in absence or presence of anti–Notch-1/-3 and -4 Abs and GSI XII for 3 days. Percentage of viable B-ALL cells was measured by annexin V−/7-AAD− (bottom left quadrant) flow cytometry after electronic gating on CD19+ cells in 2-color flow cytometric analysis.
Examples of flow cytometric measurements showing the percentage of viable B-ALL cells cultured alone and cocultured with hBM-MSCs in absence or presence of neutralizing Notch receptor Abs and GSI XII. B-ALL cells were cultured alone or cocultured with hBM-MSCs (10:1 ratio) in absence or presence of anti–Notch-1/-3 and -4 Abs and GSI XII for 3 days. Percentage of viable B-ALL cells was measured by annexin V−/7-AAD− (bottom left quadrant) flow cytometry after electronic gating on CD19+ cells in 2-color flow cytometric analysis.
Jagged-1, Jagged-2, and DLL-1 are involved in hBM-MSC–induced survival of B-ALL cells
By blocking either Jagged-1, -2, or DLL-1, a significant reduction of the survival of B-ALL cells in both culture conditions was achieved (Table 2, Figure 6). By contrast, blockade of either DLL-3 or -4 had little effect on B-ALL cell survival in both culture conditions (Table 2, Figure 6). The combination of anti–Jagged-1, -2, and DLL-1 was more efficient than the use of each single neutralizing Ab in the promotion of survival of B-ALL cells cultured in both conditions (Table 2, Figure 6).
Examples of flow cytometric measurements showing the percentage of viable B-ALL cells cultured alone and cocultured with hBM-MSCs in absence or presence of neutralizing Notch ligands Abs and GSI XII. B-ALL cells were cultured alone or cocultured with hBM-MSCs (10:1 ratio) in absence or presence of anti–Jagged-1/-2 (Jag-1/-2), DLL-1/-3 and -4 Abs and GSI XII for 3 days. Percentage of viable B-ALL cells was measured by annexin V−/7-AAD− (bottom left quadrant) flow cytometry after electronic gating on CD19+ cells in 2-color flow cytometric analysis.
Examples of flow cytometric measurements showing the percentage of viable B-ALL cells cultured alone and cocultured with hBM-MSCs in absence or presence of neutralizing Notch ligands Abs and GSI XII. B-ALL cells were cultured alone or cocultured with hBM-MSCs (10:1 ratio) in absence or presence of anti–Jagged-1/-2 (Jag-1/-2), DLL-1/-3 and -4 Abs and GSI XII for 3 days. Percentage of viable B-ALL cells was measured by annexin V−/7-AAD− (bottom left quadrant) flow cytometry after electronic gating on CD19+ cells in 2-color flow cytometric analysis.
The addition of anti–DLL-3 and -4 to the mixture of anti–Jagged-1/-2 and anti–DLL-1 did not modify B-ALL cells survival, thus suggesting the major role of the loop between Notch-3 and -4 and their ligands Jagged-1/-2 and DLL-1 in B-ALL cell survival in both culture conditions (Table 2, Figure 6). These results indicate that Jagged-1/-2 and DLL-1 or Notch-3 and -4 pathways have some different downstream targets involved in the prevention of apoptosis in B-ALL cells.
To confirm the role of Notch ligands in B-ALL cells survival, we directly stimulated the Notch receptors by adding exogenously their recombinant Notch ligands. We observed that biologically active Jagged-1/-2 and DLL-1 significantly enhanced B-ALL cells survival in both culture conditions, whereas DLL-3 and -4 did not (Figure 7A-B).
Effects of recombinant Notch ligands Jagged-1/-2, DLL-1/-3 and -4 on the survival of B-ALL cells. (A) B-ALL cells were cultured alone and (B) cocultured with hBM-MSCs for 3 days (10:1 ratio) in absence and presence of recombinant Notch ligands Jagged-1/-2, DLL-1/-3 and -4. DLL indicates Delta-like ligand; and Jag, Jagged. Percentage of viable B-ALL cells cocultured with hBM-MSCs in Transwell conditions. (C) B-ALL cells were cocultured with hBM-MSCs (10:1 ratio) in Transwell conditions in absence or presence of anti-Notch-1/-3 and -4 Abs and GSI XII for 3 days. Percentage of viable B-ALL (CD19+) cells was obtained by using flow cytometry. Statistical analysis was done using 1-way ANOVA, Holm-Sidak test. *P < .05. Data were represented as the mean ± SD of 10 independent experiments. DLL indicates Delta-like ligand; and Jag, Jagged ligand.
Effects of recombinant Notch ligands Jagged-1/-2, DLL-1/-3 and -4 on the survival of B-ALL cells. (A) B-ALL cells were cultured alone and (B) cocultured with hBM-MSCs for 3 days (10:1 ratio) in absence and presence of recombinant Notch ligands Jagged-1/-2, DLL-1/-3 and -4. DLL indicates Delta-like ligand; and Jag, Jagged. Percentage of viable B-ALL cells cocultured with hBM-MSCs in Transwell conditions. (C) B-ALL cells were cocultured with hBM-MSCs (10:1 ratio) in Transwell conditions in absence or presence of anti-Notch-1/-3 and -4 Abs and GSI XII for 3 days. Percentage of viable B-ALL (CD19+) cells was obtained by using flow cytometry. Statistical analysis was done using 1-way ANOVA, Holm-Sidak test. *P < .05. Data were represented as the mean ± SD of 10 independent experiments. DLL indicates Delta-like ligand; and Jag, Jagged ligand.
hBM-MSC–mediated survival of B-ALL cells requires both cell contact and soluble factors
We observed a significant decrease in B-ALL cells survival in coculture with hBM-MSCs in Transwell conditions compared with the results obtained by coculturing B-ALL cells in direct contact with hBM-MSCs (Figure 7C). However, the supernatant obtained from 3-day culture of hBM-MSCs increased the survival of B-ALL cells compared with the results obtained by culturing B-ALL cells alone (46.3% ± 3.9% [culture + RPMI] vs 57.9% ± 1.5% [culture + supernatant], P < .05). Furthermore, we found a significant increase in B-ALL cell survival in coculture with hBM-MSCs* in Transwell conditions and in culture with the supernatant obtained from 3-day culture of hBM-MSCs*, compared with that obtained with hBM-MSCs in the same conditions (supplemental Table 4). Indeed, blocking Abs significantly decreased the survival of B-ALL cells in both Transwell coculture conditions (Figure 7C). Similar results were obtained by coculturing B-ALL cells with hBM-MSCs* (data not shown). Thus, both cell contact and soluble factors are involved in hBM-MSC* and hBM-MSC–mediated rescue of B-ALL cells from apoptosis through Notch signaling.
Notch-3– and Notch-4–dependent antiapoptotic effect of MSCs on B-ALL cells is associated with down-regulation of active Caspase-3 and up-regulation of Bcl-2 and Hes-1 in the persistence of B-ALL
To identify some different downstream targets of Notch signaling pathway involved in the prevention of apoptosis in B-ALL cells in direct contact with either hBM-MSCs or hBM-MSCs*, we assessed by flow cytometry the expression of active Caspase-3, VEGFR2, and IL-7R in B-ALL cells cocultured for 3 days. We observed that active Caspase-3, VEGFR2, and IL-7R are weakly expressed in B-ALL cells at basal conditions, whereas Bcl-2 is highly expressed as previously shown.28 Active Caspase-3 down-regulation and Bcl-2 overexpression in B-ALL cells were further even more evident after coculture of B-ALL cells with hBM-MSCs. By contrast, active Caspase-3 down-regulation and Bcl-2 overexpression were reverted by adding either anti-Notch-3 + anti–Notch-4 Abs or GSI XII (supplemental Figure 5A-D). Treatment with anti–Notch-3 + anti–Notch-4 or GSI XII resulted in > 50% of the B-ALL cells expressing activated caspase-3 (supplemental Figure 5A). The addition of blocking anti–Notch-1 had little effect on the expression of active Caspase-3, IL-7R, VEGFR2, and Bcl-2 by B-ALL cells in coculture (data not shown). IL-7R and VEGFR2 expression did not significantly increase on coculture whereas only IL-7R was up-regulated by adding either anti–Notch-3 and -4 Abs or GSI XII (supplemental Figure 5B-C).
To verify the pathway inhibition triggered by Notch, Western blot analysis was performed to verify that downstream target of the Notch pathway was indeed inhibited. As shown in supplemental Figure 5E, the treatment of B-ALL cells with either anti–Notch-3 + Notch-4 Abs or GSI XII resulted in the loss of Hes-1 and Bcl-2 protein expression. These data confirm the inhibition of the Notch pathway in B-ALL cells after the treatment with inhibitors. Hes-1 and Bcl-2 were 2- and 3.5-fold overexpressed, respectively, in B-ALL cells cocultured with hBM-MSCs*, compared with B-ALL cells cocultured with hBM-MSCs. Moreover, the addition of recombinant IL-6 and IL-7 did not affect the survival of B-ALL cells cocultured with hBM-MSCs or hBM-MSCs* (data not shown).
Notch signaling promotes the chemoresistance of human B-ALL cells in direct contact with stromal cells
The effect of chemotherapeutical agents on B-ALL cell survival was assessed by treating B-ALL cells with increasing doses of hydrocortisone. We evaluated the lowest dose of hydrocortisone that induced specific apoptosis in B-ALL cells without modifying hBM-MSCs in 1-day culture. We found that treatment with increasing concentrations of hydrocortisone resulted in a dose-dependent decrease in B-ALL cells viability. We then cultured B-ALL cells without or with hBM-MSCs in absence or presence of hydrocortisone, by adding either anti–Notch-3, -4, or GSI XII for 3 days period. Hydrocortisone promoted apoptosis of B-ALL cells in culture alone, but a consistent increase in the overall number of live B-ALL cells was observed in coculture (supplemental Table 5, supplemental Figure 6A). Interestingly, blockade of Notch-3 and -4 or all Notch signaling by GSI XII in presence of hydrocortisone dramatically decreased the level of overall live B-ALL cells in coculture (supplemental Table 5, supplemental Figure 6A).
On the other hand, we asked whether there is a direct functional link between the Notch pathway and corticosteroid-induced apoptosis. To address this question, we performed flow cytometric analysis to determine whether the blockade of Notch-3 and Notch-4 or whole Notch signaling affects the expression of active Caspase-3 and Bcl-2 in B-ALL cells in culture and coculture conditions in presence of hydrocortisone for 3 days. We observed that active Caspase-3 is overexpressed in B-ALL cells in culture alone in presence of hydrocortisone, whereas Bcl-2 is weakly expressed (supplemental Figure 6B-C). Active Caspase-3 down-regulation and Bcl-2 over-expression in B-ALL cells were further observed after coculture of B-ALL cells with hBM-MSCs in presence of hydrocortisone.
By contrast, active Caspase-3 down-regulation and Bcl-2 overexpression were reverted by adding either anti-Notch-3 + anti–Notch-4 Abs or GSI XII in presence of hydrocortisone (supplemental Figure 6B-C). Similar results were obtained by coculturing B-ALL cells with hBM-MSCs* in presence of hydrocortisone with or without blockade of Notch pathway (data not shown).
Furthermore, we did not find B-ALL cell proliferation in coculture with either hBM-MSCs* or hBM-MSCs in presence of hydrocortisone with or without blockade of Notch pathway (supplemental Table 6). In the whole samples, we observed a significant decrease of the percentage of B-ALL cells in S-phase (P < .02) and a significant increase of the percentage of B-ALL cells in G0G1-phase peak induced by treatment.
When grown on a layer of either hBM-MSCs* or hBM-MSCs in presence of hydrocortisone with or without blockade of Notch pathway, we observed a higher proportion of cells in G0G1 phase with a very low proportion of B-ALL cells in S and G2/M phases, without significant differences between hBM-MSCs and hBM-MSCs* (supplemental Table 6)
MSC functional characterization after coculture with B-ALL cells and regulatory properties of MSCs after coculture with B-ALL cells are described in supplemental Results.
Discussion
The interactions between BM stromal cells and lymphohematopoietic precursors are essential for the regulation of survival, proliferation, and differentiation of normal and neoplastic hematopoietic precursor cells.1,10,16,23,29-34 After coculture of AML and ALL cells with stromal cell layers, the expression of genes involved in leukemic cell survival/growth, such as Bcl-2, is significantly enhanced, accounting in part for protection of leukemic cells from chemotherapeutic agents.29,30 From a general point of view, the identification of signaling pathways involved in the stromal cell–dependent protection of neoplastic cells from apoptosis is crucial for the development of novel therapeutic targets. Many different signaling molecules are involved in the reciprocal interactions between BM stroma and neoplastic cells. Among them, the stromal Notch-1 pathway activation represents a common feature in T-ALL development, compared with AML and B-ALL.22 Notch signaling may induce cell-cycle arrest in a variety of neoplastic cells.35-38 For instance, culture BM-MSCs from multiple myeloma patients and normal donors may create a very efficient niche that supports the survival and proliferation of the myeloma cells24 ; in this disease, BM stroma–mediated activation of Notch-1 signaling up-regulates p21, resulting in growth inhibition and protection from chemotherapy-induced apoptosis in myeloma cells.33,38 In addition, myeloma cells overexpress Jagged-2 when in direct contact with stromal cells, and escape from apoptosis; Jagged-2 triggering induce the secretion of IL-6, VEGF, and insulin like growth factor-1 (IGF-1) in stromal cells.38
By contrast, in B-ALL the potential role of the Notch signaling pathway in the interaction of leukemic cells with BM stromal cells during the leukemogenic process is mostly unclear. Thus, we assessed in this study the role of Notch signaling in stroma-dependent B-ALL cell growth and survival. To this purpose, we used hBM-MSCs that represent quite a homogeneous stromal cell population with hematopoiesis-supporting capabilities and immune regulatory properties, shared by all their BM stromal cell progeny.14-16,39 In fact, hBM-MSCs are capable of promoting the growth and accumulation of normal lymphocytes and leukemic lymphoblasts.4-9 hBM-MSCs normally express molecules of the Notch family that are strictly associated with some of their functions.15
In our experiments, B-ALL cells cultured in vitro without MSCs underwent progressive and time-dependent apoptosis; by contrast, MSCs dramatically increased the survival of B-ALL cells, as previously shown.4-9 In particular, MSCs obtained from B-ALL patients (hBM-MSCs*) and cocultured in autologous conditions supported B-ALL cells survival even better than MSCs from normal donors (hBM-MSCs). By contrast, the ability to support the survival of normal B cells, as well as the immune-regulatory properties toward T cells, did not differ in MSCs from normal and B-ALL patients. This effect was reverted by inhibiting the entire Notch signaling pathways with the GSI XII, thus suggesting that some molecules of the Notch family were involved in stromal cell–mediated rescue of B-ALL cells from apoptosis. Actually, Notch signaling seems to have a role in cell survival also independently from the interaction with hBM-MSCs or hBM-MSCs*. However, the presence of hBM-MSCs or hBM-MSCs* significantly enhanced this phenomenon. In fact, B-ALL cell and hBM-MSC coculture led to a significant overexpression of Notch ligands on B-ALL cells, as well as a more dramatic overexpression of Notch-1/-3 and -4 on cocultured B-ALL cells and hBM-MSCs*, compared with that obtained by coculturing B-ALL cells and hBM-MSCs. Blocking and stimulating experiments demonstrated that Notch ligands Jagged-1/-2 and DLL-1 synergistically interact with Notch-3 and -4 on both cell types and promote B-ALL cell survival. Consequently, Notch-3 and Notch-4 blocking led to the same effect obtained by using GSI XII and were mostly responsible for the stromal cell–mediated antiapoptotic effect observed in cocultured B-ALL. Other receptors of the Notch family, such as Notch-1, seem to be less effective in preventing B-ALL cell from spontaneous apoptosis, as previously described by other authors.16
Coculture experiments of B-ALL cells with hBM-MSCs or hBM-MSCs* in Transwell conditions show that the enhancement of B-ALL cell survival is not only because of the direct contact of B-ALL cells with hBM-MSCs or hBM-MSCs* but also to soluble molecules that are released by MSCs. On the other hand, we demonstrated that Notch signaling decreased active Caspase-3 expression, inhibited IL-7R expression, and did not affect significantly VEGFR2 expression in B-ALL cells, and that soluble factors such as IL-6, IL-7, and VEGF, whose role has been previously described in other hematologic malignancies and experimental conditions,11,37 did not affect B-ALL cell survival. On the other hand, Notch-3– and -4–dependent prosurvival effect of hBM-MSCs or hBM-MSCs* was associated with the maintenance of Hes-1 and Bcl-2 expression in B-ALL cells, together with the down-regulation of active Caspase-3, as shown by the blocking experiments with anti–Notch-3 and -4 Abs. These findings are in agreement with previous data showing the role of Bcl-2 signaling in the persistence of B-ALL residual clones after chemotherapy.11,12 Our data suggest that Notch-3 and -4 signaling are involved in the chemo-resistance of B-ALL cells in direct contact with hBM-MSC' or hBM-MSC after hydrocortisone exposure. Indeed, classic inhibitors of mitochondrial-dependent cell death, such as Bcl-2 and Bcl-xL as well as inhibitors of apoptosis (IAPs), are reported to work at least in part by directly binding and inhibiting some caspases, including caspase-3, -7, and -9.40-48 We found that Notch-3 and -4 signaling up-regulated Bcl-2 and down-regulated active Caspase-3 in B-ALL cells cocultured with hBM-MSCs or hBM-MSCs* in absence or presence of hydrocortisone. Furthermore, the ability of Notch-3 and -4 signaling to confer resistance to hydrocortisone by up-regulating Bcl-2 in all cases of B-ALL cells cocultured with hBM-MSCs or hBM-MSCs* indicates that this effect may occur at least in part through a Bcl-2–dependent mechanism.
Considered together, hBM-MSCs and hBM-MSCs* contribute to the survival of B-ALL cells by activating Notch signaling, which plays a role also in reciprocal interactions among B-ALL cells. Notch-3 and -4 are mainly responsible for these phenomena and could become specific targets for the treatment of human B-ALL aimed to lower leukemic cell survival after proapoptotic stimuli, such as chemotherapy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by Italian Ministry of University and Scientific Research (PRIN 2005, PRIN 2007), and Fondazione CARIVERONA, Bando 2003 and 2008.
Authorship
Contribution: A.H.N.K. designed and performed research, analyzed data, and wrote the paper; F.M. performed research and analyzed data; F.B., V.L., G.B., G.M., M.R., and O.P. performed research; M.T.S. and G.P. analyzed data and contributed to the writing of the paper; and M.K. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mauro Krampera, MD, PhD, Stem Cell Research Laboratory, Section of Hematology, Department of Medicine, University of Verona, Policlinico G.B. Rossi, P.le L. Scuro, 10, 37134 Verona, Italy; e-mail: mauro.krampera@univr.it.

![Figure 3. Specific relative cell viability (%) of B-ALL cells cultured alone with increasing concentrations of the GSI XII for 1 day. Percentage of live human B-ALL cells (annexin V−/7-AAD−; bottom left quadrant) was analyzed by using flow cytometric analysis. Percentage of specific apoptosis was calculated according to the following formula: (test [inhibitor-induced apoptosis] − control [spontaneous apoptosis]) × 100/(100 − control). Statistical analysis was carried out by using 1-way ANOVA, Holm-Sidak test. **P < .01. Data were represented as the mean ± SD of 10 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/2/10.1182_blood-2010-12-326694/4/m_zh89991174350003.jpeg?Expires=1769234204&Signature=yxQ-6l~LjkEP-cSt36xoGGhY9PYkHWdysmkVyzLPvLFWVkUkASs3Vxo~DD3wE5S0otIpfEZP6Qw52g68xli5uXazRlL9GEz8ZAXKYvPJbOvhuS8D1D~b3aMl8DGeDt-NyJsmTRPUbVbRo6LMQ3AT1Oca58UVmiUVPKulBU1lfufICaGstTywAyx4sOp0hL1OWOqBDW9BlKUsSgPOKnMT5UWAeGzgeIypdFvpq4YBkktN06QJrsun~nuY7p7VU3uvAFfcMimQK-quZykVTMcOnAyYh1tnPu6XwGDonxNhrHP3gPbX9vA~tSeEU2lDriUadh5L8ZucPUIH4II8RdAd1w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal