Abstract
In common variable immunodeficiency (CVID) defects in early stages of B-cell development, bone marrow (BM) plasma cells and T lymphocytes have not been studied systematically. Here we report the first morphologic and flow cytometric study of B- and T-cell populations in CVID BM biopsies and aspirates. Whereas the hematopoietic compartment showed no major lineage abnormalities, analysis of the lymphoid compartment exhibited major pathologic alterations. In 94% of the patients, BM plasma cells were either absent or significantly reduced and correlated with serum immunoglobulin G levels. Biopsies from CVID patients had significantly more diffuse and nodular CD3+ T lymphocyte infiltrates than biopsies from controls. These infiltrates correlated with autoimmune cytopenia but not with other clinical symptoms or with disease duration and peripheral B-cell counts. Nodular T-cell infiltrates correlated significantly with circulating CD4+CD45R0+ memory T cells, elevated soluble IL2-receptor and neopterin serum levels indicating an activated T-cell compartment in most patients. Nine of 25 patients had a partial block in B-cell development at the pre-B-I to pre-B-II stage. Because the developmental block correlates with lower transitional and mature B-cell counts in the periphery, we propose that these patients might form a new subgroup of CVID patients.
Introduction
Common variable immunodeficiency (CVID) is the most frequent primary humoral immunodeficiency in adults associated with a high morbidity and elevated mortality.1 The disease is defined by low serum antibody concentrations, increased susceptibility to respiratory and gastrointestinal tract infections, and poor response to vaccines (www.esid.org). Additional features are autoimmunity, lymphoproliferation, occurrence of noncaseating granulomas, and elevated incidence of malignant tumors.2-4 A large body of evidence favors a disturbed peripheral B-cell development as the major underlying immunopathologic condition in CVID.3,5-8 Despite important progress in immunopathology, molecular genetics,9,10 diagnosis, and treatment,11 very little is known in CVID about changes of T and B lymphocyte populations in the bone marrow (BM).12,13 Here we report a retrospective analysis of BM biopsies from 48 well-classified CVID patients. Although the hematopoietic compartment showed no major abnormalities, we confirmed the absence of plasma cells12 and observed a highly significant increase of diffuse and nodular CD3+ T-cellular infiltrates in the majority of CVID BM biopsies. In a subset of 25 CVID patients, a prospective analysis of B-cell precursors was performed using fresh BM aspirates. The results were compared with histologic findings in the concomitant BM biopsies. Nine of these patients exhibited an unexpected partial arrest of BM dependent early B-cell development leading to low peripheral B-cell counts. Possible immunopathologic implications, notably the role of BM-infiltrating T cells, are discussed.
Methods
Patients and controls
A total of 48 CVID patients (mean age, 40 years; 23 females, 25 males), regularly seen in the CVID outpatient clinic of the Freiburg University Medical Center, were included in our study. All patients were examined for the presence of switched memory B cells following the Freiburg CVID classification protocol14 : 43 patients (90%) were classified as CVID type I. They are characterized by a significant decrease of switched memory B cells in peripheral blood and an impaired vaccination response.15 The subset CVID Ia (22 patients; 46%) exhibited an additional expansion of CD21low B cells not seen in the subset CVID Ib (21 patients; 44%). Patients of CVID type II (5 patients; 10%) have still switched memory B cells in the lower normal range and mount usually some vaccination response to polypeptide and polysaccharide antigens.15 Patients of CVID type Ia exhibit usually the more severe course of disease often associated with splenomegaly, granuloma formation in different organs, nodular lymphoid hyperplasia (NLH) of the gut, lymphoproliferation, or autoimmune cytopenias (autoimmune hemolytic anemia [AIHA]; immune thrombocytopenia [ITP]; 1 case had autoimmune neutropenia).
To exclude malignant lymphoma and other serious causes of cytopenia, our CVID patients underwent a BM biopsy from the spina iliaca posterior superior under local anesthesia. In 25 of the 48 CVID patients, additional BM aspirates were obtained during the biopsy procedure and the precursor B-cell development was analyzed by FACS. In patient 24, only an aspirate could be obtained reducing the evaluable number of BM biopsies to 47 (Table 1). Informed consent was obtained on the basis of our protocol 23999 and 782001, approved by the ethics committee of the Freiburg Medical School.
Clinical and laboratory findings in patients
| Patient no. . | Freiburg classification . | Genetic deficiency . | Sex . | Age at BM analysis, y . | Onset symptoms, age . | Duration of disease, y . | Splenomegaly . | Lymphadenopathy . | NLH of gut . | Granuloma . | Autoimmune cytopenia . | Other autoimmunity . | BM plasma cells: category . | CD19, cell/μL . | CD4+45RO+, % of CD4+ . | CD28−CD27− of CD8+CD3+, % . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1* | Ia | BAFF-R (SNP P21R het, G64V het) | M | 38 | 37 | 1 | Yes | Yes | No | No | No | No | 1 | 50 | 57.7 | 82.7 |
| 2* | Ia | M | 45 | ID = 24 | 21 | Yes | Yes | No | No | Yes | No | 1 | 15 | 90.4 | 47 | |
| 3* | Ia | F | 29 | 28 | 1 | Yes | No | No | No | No | No | 2 | 94 | 54.8 | 5 | |
| 4 | Ia | M | 44 | 38 | 6 | No | No | NA | No | Yes | Yes | 1 | 315 | 74.4 | 30.3 | |
| 5 | Ia | F | 35 | ID = 35 | 0 | Yes | Yes | No | No | No | No | 1 | 150 | 74.6 | 42.2 | |
| 6 | Ia | M | 57 | 15 | 42 | Yes | Yes | No | Yes | No | No | 1 | 300 | 68.5 | 12.8 | |
| 7* | Ia | M | 34 | 29 | 5 | Yes | No | No | Yes | Yes | Yes | 1 | 140 | 81.5 | 27.8 | |
| 8 | Ia | F | 66 | 62 | 4 | Yes | No | No | Yes | No | No | 3 | 270 | 89.8 | 22 | |
| 9 | Ia | F | 17 | 8 | 9 | Yes | No | No | No | Yes | Yes | 1 | 13 | 71.0 | 40.3 | |
| 10* | Ia | M | 22 | 9 | 13 | Yes | Yes | Yes | No | Yes | Yes | 3 | 100 | NA | 6.7 | |
| 11* | Ia | M | 39 | ID = 39 | 0 | Yes | No | No | No | No | No | 2 | 18 | 29.7 | 4 | |
| 12 | Ia | F | 51 | ID = 38 | 13 | Yes | No | No | No | No | No | 1 | 152 | 92.7 | 63.3 | |
| 13* | Ib | ICOS | M | 26 | 18 | 8 | Yes | No | No | No | No | No | 2 | 5 | 60.0 | 38.2 |
| 14 | Ib | M | 21 | 14 | 7 | Yes | Yes | No | No | No | Yes | 2 | 100 | 51.3 | 9.6 | |
| 15* | Ib | M | 52 | 7 | 45 | Yes | Yes | No | Yes | Yes | No | 1 | 24 | 70.2 | 57 | |
| 16 | Ib | F | 62 | ID = 60 | 2 | No | No | Yes | No | No | No | 2 | 250 | 78.5 | 42.1 | |
| 17 | Ib | M | 36 | ID = 28 | 8 | Yes | Yes | No | Yes | Yes | Yes | 1 | 190 | 56.1 | ND | |
| 18 | Ib | TACI (C104R het) | F | 25 | ID = 25 | 0 | Yes | Yes | No | No | No | No | 2 | 40 | 50.2 | ND |
| 19 | Ib | BAFF-R (SNP P21R) | F | 37 | 5 | 32 | No | No | Yes | No | No | No | 2 | 190 | 53.7 | 32.6 |
| 20 | Ib | F | 42 | 32 | 10 | Yes | Yes | Yes | No | Yes | No | 2 | 198‡ | 77.1 | 44.6 | |
| 21* | Ib | F | 37 | 5 | 32 | Yes | No | Yes | NA | No | No | 1 | 120 | 67.3 | 60 | |
| 22 | II | TACI (comp het: Ins104A/C104R) | F | 51 | 41 | 10 | No | No | NA | No | No | No | 2 | 110 | 82.9 | 28.3 |
| 23 | Ib | F | 35 | 6 | 29 | Yes | No | No | No | No | No | 2 | 330 | 54.0 | 4.5 | |
| 24 | Ib | M | 35 | 3 | 32 | Yes | No | No | NA | No | No | NA | 260 | NA | 49.3 | |
| 25 | Ib | F | 29 | ID = 16 | 13 | Yes† | Yes | NA | NA | Yes | No | 2 | 120 | 74.6 | 2 | |
| 26 | Ia | ICOS | M | 21 | 20 | 1 | Yes | No | Yes | No | No | No | 1 | 11† | NA | 5.1 |
| 27 | Ia | F | 41 | 39 | 2 | Yes | Yes | No | Yes | No | Yes | 1 | 100† | NA | 55.5 | |
| 28 | Ia | F | 26 | 6 | 20 | No | No | No | No | No | Yes | 2 | 55 | NA | 0.9 | |
| 29 | Ia | M | 30 | 21 | 9 | Yes† | Yes | No | No | Yes | Yes | 2 | 102† | NA | ND | |
| 30 | Ia | F | 21 | 20 | 1 | Yes | No | No | Yes | No | No | 2 | 281† | NA | 20.1 | |
| 31 | Ia | BAFF-R (SNP P21R het) | M | 37 | 28 | 9 | Yes | Yes | No | No | No | Yes | 2 | 107† | NA | 1.8 |
| 32 | Ia | M | 39 | 30 | 9 | Yes | No | Yes | No | No | No | 2 | 106 | NA | 40.8 | |
| 33 | Ia | F | 52 | 11 | 41 | Yes† | No | No | No | Yes | No | 1 | 1 | NA | ND | |
| 34 | Ia | BAFF-R (SNP P21R het) | M | 36 | 32 | 4 | Yes | Yes | NA | Yes | Yes | No | 2 | 104 | NA | 32.9 |
| 35 | Ia | M | 75 | ID = 61 | 14 | Yes | No | No | Yes | No | No | 2 | 16 | NA | 63.6 | |
| 36 | Ib | F | 36 | 29 | 7 | No | No | Yes | No | No | Yes | 2 | 91 | NA | 6.1 | |
| 37 | Ib | F | 30 | 10 | 20 | No | No | Yes | No | No | No | 2 | 170† | NA | 34.5 | |
| 38 | Ib | M | 27 | 25 | 2 | No | No | Yes | No | No | No | 2 | 589 | NA | ND | |
| 39 | Ib | BAFF-R (SNP P21R) | M | 74 | 53 | 21 | Yes† | No | No | Yes | No | Yes | 2 | 598 | NA | ND |
| 40 | Ib | F | 67 | 36 | 31 | Yes | No | No | No | No | No | 1 | 33 | NA | ND | |
| 41 | Ib | BAFF-R (SNP P21R) | M | 32 | 6 | 26 | No | No | No | No | No | No | 2 | 251 | NA | ND |
| 42 | Ib | F | 39 | 35 | 4 | No‡ | No | Yes | No | No | No | 2 | 138 | 57.2 | 10.7 | |
| 43 | Ib | ICOS | M | 39 | 15 | 24 | Yes | No | NA | No | Yes | No | 2 | 29 | 48.2 | 75 |
| 44 | Ib | ICOS | M | 46 | 20 | 26 | Yes | No | No | No | No | No | 2 | 133 | 38.1 | 37.5 |
| 45 | II | M | 44 | 44 | 0 | Yes | No | Yes | No | No | No | 2 | 121 | NA | ND | |
| 46 | II | F | 74 | 44 | 30 | No | No | Yes | No | No | Yes | 2 | 447 | NA | ND | |
| 47 | II | F | 49 | 29 | 20 | No | Yes | No | Yes | No | No | 1 | 147 | NA | ND | |
| 48 | II | TACI (C104R het) | M | 36 | 10 | 26 | Yes | No | Yes | Yes | No | Yes | 3 | 379† | NA | ND |
| Patient no. . | Freiburg classification . | Genetic deficiency . | Sex . | Age at BM analysis, y . | Onset symptoms, age . | Duration of disease, y . | Splenomegaly . | Lymphadenopathy . | NLH of gut . | Granuloma . | Autoimmune cytopenia . | Other autoimmunity . | BM plasma cells: category . | CD19, cell/μL . | CD4+45RO+, % of CD4+ . | CD28−CD27− of CD8+CD3+, % . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1* | Ia | BAFF-R (SNP P21R het, G64V het) | M | 38 | 37 | 1 | Yes | Yes | No | No | No | No | 1 | 50 | 57.7 | 82.7 |
| 2* | Ia | M | 45 | ID = 24 | 21 | Yes | Yes | No | No | Yes | No | 1 | 15 | 90.4 | 47 | |
| 3* | Ia | F | 29 | 28 | 1 | Yes | No | No | No | No | No | 2 | 94 | 54.8 | 5 | |
| 4 | Ia | M | 44 | 38 | 6 | No | No | NA | No | Yes | Yes | 1 | 315 | 74.4 | 30.3 | |
| 5 | Ia | F | 35 | ID = 35 | 0 | Yes | Yes | No | No | No | No | 1 | 150 | 74.6 | 42.2 | |
| 6 | Ia | M | 57 | 15 | 42 | Yes | Yes | No | Yes | No | No | 1 | 300 | 68.5 | 12.8 | |
| 7* | Ia | M | 34 | 29 | 5 | Yes | No | No | Yes | Yes | Yes | 1 | 140 | 81.5 | 27.8 | |
| 8 | Ia | F | 66 | 62 | 4 | Yes | No | No | Yes | No | No | 3 | 270 | 89.8 | 22 | |
| 9 | Ia | F | 17 | 8 | 9 | Yes | No | No | No | Yes | Yes | 1 | 13 | 71.0 | 40.3 | |
| 10* | Ia | M | 22 | 9 | 13 | Yes | Yes | Yes | No | Yes | Yes | 3 | 100 | NA | 6.7 | |
| 11* | Ia | M | 39 | ID = 39 | 0 | Yes | No | No | No | No | No | 2 | 18 | 29.7 | 4 | |
| 12 | Ia | F | 51 | ID = 38 | 13 | Yes | No | No | No | No | No | 1 | 152 | 92.7 | 63.3 | |
| 13* | Ib | ICOS | M | 26 | 18 | 8 | Yes | No | No | No | No | No | 2 | 5 | 60.0 | 38.2 |
| 14 | Ib | M | 21 | 14 | 7 | Yes | Yes | No | No | No | Yes | 2 | 100 | 51.3 | 9.6 | |
| 15* | Ib | M | 52 | 7 | 45 | Yes | Yes | No | Yes | Yes | No | 1 | 24 | 70.2 | 57 | |
| 16 | Ib | F | 62 | ID = 60 | 2 | No | No | Yes | No | No | No | 2 | 250 | 78.5 | 42.1 | |
| 17 | Ib | M | 36 | ID = 28 | 8 | Yes | Yes | No | Yes | Yes | Yes | 1 | 190 | 56.1 | ND | |
| 18 | Ib | TACI (C104R het) | F | 25 | ID = 25 | 0 | Yes | Yes | No | No | No | No | 2 | 40 | 50.2 | ND |
| 19 | Ib | BAFF-R (SNP P21R) | F | 37 | 5 | 32 | No | No | Yes | No | No | No | 2 | 190 | 53.7 | 32.6 |
| 20 | Ib | F | 42 | 32 | 10 | Yes | Yes | Yes | No | Yes | No | 2 | 198‡ | 77.1 | 44.6 | |
| 21* | Ib | F | 37 | 5 | 32 | Yes | No | Yes | NA | No | No | 1 | 120 | 67.3 | 60 | |
| 22 | II | TACI (comp het: Ins104A/C104R) | F | 51 | 41 | 10 | No | No | NA | No | No | No | 2 | 110 | 82.9 | 28.3 |
| 23 | Ib | F | 35 | 6 | 29 | Yes | No | No | No | No | No | 2 | 330 | 54.0 | 4.5 | |
| 24 | Ib | M | 35 | 3 | 32 | Yes | No | No | NA | No | No | NA | 260 | NA | 49.3 | |
| 25 | Ib | F | 29 | ID = 16 | 13 | Yes† | Yes | NA | NA | Yes | No | 2 | 120 | 74.6 | 2 | |
| 26 | Ia | ICOS | M | 21 | 20 | 1 | Yes | No | Yes | No | No | No | 1 | 11† | NA | 5.1 |
| 27 | Ia | F | 41 | 39 | 2 | Yes | Yes | No | Yes | No | Yes | 1 | 100† | NA | 55.5 | |
| 28 | Ia | F | 26 | 6 | 20 | No | No | No | No | No | Yes | 2 | 55 | NA | 0.9 | |
| 29 | Ia | M | 30 | 21 | 9 | Yes† | Yes | No | No | Yes | Yes | 2 | 102† | NA | ND | |
| 30 | Ia | F | 21 | 20 | 1 | Yes | No | No | Yes | No | No | 2 | 281† | NA | 20.1 | |
| 31 | Ia | BAFF-R (SNP P21R het) | M | 37 | 28 | 9 | Yes | Yes | No | No | No | Yes | 2 | 107† | NA | 1.8 |
| 32 | Ia | M | 39 | 30 | 9 | Yes | No | Yes | No | No | No | 2 | 106 | NA | 40.8 | |
| 33 | Ia | F | 52 | 11 | 41 | Yes† | No | No | No | Yes | No | 1 | 1 | NA | ND | |
| 34 | Ia | BAFF-R (SNP P21R het) | M | 36 | 32 | 4 | Yes | Yes | NA | Yes | Yes | No | 2 | 104 | NA | 32.9 |
| 35 | Ia | M | 75 | ID = 61 | 14 | Yes | No | No | Yes | No | No | 2 | 16 | NA | 63.6 | |
| 36 | Ib | F | 36 | 29 | 7 | No | No | Yes | No | No | Yes | 2 | 91 | NA | 6.1 | |
| 37 | Ib | F | 30 | 10 | 20 | No | No | Yes | No | No | No | 2 | 170† | NA | 34.5 | |
| 38 | Ib | M | 27 | 25 | 2 | No | No | Yes | No | No | No | 2 | 589 | NA | ND | |
| 39 | Ib | BAFF-R (SNP P21R) | M | 74 | 53 | 21 | Yes† | No | No | Yes | No | Yes | 2 | 598 | NA | ND |
| 40 | Ib | F | 67 | 36 | 31 | Yes | No | No | No | No | No | 1 | 33 | NA | ND | |
| 41 | Ib | BAFF-R (SNP P21R) | M | 32 | 6 | 26 | No | No | No | No | No | No | 2 | 251 | NA | ND |
| 42 | Ib | F | 39 | 35 | 4 | No‡ | No | Yes | No | No | No | 2 | 138 | 57.2 | 10.7 | |
| 43 | Ib | ICOS | M | 39 | 15 | 24 | Yes | No | NA | No | Yes | No | 2 | 29 | 48.2 | 75 |
| 44 | Ib | ICOS | M | 46 | 20 | 26 | Yes | No | No | No | No | No | 2 | 133 | 38.1 | 37.5 |
| 45 | II | M | 44 | 44 | 0 | Yes | No | Yes | No | No | No | 2 | 121 | NA | ND | |
| 46 | II | F | 74 | 44 | 30 | No | No | Yes | No | No | Yes | 2 | 447 | NA | ND | |
| 47 | II | F | 49 | 29 | 20 | No | Yes | No | Yes | No | No | 1 | 147 | NA | ND | |
| 48 | II | TACI (C104R het) | M | 36 | 10 | 26 | Yes | No | Yes | Yes | No | Yes | 3 | 379† | NA | ND |
Sex and age distribution of patients: 23 females, 25 males (mean age, 40.3 years). Patients 1 to 25 were subjected to BM biopsy plus pre-B-cell analysis in aspirate samples; patients 26 to 48 were only biopsied. CVID type Ia, n = 22; type Ib, n = 21; and type II, n = 5. Genetic deficiencies: The indicated patients have proven ICOS deficiency or polymorphisms in the TACI and BAFF-R genes. Age at onset of symptoms: Date of ID was used in cases where onset of symptoms was unknown. Clinical findings: Yes indicates present; and No, not present. Splenomegaly was measured by abdominal ultrasound in 47 of 48 patients. BM plasma cell content was semiquantitatively graded into 4 categories: 1 indicates absent; 2, reduced (< 3%); 3, normal (3%- < 5%); and 4, increased (≥ 5%). CD19+ B cells in blood were determined at or within 2 months after BM biopsy.
ID indicates initial diagnosis; NA, data not available; TACI, transmembrane activator and modulator and cyclophilin ligand interactor; BAFF-R, B-cell activating factor receptor; ND, not done; Yes†, documented before splenectomy; and No‡, spleen size was not verified by ultrasound but clinically not palpable.
Numbers of patients with partial pre-B-I to pre-B-II block in BM-dependent B-cell differentiation.
B-cell numbers were determined 1 to 12 years after BM biopsy.
B-cell number was determined 3 years before BM biopsy.
From our 48 CVID patients, the following clinical symptoms were recorded: splenomegaly, lymphadenopathy, NLH of the gut, sarcoid-like granulomas, autoimmune cytopenia, and other autoimmune phenomena. The findings are given in Table 1 together with onset and duration of disease, BM plasma cells, and peripheral T- and B-cell subsets. The following additional findings are recorded in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article): serum concentrations of soluble IL-2 receptor (sIL-2R), neopterin, and IL-10, as well as IgG, IgA, and IgM serum levels before substitution and results from chest x-ray or computed tomography to exclude a mediastinal mass/thymoma. Fifteen BM biopsies from normal persons (mean age, 51 years; 5 females, 10 males) and 13 BM aspirates (mean age, 51.7 years; 6 females, 7 males) served as controls. In these 28 persons, BM biopsies or aspirates were performed for exclusion of hematologic diseases. None of the CVID patients and the controls had a history of long-term immunosuppressive treatment.
Staining and evaluation of BM biopsies
Conventional histology.
Three BM biopsies were fixed in buffered formalin and 44 in a mixed solution of 0.1M calcium acetate, 1.1 volume % formaldehyde, and 0.5 volume % glutaraldehyde, as described by Mufti et al.16 For conventional light microscopy, 2-μm sections were analyzed using naphthyl AS-D chloroacetate esterase, Giemsa, Prussian blue, and reticulin fiber stains.
Immunohistochemistry.
The 2- to 3-μm sections of the paraffin-embedded biopsies were exposed to the Dako Denmark AutostainerPlusLink and stained for CD3 (clone F 7.238), CD20 (clone L26), polyclonal goat antilight chain antibodies (all reagents from Dako Denmark), and a monoclonal anti-CD38 (clone SPC32, Novocastra). Deparaffinized tissue sections were pretreated with proteinase K (Dako Denmark) for 5 minutes (anti-CD3, anti-kappa) or 10 minutes (anti-lambda) and were incubated in a diluted solution (1:10) of target retrieval solution pH 6.1 (Dako Denmark) for 20 minutes at 95°C. Subsequently, the sections were incubated with the primary antibodies. The results were visualized using the Dako Denmark REAL Detection System, according to the manufacturer's instructions.
Evaluation of plasma cells in BM biopsies.
The plasma cell content of BM biopsies was quantified on the basis of standard histochemical coloration and verified by additional immunohistochemical staining for anti-CD38, anti-kappa, and anti-lambda light chains. A semiquantitative grading scheme was adopted expressing plasma cells as percentage of total nucleated cells: grade 1, absent, 0%; grade 2, reduced, < 3%; grade 3, normal, 3% to 5%; grade 4, increased, ≥ 5%.
Evaluation of T cells in BM biopsies.
CD3+ T cells were classified as diffuse or nodular BM infiltrates according to their appearance in BM punch biopsies. The diffuse T-cell infiltrations were quantified by visual analysis using a Photoshop-based image analysis program.17 Five representative digital photographs were obtained from each biopsy (Sony, 3CCD Color Video Camera attached to a diagnostic microscope; Carl Zeiss, Axioskop 50). The images were automatically analyzed by Adobe Photoshop CS Version 8.0.1, which allows recognition, selection, and distinction of cells on the basis of size, shape, and color. The CD3+ T cells were quantified by numbers of pixels and expressed as percentage of the total nucleated cell counts in 5 imaged fields of each biopsy. A further differentiation of T cells into CD4+ and CD8+ subsets was attempted but proved only to be possible in the 3 formalin-fixed biopsies. In addition, a T-cell subset analysis in BM aspirates provided no alternative because of an unpredictable proportion of admixed peripheral blood T lymphocytes.
Nodular T-cell infiltrates were defined as lymphocyte clusters of more than 50 cells. For this analysis, the entire biopsy specimen was screened at low magnification (×50). A subgrading in T-cell aggregates of 50 to 100 cells (grade 1), 100 to 500 cells (grade 2), and > 500 cells (grade 3) was performed. For statistical analysis, the 3 cluster grades were combined and expressed as total number of nodular CD3+ T-cell infiltrates per whole punch biopsy and per millimeter length of the biopsy.
Phenotyping of peripheral blood lymphocytes and of precursor B cells in BM aspirates
Peripheral blood.
Analysis of peripheral blood lymphocyte subpopulations was performed by standard 4-color flow cytometry (FACSCalibur; BD Biosciences) using a lyse-no-wash protocol (Optilyse B; Beckman Coulter). Circulating B-cell subpopulations were analyzed in mononuclear cell fractions obtained from Ficoll density gradient centrifugation as described previously.3,18 Transitional B cells were defined as CD19+IgMhighCD38highCD24high. CD4+CD45R0+ memory T helper cells were expressed as percentage of CD4+ T cells (normal range, 30%-57%). CD8+CD28−CD27− effector/memory T killer cells were expressed as percentage of CD3+CD8+ T cells (normal range, 2%-41%).
BM.
EDTA-anticoagulated BM aspirates were subjected to Ficoll density gradient centrifugation to enrich the mononuclear cell fraction. The protocol for analyzing B-cell precursors is essentially based on the work of the van Dongen group19 and has been described in detail.20 Four-color flow cytometric analysis (FACSCalibur; BD Biosciences) was performed using FITC, phycoerythrin (PE), peridinin chlorophyll protein (PerCP), phycoerythrin-cyanin 7, or allophycocyanin-conjugated antibodies: CD3 (clone SK7, PerCP, or allophycocyanin), CD19 (clone SJ25C1, allophycocyanin), CD20 (clone Leu-16, PE), CD33 (clone P67.6; PerCP), CD34 (clone 8G12, FITC), CD45 (clone 2D1, PerCP) by BD Biosciences; CD10 (clone ALB1, FITC), CD16 (clone 3G8, phycoerythrin-cyanin 7), CD19 (clone J4.119, FITC, or phycoerythrin-cyanin 7), CD22 (clone SJ10.1H11, PE), CD24 (clone ALB9, PE), CD36 (clone FA6.152, FITC), CD79a (HM47, PE), CD179a (VpreB, clone 4G7, PE) by Beckman Coulter; TdT (clone HT-6; FITC), anti-IgD and anti-IgM [rabbit F(ab′)2, FITC] by Dako Denmark; anti-IgD and anti-IgM [goat F(ab′)2, PE] by Southern Biotechnology. For intracellular staining, the IntraPrep kit (Beckman Coulter) was used. For all staining procedures, a mixture of anti-CD3, anti-CD16, and anti-CD33 antibodies served as exclusion marker.
Pro-B cells are characterized by the absence of CD19 and a dim surface expression of CD22 or of intracellular CD79a in combination with surface expression of CD34. Pre-B-I cells exhibit a characteristic surface pattern of CD19+ CD10+ CD20− CD34+ and intracellular TdT+ CD179a(VpreB)+ μ−. Pre-B-II cells are CD19+ CD10+ CD20− to dim and express intracellular μ-chains but no surface IgM. Finally, the immature B-cell subset is characterized by surface expression of CD20 and IgM but missing or faint IgD expression; the immature markers CD10, CD24, and CD38 are still highly expressed. B-cell precursor subpopulations are given as percentage of all precursor B cells, which are defined as CD19+CD10+ cells plus CD19− CD22dim pro-B cells.
BTK cDNA sequencing and protein detection (FACS, Western blot)
Total RNA was extracted from Epstein-Barr virus cell lines of patients and a control with the Roche RNA High Pure RNA Isolation Kit (Roche Diagnostics) according to the manufacturer's instructions. After RNA quantification, equal amounts of RNA per patient or control were reverse-transcribed using the RevertAid First Strand cDNA Synthesis Kit (Fermentas) according to the manufacturer's instructions. The complete human BTK coding sequence was then amplified with QIAGEN Taq Polymerase from equal amounts of cDNA in 3 separate polymerase chain reaction products using the following primer pairs: btk_1_forward: 5′-CTG CGA TCG AGT CCC ACC TTC C-3′, btk_1_reverse: 5′-AAC CTC CTT CTT TCC CCT CTT GCT TTA G-3′; btk_2_forward: 5′-AGC CAG CAG CAG CAC CAG TCT CC-3′, btk_2_reverse: 5′-GGC GCA TCT CCC TCA GGT AGT TCA -3′; btk_3_forward: 5′-ATC CAG GCT CAA ATA TCC AGT GTC-3′, btk_3_reverse: 5′-GAG AGG GGC CTT TTT GTA TTG AGT-3′. After visualization on agarose gel, PCR products were purified using Exonuclease I and Shrimp Alkaline Phosphatase (both from Fermentas) and then sequenced using the same primers listed and the ABI PRISM BigDye Terminator cycle sequencing ready reaction kit Version 1.1 (Applied Biosystems) on an ABI Prism 3130 DNA Sequencer (Applied Biosystems). The sequence reads were analyzed with Sequencing Analysis software (Version 5.4; Applied Biosystems), and Sequencher software (Version 4.10.1; Genecodes).
For Western blot analysis of BTK, 5 × 106 cells were lysed in lysis buffer (50mM Hepes, 250mM NaCl, 5mM EDTA, 1% NP40) containing protease inhibitor (Roche Diagnostics). Protein amount was quantified by bicinchoninic acid assay (Pierce Chemical) following the manufacturer's instructions. Protein lysates were incubated for 5 minutes at 95°C with Laemmli buffer. A total of 50 μg of protein lysate for each Epstein-Barr virus line was loaded on a 12% SDS–polyacrylamide gel and then transferred to polyvinylidene difluoride membranes (Millipore). Membranes were incubated with rabbit anti–human BTK antibody (Cell Signaling), developed using peroxidase-conjugated goat anti–rabbit IgG (Jackson ImmunoResearch Laboratories) and ECL substrate (Pierce Chemical).
Statistical analysis
Statistical differences between control and patient groups were analyzed by the nonparametric Wilcoxon-Mann-Whitney test or the Kruskal-Wallis test at a 2-sided significance level of 5%. In analogy, correlations between variables were analyzed by the Spearman correlation test. The statistical software package StatXact (Version 8; Cytel Statistical Software) or SigmaStat (Version 3.1; Systat Software) was used.
Results
Decrease of plasma cells in BM biopsies of CVID patients
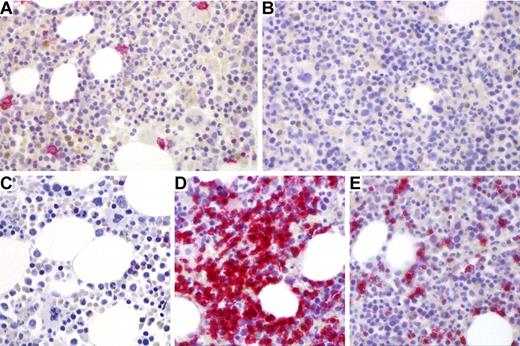
The lack of serum antibodies is a hallmark of CVID. Because long-lived plasma cells reside in the BM,21 we first screened BM biopsies by immunohistochemistry for the presence of CD38+ Ig-κ/λ+ plasma cells (Figure 1A-B). As summarized in Table 2, in 44 of 47 biopsies (94%), we found highly reduced or no plasma cells extending a previous observation from 12 BM samples.12 Interestingly, there was a significant positive correlation between serum IgG concentration before IVIg substitution (supplemental Table 1) and the BM plasma cell content (Spearman correlation coefficient, P < .034); the respective correlations for IgA and IgM were not significant (supplemental Table 2).
Plasma cells and T cells in BM biopsies of a control case and a CVID patient (patient 2 in Table 1). (A) Normal CD38+ plasma cells (red color) in a control biopsy. (B) Absence of CD38+ plasma cells in a typical CVID Ia BM biopsy. (C) Sparsely scattered CD3+ T cells in typical control biopsy in contrast to a nodular T-cell cluster (D) and diffuse interstitial T-cell infiltrates (E) in a representative CVID bone marrow biopsies. Immunostains: CD38 (A-B), original magnification ×200; and CD3 (C-E), original magnification ×200. Images were obtained with a Zeiss Axioskop 50 microscope equipped with a digital camera (3CCDColor Video Camera; Sony) using Cell P Imaging Software Version 3.3 (Olympus). The figure was finalized applying Adobe Photoshop CS2 Version 9.0. Original magnification 400× (Plan-Neofluar objective 40×/0.75; Zeiss).
Plasma cells and T cells in BM biopsies of a control case and a CVID patient (patient 2 in Table 1). (A) Normal CD38+ plasma cells (red color) in a control biopsy. (B) Absence of CD38+ plasma cells in a typical CVID Ia BM biopsy. (C) Sparsely scattered CD3+ T cells in typical control biopsy in contrast to a nodular T-cell cluster (D) and diffuse interstitial T-cell infiltrates (E) in a representative CVID bone marrow biopsies. Immunostains: CD38 (A-B), original magnification ×200; and CD3 (C-E), original magnification ×200. Images were obtained with a Zeiss Axioskop 50 microscope equipped with a digital camera (3CCDColor Video Camera; Sony) using Cell P Imaging Software Version 3.3 (Olympus). The figure was finalized applying Adobe Photoshop CS2 Version 9.0. Original magnification 400× (Plan-Neofluar objective 40×/0.75; Zeiss).
Plasma cell content in BM biopsies
| Plasma cells in BM biopsy* (no. of biopsies) . | CVID Ia (n = 22) . | CVID Ib (n = 20) . | CVID II (n = 5) . | Controls (n = 14) . |
|---|---|---|---|---|
| Grade 1: absent | 11 | 4 | 1 | 0 |
| Grade 2: reduced < 3% | 9 | 16 | 3 | 0 |
| Grade 3: normal 3%- < 5% | 2 | 0 | 1 | 10 |
| Grade 4: increased ≥ 5% | 0 | 0 | 0 | 4 |
| Statistical significance (2-sided Wilcoxon-Mann-Whitney test), P | < .001 | < .001 | .006 |
| Plasma cells in BM biopsy* (no. of biopsies) . | CVID Ia (n = 22) . | CVID Ib (n = 20) . | CVID II (n = 5) . | Controls (n = 14) . |
|---|---|---|---|---|
| Grade 1: absent | 11 | 4 | 1 | 0 |
| Grade 2: reduced < 3% | 9 | 16 | 3 | 0 |
| Grade 3: normal 3%- < 5% | 2 | 0 | 1 | 10 |
| Grade 4: increased ≥ 5% | 0 | 0 | 0 | 4 |
| Statistical significance (2-sided Wilcoxon-Mann-Whitney test), P | < .001 | < .001 | .006 |
Increase of diffuse and nodular T-cell infiltrates in BM biopsies of CVID patients
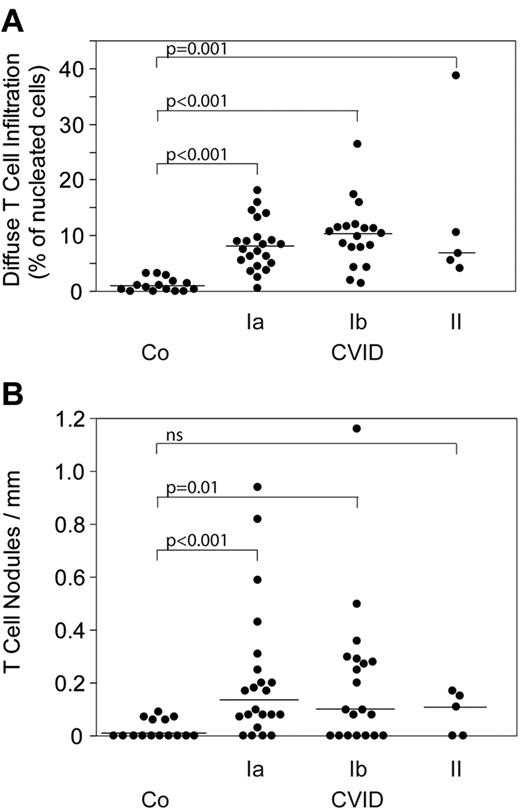
The immunohistologic hallmark of 47 diagnostic BM biopsies from CVID patients is a highly significant increase of diffuse and nodular CD3+ T-cell infiltrates. Figure 1C through E shows representative morphologic examples of this finding. In the 3 formalin-fixed biopsies (patients 21, 23, and 25), we could stain for CD4 and CD8 showing a roughly equal distribution of both T-cell subsets within the diffuse and nodular infiltrates (data not shown). The increase in diffuse and nodular T-cell infiltrates was seen in all CVID patients irrespective of the subtypes Ia, Ib, and II (Figure 2). Moreover, there was no correlation of T-cell infiltrates with the circulating B-cell counts (estimate Tnod: −0.040, P = .788; estimate Tdiffuse: 0.032, P = .83). Nodular T-cell infiltrates of all sizes were observed in BM biopsies of 18 of 22 type Ia, 13 of 20 type Ib, and 3 of 5 type II CVID patients; large grade 3 nodules (> 500 cells) were seen in 4 of 18 Ia, 3 of 13 Ib, and 0 of 3 type II CVID patients, respectively. Similarly, nodular T-cell infiltrates in BM biopsies did not exhibit a significant relationship to CD4/CD8 ratios (estimate Tnod: 0.150, P = .314; data not shown). However, there was a significant correlation between nodular T-cell infiltrates and circulating CD4+CD45R0+ memory T helper cells (estimate Tnod: 0.408, P = .038). No correlation was observed between nodular and diffuse T-cell infiltrates and circulating CD8+CD28−CD27− effector/memory T killer cells (estimate Tnod: 0.055, P = .751; estimate Tdiffuse: −0.027, P = .878; Table 1).
T-cell infiltrates. Diffuse (A) and nodular T-cell infiltrates (B) in 47 BM biopsies from classified CVID patients and 15 controls (“Staining and evaluation of BM biopsies”). ns indicates not significant.
T-cell infiltrates. Diffuse (A) and nodular T-cell infiltrates (B) in 47 BM biopsies from classified CVID patients and 15 controls (“Staining and evaluation of BM biopsies”). ns indicates not significant.
sIL-2R is a serologic marker of an activated adaptive immune system. In this study, it was elevated in 38 of 40 CVID patients (supplemental Table 1) and proved to be positively correlated with most CVID specific clinical symptoms (C.S., manuscript in preparation). Of particular interest was the finding that sIL-2R serum concentrations were significantly correlated with nodular T-cell infiltrates (estimate Tnod: 0.462,P = .003) and with autoimmune cytopenia (P = .008). Neopterin, a phagocyte activation marker, was found to be elevated in 28 of 36 patients (supplemental Table 1) and showed a significant correlation to sIL-2R serum levels (P = .039) and to nodular T-cell infiltrates in the BM (P = .009). Unlike sIL-2R and neopterin, the anti-inflammatory cytokine IL-10 measured in the same serum samples was undetectable except in 3 patients (patients 10, 20, and 36), 2 of whom presented with signs of strong lymphoproliferation and belong to a recently postulated CVID/autoimmune lymphoproliferative syndrome overlap subset22 (supplemental Table 1).
It should be noted that none of the 47 biopsies showed major abnormalities in the composition of the hematopoietic lineages; notably, there were no signs of myelodysplasia, myeloproliferative disorders, or granulomatous infiltrates. The frequently observed peripheral autoimmune cytopenias were regularly associated with a mild reactive expansion of the respective hematologic precursors in the BM.
Relationship of plasma cell and T lymphocyte content in BM biopsies with clinical symptoms of CVID
Next, we wanted to know whether T-cellular infiltration of the BM is associated with the manifestation of splenomegaly, noncaseating granulomas in different organs, lymphoproliferation, NLH of the gut, autoimmune cytopenias, or other autoimmune phenomena (Table 3).2-4 Thymoma as possible cause of Good syndrome (GS)23 was excluded by chest x-ray and computed tomography (supplemental Table 1). Interestingly, diffuse and nodular CD3+ T-cell infiltrates were significantly associated with autoimmune cytopenia; this was particularly evident for large grade 3 nodules (P = .009). Interestingly, other autoimmune phenomena (eg, atrophic gastritis, arthritis, Hashimoto thyroiditis, and others) did not show this association (Table 3). There was no statistically significant relationship between the extent of diffuse or nodular BM T-cell infiltration and disease duration in the 47 patients (estimate Tnod: 0.182, P = .219, estimate Tdiffuse: 0.093, P = .539). The correlation of plasma cell counts with clinical manifestations revealed that patients with NLH of the gut had higher plasma cell counts in their BM biopsies than CVID patients without NLH, albeit lower than in the control biopsies. Interestingly, NLH of the gut occurred rarely together with granulomas (7%) or lymphadenopathy (14%) and only 7 of 14 NLH patients (50%) had concomitant splenomegaly. This was in contrast to the high rate (> 90%) of concomitant splenomegaly in CVID patients presenting with lymphadenopathy and/or granulomas (Tables 1,3).
Association of clinical CVID phenotypes with BM plasma cells and nodular T-cell infiltrates
| CVID clinical phenotype* . | Splenomegaly . | Lymphadenopathy . | NLH of gut . | Granulomas . | Autoimmune cytopenia . | Other autoimmunity . |
|---|---|---|---|---|---|---|
| No. of patients† | 12 (no) vs 36 (yes) | 32 (no) vs 16 (yes) | 29 (no) vs 14 (yes) | 33 (no) vs 12 (yes) | 35 (no) vs 13 (yes) | 34 (no) vs 14 (yes) |
| BM: plasma cells‡ | NS | NS | .04‡ | NS | NS | NS |
| BM: diffuse CD3+ T-cell infiltrates§ | NS | NS | NS | NS | .04§ | NS |
| BM: nodular CD3+ T-cell infiltrates/mm biopsy§ | NS | NS | NS | NS | .014§ | NS |
| CVID clinical phenotype* . | Splenomegaly . | Lymphadenopathy . | NLH of gut . | Granulomas . | Autoimmune cytopenia . | Other autoimmunity . |
|---|---|---|---|---|---|---|
| No. of patients† | 12 (no) vs 36 (yes) | 32 (no) vs 16 (yes) | 29 (no) vs 14 (yes) | 33 (no) vs 12 (yes) | 35 (no) vs 13 (yes) | 34 (no) vs 14 (yes) |
| BM: plasma cells‡ | NS | NS | .04‡ | NS | NS | NS |
| BM: diffuse CD3+ T-cell infiltrates§ | NS | NS | NS | NS | .04§ | NS |
| BM: nodular CD3+ T-cell infiltrates/mm biopsy§ | NS | NS | NS | NS | .014§ | NS |
Data are P values of exact 2-sided Wilcoxon-Mann-Whitney test.
NS indicates not significant.
The clinical phenotype is independent of the Freiburg classification,18 which showed no significant association with BM plasma cells or with diffuse and nodular T-cell infiltrates (data not shown).
Number of patients without symptoms vs patients with symptoms.
BM plasma cells classified as absent (1), reduced (2), normal (3), increased (4). CVID patients with NLH of the gut had higher plasma cell counts than CVID patients without NLH, albeit lower than control biopsies.
Nodular CD3+ T-cell infiltrates expressed per millimeter length of BM biopsy cylinder were significantly elevated in CVID patients with autoimmune cytopenias (autoimmune hemolytic anemia, immune thrombocytopenia, or autoimmune neutropenia). The result was equally significant with the absolute number of nodules per whole biopsy, and there was a clear trend for large grade III nodules (P < .009) vs smaller ones (grade II, P < .07; and grade I, P < .06).
Changes in pre-B-cell development in a subset of CVID patients
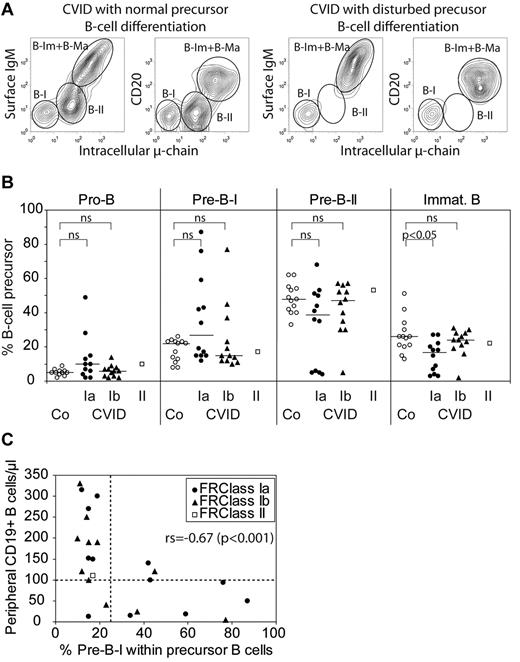
B-cell progenitor analysis has been performed in BM aspirates of 25 patients (Table 1, patients 1-25), including 1 inducible costimulator (ICOS)–deficient patient, and 2 patients each carrying transmembrane activator and modulator and cyclophilin ligand interactor or B-cell activating factor receptor polymorphisms. Figure 3A shows cytofluorimetric examples of normal and disturbed BM-dependent B-cell differentiation pattern. Notably, in a subgroup of 9 CVID patients (Table 1: patients 1, 2, 3, 7, 10, 11, 13, 15, and 21; 7 males, 2 females; Freiburg classification: 6× Ia, 3× Ib), we observed an increase in pre-B-I cells accompanied by a decrease in the percentage of more mature stages (pre-B-II, immature, transitional) and reduced peripheral B-cell counts (Figure 3B-C; Table 4). Whereas an increase in pre-B-I cells in BM aspirates is inversely correlated with the absolute peripheral B-cell counts (Figure 3C), the reverse is not true: there exist also patients with a normal early B-cell differentiation pattern and low peripheral B-cell counts indicating that other mechanisms can account for low numbers of circulating B cells as well.
Precursor B cells in CVID bone marrow. Altered B-cell precursor composition in BM biopsies of CVID patients grouped according to the Freiburg classification.14 (A) Two examples showing the flow cytometric analysis of bone marrow samples from CVID patients with normal (left panels) and disturbed (right panels) B-cell precursor development. CD19+ events were gated using CD3/CD16/CD33 as exclusion markers. B-I indicates pre-B-I; B-II, pre-B-II; and B-Im + B-Ma, immature plus mature B-cell populations. (B) B-cell precursors were phenotypically differentiated into pro-B, pre-B-I, pre-B-II, and immature B cells by flow cytometry as described in “Phenotyping of peripheral blood lymphocytes and of precursor B cells in BM aspirates.” Presented are B-cell precursor analyses of BM aspirates from controls (Co, ○, n = 13) and of CVID patients grouped according to the Freiburg classification Ia (●, n = 12), Ib (▴, n = 12), or II (□, n = 1). Lines indicate median values; statistical differences between controls and patient groups were tested by the Wilcoxon test. ns indicates not significant. (C) The percentage of pre-B-I precursors in bone marrow samples of CVID patients is plotted against the absolute peripheral B-cell counts. Symbols represent CVID subgroups as depicted in panel B. For the whole CVID group, the Spearman rank order correlation test yielded a statistically significant (P < .001) correlation coefficient of −0.67. For a separate analysis of group Ib, the correlation coefficient was 0.78 (P = .004), whereas for the group Ia the correlation did not reach statistical significance.
Precursor B cells in CVID bone marrow. Altered B-cell precursor composition in BM biopsies of CVID patients grouped according to the Freiburg classification.14 (A) Two examples showing the flow cytometric analysis of bone marrow samples from CVID patients with normal (left panels) and disturbed (right panels) B-cell precursor development. CD19+ events were gated using CD3/CD16/CD33 as exclusion markers. B-I indicates pre-B-I; B-II, pre-B-II; and B-Im + B-Ma, immature plus mature B-cell populations. (B) B-cell precursors were phenotypically differentiated into pro-B, pre-B-I, pre-B-II, and immature B cells by flow cytometry as described in “Phenotyping of peripheral blood lymphocytes and of precursor B cells in BM aspirates.” Presented are B-cell precursor analyses of BM aspirates from controls (Co, ○, n = 13) and of CVID patients grouped according to the Freiburg classification Ia (●, n = 12), Ib (▴, n = 12), or II (□, n = 1). Lines indicate median values; statistical differences between controls and patient groups were tested by the Wilcoxon test. ns indicates not significant. (C) The percentage of pre-B-I precursors in bone marrow samples of CVID patients is plotted against the absolute peripheral B-cell counts. Symbols represent CVID subgroups as depicted in panel B. For the whole CVID group, the Spearman rank order correlation test yielded a statistically significant (P < .001) correlation coefficient of −0.67. For a separate analysis of group Ib, the correlation coefficient was 0.78 (P = .004), whereas for the group Ia the correlation did not reach statistical significance.
CVID patients with elevated pre-B-I precursors show significantly reduced peripheral B-cell counts and a tendency to more nodular T-cell infiltrates in BM
| . | Pre-B-I normal (n = 16) . | Pre-B-I high* (n = 9) . | P† . |
|---|---|---|---|
| Freiburg classification groups Ia, Ib, and II | Ia: 6 | Ia: 6 | NS |
| Ib: 9 | Ib: 3 | ||
| II: 1 | |||
| Pre-B-II, % | 51.5‡ | 6 | < .001 |
| Immature B, % | 24 | 7 | < .001 |
| Peripheral CD19+, 1/μL | 190 | 50 | .003 |
| Peripheral transitional B cells, 1/μL | 5.3 | 0.2 | .002 |
| Peripheral CD4/CD8 ratio | 1.9 | 1.7 | NS |
| Peripheral CD45RO+ in CD4+ T cells, % | 74.4 | 63.7 | NS |
| sIL-2R, U/mL | 1033 | 1503 | NS |
| CD3+ T-cell nodules/mm BM biopsy | 0.1 | 0.3 | NS (.1) |
| . | Pre-B-I normal (n = 16) . | Pre-B-I high* (n = 9) . | P† . |
|---|---|---|---|
| Freiburg classification groups Ia, Ib, and II | Ia: 6 | Ia: 6 | NS |
| Ib: 9 | Ib: 3 | ||
| II: 1 | |||
| Pre-B-II, % | 51.5‡ | 6 | < .001 |
| Immature B, % | 24 | 7 | < .001 |
| Peripheral CD19+, 1/μL | 190 | 50 | .003 |
| Peripheral transitional B cells, 1/μL | 5.3 | 0.2 | .002 |
| Peripheral CD4/CD8 ratio | 1.9 | 1.7 | NS |
| Peripheral CD45RO+ in CD4+ T cells, % | 74.4 | 63.7 | NS |
| sIL-2R, U/mL | 1033 | 1503 | NS |
| CD3+ T-cell nodules/mm BM biopsy | 0.1 | 0.3 | NS (.1) |
NS indicates not significant.
Patients with a percentage of pre-B-I cells > 25% were classified as pre-B-I high.
Wilcoxon test (2-sided P values).
Median values.
Because the observed developmental block was similar to that seen in BTK deficiency19 and the patients with the block showed a skewed male/female ratio of 7:2, we suspected that some of the male patients might carry hypomorphic mutations in the BTK gene on chromosome Xq21.33-q22. Recently, a hypomorphic BTK mutation has been shown to result in low B-cell numbers.24 In 3 of 7 male patients in whom material and consent to genetic testing were available, we detected a normal BTK protein as well as normal transcript sequence (data not shown). Interestingly, the CVID patients with high counts of pre-B-I cells showed a tendency (P = .1) to higher nodular T-cell infiltrates in the corresponding BM biopsies (Table 4). There was no significant association of a given early B-cell phenotype with clinical CVID symptoms, such as splenomegaly, lymphadenopathy, NLH of the gut, granulomas, and autoimmune cytopenias (data not shown).
Discussion
Since a first report on BM analysis of pre-B cells in X-linked agammaglobulinemia and other forms of hypogammaglobulinemia,25 BM has rarely been in the focus of antibody deficiency research.12 Several case reports have shown that B- or T-cell lymphomas with BM involvement may complicate CVID, notably in the subset with granulomatous disease26,27 or in X-linked lymphoproliferative disease mimicking CVID.28 Monocyte lineage abnormalities in CVID have been associated with dysregulated monocyte-derived cytokine production skewing the immune system away from antibody synthesis.29,30 Neutropenia in CVID and other types of primary immune deficiency31 has been attributed to different etiologies ranging from autoimmune cytopenia to cyclic neutropenia32 and idiopathic forms, the latter being accompanied by an expansion of oligoclonal CD8+ T cells, increased numbers of large granular lymphocytes, and elevated levels of soluble Fas-ligand, splenomegaly, and lymphoproliferation.33 A relatively large body of evidence on BM abnormalities has been reported for GS, an unexplained association of thymoma, hypogammoglobulinemia, and high susceptibility to infections.23 Because of the presence of B- and T-cell lymphopenia, impaired maturation of erythroid and myeloid precursors, pure red cell aplasia, myelodysplasia with neutropenia, and eosinopenia in many cases of GS, it has been suggested that the basic defect may reside in the BM. The absence of pre-B cells or a B-cell arrest have also been reported in BM samples from GS patients34 as well as an increase of BM-infiltrating T cells, notably in GS patients with autoimmune phenomena and pure red cell aplasia.35 None of our patients presented with chest x-rays or computed tomography scans suspicious of a mediastinal tumor (supplemental Table 1). Moreover, the fact that in 80% of GS thymoma precedes or coincides with the diagnosis of immunodeficiency23 makes subclinical thymoma a rather unlikely pathogenic factor in our long-term follow-up cohort of CVID patients.
Isgro et al13 found in BM aspirates from 11 unclassified CVID patients significantly reduced numbers of hematopoietic lineage progenitors and a skewed cytokine production toward proapoptotic TNF-α at the expense of antiapoptotic IL-2 and reduced IL-7. The authors postulated a primary progenitor cell defect or a secondary influence of chronic recurrent infections leading to elevated TNF-α production by inflammatory cells in the BM.13
In view of these intriguing findings, we felt that a systematic analysis of T and B lymphocytes in BM biopsies might provide new clues for an involvement of hematopoietic and lymphoid progenitor cells as well as BM-infiltrating T lymphocytes in the physiopathology of CVID. Our study embarked on 48 diagnostically indicated BM biopsies from clinically well-classified CVID patients. The material has been subjected to conventional and immunohistologic staining procedures and was quantitatively analyzed for diffuse and nodular T-cell infiltrates and CD38+ plasma cells. Twenty-five biopsies were accompanied by an analysis of early B-cell differentiation markers in BM aspirates using flow cytometry.20 Whereas the hematopoietic compartment of the 47 evaluable BM biopsies was inconspicuous, the key findings relate to the T- and B-cell compartment of the BM. First, in line with the pronounced hypogammaglobulinemia, BM plasma cells were either absent or greatly reduced in 94% of the biopsies confirming a previous study.12 Interestingly, the residual plasma cell counts correlated significantly with the serum IgG levels before IVIg substitution supporting the notion that IgG-producing plasma cells home preferentially to the BM,21 whereas IgA- and IgM-secreting plasma cells are more often found in gut, tonsils, or spleen. Second, there was a highly significant diffuse and nodular T-cell infiltration in the majority of BM biopsies from CVID patients compared with control biopsies. Particularly, the large nodular T-cell aggregates were most prominent in BM biopsies from CVID patients with autoimmune cytopenia somewhat resembling findings in GS. They did neither correlate with disease duration, nor with the circulating B-cell counts but with circulating CD4+CD45R0+ T helper memory cells, elevated sIL-2R, and neopterin levels. The fact that CD4+CD45R0+ T helper memory cells were significantly elevated together with sIL-2R levels (supplemental Table 1) suggests an activated state of the BM-infiltrating T cells. They may locally recognize putative (auto)antigens or influence by their altered cytokine signature the surrounding stromal cell milieu, as suggested by Isgro et al.13 Third, BM-dependent, early B-cell differentiation was severely disturbed in 9 of 25 patients, leading to an increase of pro-B and pre-B-I cells because of a bottleneck at the pre-B-I to pre-B-II differentiation stage. Accordingly, the number of immature, transitional, and mature B cells of these 9 patients was reduced. Because 7 of the 9 patients were male and the differentiation block was less severe than in classic X-linked agammaglobulinemia,19 we had to exclude hypomorphic BTK mutations as cause of the pre-B-cell block.24 We found in 3 male patients normal BTK protein and unmutated sequence transcripts suggesting another intrinsic B-cell defect than X-linked agammaglobulinemia; alternatively, an altered local stroma cell environment may no longer be able to promote pre-B-cell development. Interestingly, the respective BM biopsies exhibited a tendency (P = .1) to higher numbers of nodular T-cell infiltrates compared with biopsies without a differentiation arrest at the pre-B-I stage (Table 4).
An interpretation of our findings has to recur to several animal models, which clearly demonstrated that antigen-specific CD8+ and CD4+ T cells compete for seeding to the BM.36-38 In that respect, they resemble plasma cells, which compete for niches of longevity in the BM.21,39,40 In a situation of an impaired humoral immunity with lack of switched memory B cells in the periphery (definition of CVID type I) and absence or severe reduction of plasma cells in blood and BM, it is probable that the T-cell compartment is more often challenged and activated; therefore, more memory T cells probably settle in the BM. This assumption was supported by an increase of sIL-2R concentration in serum and a significant increase of circulating CD4+CD45RO+ T memory cells. In a recent report, plasma cells have been shown to negatively regulate follicular T helper cells.41 Consonant with this finding is the observation by several groups that the T-cell compartment in CVID exhibits up-regulated memory and activation markers at the expense of markers typical for naive T cells (eg, CD45RA+ CD62L+).18,42-44 Particularly in CVID Ia patients, oligoclonal expansions of activated T cells have been noted.18,43,44
Whether or not there is a functional difference between diffuse and nodular T-cell infiltrates remains unclear and needs further studies. Clearly, nodular lymphoid aggregates have been observed in other types of immune-mediated diseases and are by no means specific for CVID,45 nor do they correlate in our cohort with disease duration. However, they show a highly significant association with autoimmune cytopenias, suggesting that some interaction with the B-cell lineage development takes place. One may speculate that the clusters of T cells result from local proliferation of individual clones reacting to intruded pathogens, which are normally being prevented from entering the BM by antibodies and complement. Alternatively, the nodular T-cell infiltrates may represent autoreactive T-cell clones impeding the early B-cell differentiation in analogy to the well-known T lymphoproliferative disorders leading to autoimmune cytopenia, pure red cell aplasia, or neutropenia.46,47 We favor the hypothesis of activated T cells playing a decisive pathogenic role in our 9 CVID patients presenting with a partial differentiation block at the pre-B-I stage, although the association with nodular T-cell infiltrates did not reach statistical significance (P = .1). We think that this is mainly because of the limited number of cases rather than to the inclusion of patients with B-cell activating factor receptor (patients 1 and 19) or transmembrane activator and modulator and cyclophilin ligand interactor polymorphisms (patients 18 and 22) and ICOS deficiency (patient 13); at least recalculation without the ICOS-deficient patient did not change the statistics. Furthermore, leaky blocks at the pre-B-I to pre-B-II stage have been described in Nijmegen breakage syndrome48 and ICOS deficiency,5 suggesting that this differentiation step is particularly sensitive to perturbations. The consequence of the ensuing differentiation arrest at the pre-B-I to pre-B-II stage is a highly significant (P < .001) increase of pre-B-I cells at the expense of more mature B-cell stages (Table 4). For the CVID patients reported here, we propose that still unknown mechanisms, such as new intrinsic B-cell defects or an altered stromal cell environment, may contribute to the disturbed pre-B-cell development. BM-infiltrating T cells may influence this scenario via changes in cytokine milieu, induction of autoreactive B cells, or direct killing of B-cell progenitors. In any case, more functional studies will be needed to shed more light in the pathogenesis of this CVID subset with low B-cell counts and a differentiation block at the pre-B-I stage.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Professor Karl Winkler, Department of Clinical Chemistry and Ilka Bonzio, Center of Chronic Immunodeficiency, UMC Freiburg for measuring neopterin and IL-10 serum concentrations, respectively, Professor Stefan Weiner, Trier, for contributing clinical data, and Mara-Lynn Metzger for technical assistance.
The work was supported by Bundesministeriun für Bildung und Forschung 01 EO 0803, supporting tandem project 2 (H.H.P., M.R., S.G., M.W.); Deutsche Forschungsgemeinschaft (grant SFB620 project C1; K.W., H.H.P.), and the European 7 framework (EUROPAD.net grant, HEALTH-F2-2008-201549; H.E., M.R., K.W., U.S.).
Authorship
Contribution: M.L.G.O., S.G., A.M.M., M.R., R.D., C.S., U.S., and M.S. performed experiments, analyzed specimens, collected and evaluated clinical and immunologic data from patients, and contributed to writing of the manuscript; H.G., K.T.-I., and M.W. established critical methodology for the evaluation of primary data, analyzed results, and contributed to writing of the manuscript; K.W. and H.E. gave critical input to the manuscript; D.H. performed statistical analysis; and M.S. and H.H.P. designed the study, evaluated data, designed figures, and wrote the manuscript.
Conflict-of-interest disclosure: H.H.P. is a member of the scientific advisory board of the Pfizer/Wyeth vaccine development program (Germany) and member of an expert panel of the Abbott Rheumatology branch (Germany). The remaining authors declare no competing financial interests.
Correspondence: Hans Hartmut Peter, Abt für Rheumatologie und Klinische Immunologie, Medizinische Universitätsklinik, Hugstetterstrasse 55, D-79106 Freiburg, Germany; e-mail: hans-hartmut.peter@uniklinik-freiburg.de.
References
Author notes
M.L.G.O. and S.G. contributed equally to this study as first authors.
M.S. and H.H.P. contributed equally to this study as senior authors.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal