Abstract
In human inflammatory diseases, we identified endothelial angiopoietin-2 (Ang-2) expression to be strongly associated with inflammations mediated by myeloid cells but not lymphocytes. To identify the underlying mechanism, we made use of a transgenic mouse model with inducible endothelial cell-specific expression of Ang-2. In this model, in the absence of inflammatory stimuli, long-term expression of Ang-2 led to a time-dependent accumulation of myeloid cells in numerous organs, suggesting that Ang-2 is sufficient to recruit myeloid cells. In models of acute inflammation, such as delayed-type hypersensitivity and peritonitis, Ang-2 transgenic animals showed an increased responsiveness. Intravital fluorescence video microscopy revealed augmented cell adhesion as an underlying event. Consequently, we demonstrated that Ang-2 is able to induce strong monocyte adhesion under shear in vitro, which could be blocked by antibodies to β2-integrin. Taken together, our results describe Ang-2 as a novel, endothelial-derived regulator of myeloid cell infiltration that modulates β2-integrin–mediated adhesion in a paracrine manner.
Introduction
In settings of pathologic angiogenesis, such as neoplasia, neovascularization is a prerequisite for sustained tumor growth. Angiopoietins (Ang-1 and Ang-2) belong to a family of growth factors that critically drive vessel growth as evidenced by genetic ablation studies in mice.1 Accumulating evidence indicates a link between chronic inflammation and the onset of cancer (ie, the recruitment of new blood vessels).2-4 In addition, it has been shown that inflammatory mediators, such as myeloid cells, also significantly contribute to tumor progression.2 Conversely, the sprouting of new blood vessels and the recruitment of inflammatory cells are triggered in acute and chronic inflammation.2,3 The close correlation of angiogenesis and inflammation suggests the existence of molecules that trigger both angiogenic sprouting and the recruitment of inflammatory cells. Evidence for the capacity of growth factors to interfere with inflammatory cell recruitment has previously been described for vascular endothelial growth factor (VEGF), which attracts bone marrow–derived, circulating cells on expression of stromal cell-derived factor 1 (SDF-1).5
More recently, common signaling pathways for the angiopoietin/Tie system and inflammation have been described.6,7 In the study presented here, we provide evidence that endothelial cell–derived Ang-2 critically controls the recruitment of myeloid cells via β2-integrins in both a genetic mouse model as well as in human inflamed tissues. Angiopoietin/Tie signaling is essential for embryonic vessel development and maturation and has a key role in mediating vessel quiescence in the adult.8 In concert with Ang-1, Ang-2 acts on the Tie2 tyrosine kinase receptor.1 Whereas Ang-1–mediated activation of the Tie2 receptor leads to blood vessel stabilization and the recruitment of mural cells,9 Ang-2 acts as an antagonist through inhibition of Ang-1–induced Tie2 phosphorylation.10 The antagonizing action of Ang-2 leads to vessel sprouting or regression depending on the presence or absence of additional growth factors (ie, VEGF).10,11 In contrast to Ang-1, which is constitutively expressed in the adult, Ang-2 is up-regulated only at sites of angiogenic remodeling as it occurs during tumor growth.11,12 Ang-1 has been described to maintain vessel integrity13,14 and act as an anti-inflammatory through the inhibition of NF-κB,15,16 and by blocking permeability agents, such as thrombin or VEGF.17 Previous studies performed in Ang-2–deficient mice revealed a defective inflammatory response in the absence of Ang-2.6 However, little is known about the mechanisms that link the signaling of angiogenic factors to inflammation. In human biopsy specimens from inflamed tissues, we made the surprising observation that Ang-2 is exclusively expressed in endothelial cells of biopsies that are dominated by myeloid cell infiltration. This observation prompted us to investigate whether Ang-2 specifically promotes the recruitment of myeloid cells. We applied a genetic mouse model to investigate the potential role of Ang-2 in triggering inflammatory responses. On prolonged expression of Ang-2 in the vasculature, transgenic mice exhibit signs of skin erythema accompanied by an accumulation of inflammatory cells. We furthermore used the dorsal skinfold chamber model to analyze leukocyte-endothelial interactions and observed increased adhesion in Ang-2 transgenic mice. By investigating cell adhesion in a flow chamber model in vitro, we showed that Ang-2 acts as a paracrine on cell adhesion and in a β2-integrin–dependent manner. In addition, contact hypersensitivity and peritonitis experiments in vivo revealed augmented responsiveness of transgenic mice to inflammatory stimuli. As such, we identified Ang-2 as a novel endogenous, endothelial cell-derived regulator of myeloid cell infiltration.
Methods
Animals
Tie1 tTA driver and Tetos human Ang-2 responder transgenes were generated as previously described.18 Genotypes of Tie1 tTA lines crossed with Tetos Ang-2 lines were determined by PCR. Mice were fed with doxycycline-containing food pellets (100 mg/kg) derived from ssniff Spezialdiaeten GmbH.
The present study was performed in accordance with the German Protections of Animals Act (Tierschutzgesetz) and the permission of the Regierungspräsidium Darmstadt.
ELISA
VEGF and Ang-2 serum levels were measured using BD Quantikine ELISA according to the manufacturer's instructions.
Embedding and immunohistochemistry of human biopsy specimen
Human specimens were fixed in 4% formaldehyde and embedded in paraffin. Diagnoses were determined by neuropathologists at the Department of Neuropathology, Edinger Institute, University of Frankfurt Medical School. The use of human material has been approved by the Frankfurt University Medical School Institutional review board. For anti–human CD3 (Dako), anti–human CD68 (Dako) and anti–human Ang-2 (R&D) immunhistochemistry, a Ventana Benchmark automated staining system was used. For antigen retrieval, cell conditioning 1 was used (prediluted; Ventana Medical Systems). After incubation with primary antibodies, signals were detected with Chromo DAB Map kit (Ventana Medical Systems; for anti-CD3 and anti-CD68) and with IHC Ultra Map AP kit (Ventana Medical Systems; anti–Ang-2).
Mouse tissue embedding
Tissue samples were fixed in 4% paraformaldehyde o/N at 4°C followed by dehydration with 10%, 20% sucrose in PBS overnight, and finally 30% sucrose for 2 days. Specimens were then embedded in Tissue Tek and frozen on dry ice; 10-μm cryosections were taken at a Microm HM550 Mikrotom and melted on Superfrost Microscope slides.
Immunohistochemistry of mouse tissues
Sections were postfixed using 4% paraformaldehyde for 10 minutes followed by peroxidase blocking with 1.5% H2O2 in methanol for an additional 10 minutes. Blocking and permeabilization were performed using 5% BSA and 20% normal goat serum, respectively, each supplemented with 0.01% Triton X-100. The following primary rat anti–mouse antibodies were used: anti-CD45, anti-CD4, anti-CD3, anti-B220, anti-CD31, anti–Ly-6G (BD Biosciences/1:100), and anti-F4/80 (Serotec/1:100). Biotin-conjugated secondary antibodies were purchased from Invitrogen. Detection was performed using Vectastain ABC Kit and AEC Kit (Vector) followed by counterstaining with Mayer hemalum solution (Merck). All pictures were taken on a Nikon80i Microscope running NIS Elements AR3.10 software. Cells in skin tissue were stereologically counted using a Zeiss microscope with Stereo Investigator Version 4.34 software from MicroBrightField Inc.
For fluorescence immunohistochemistry, we applied α-smooth muscle actin Cy3 antibody (Sigma-Aldrich) and Alexa-488–conjugated secondary antibody (Invitrogen), and counterstained with Toto3 iodide. Slides were then mounted with Aqua Poly/Mount (Polysciences). For imaging, a confocal laser scanning microscope (Nikon LSM C1S1) was applied.
Isolation of human monocytes
Human blood samples were obtained from adult healthy donors. PBMCs were isolated using Ficoll gradient and further incubated with anti–human CD14 Microbeads (Miltenyi) and magnetically isolated using MS Columns (Miltenyi). Purity was confirmed by FACS staining with anti-CD14 allophycocyanin (APC; BD Biosciences). Isolated cells were allowed to rest in HBSS plus 0.05% BSA in the incubator for at least 2 hours.
Flow chamber
Adhesion under flow was determined using Ibidi μ-Slide VI chambers coated with 10 μg/mL human ICAM-1 or 2 μg/mL human VCAM-1 (R&D Systems) and 25 μg/mL antipolyhistidine (R&D Systems) for 30 minutes. Chambers were blocked with 1% BSA for 10 minutes and then incubated with 6.25 μg/mL recombinant human Ang-2, human Ang-1 (R&D Systems), or 1% BSA in PBS for 2 hours. Human monocytes were allowed to attach for 3 minutes. Nonadherent cells were flushed away, and shear stress was then increased stepwise for 30 seconds each. To block integrins, cells were incubated with 20 μg/mL anti–human CD18 or anti–human CD29 (R&D Systems) antibody 1 hour before adhesion. Integrin α-subunits were blocked by applying 7.2 μg/mL CD11a (Thermo Scientific) or 50 μg/mL CD11b (eBioscience) antibody 30 minutes in advance. As isotype control, goat IgG (Abcam) was used at equal concentrations. To block G protein–coupled receptors, cells were treated with pertussis toxin (Sigma-Aldrich) for 1 hour at a final concentration of 0.4 μg/mL. The number of adhering cells was quantified from photographic images taken with a charge-coupled device camera (Sony) before application of the first shear stress and at each shear stress period.
FACS
Lung biopsies of Ang-2 transgenic mice were digested using collagenase D/DNAse, and single-cell suspension was obtained using Miltenyi gentleMACS dissociator and gentleMACS C-Tubes. After blocking with NGS and Fc-Block (BD Biosciences), cells were stained with anti-CD45 peridinin chlorophyll protein complex (PerCP; BD Biosciences).
Peripheral blood of wild-type (WT) and transgenic mice was collected by heart punctuation. Cells were blocked with Fc-Block (BD Biosciences) and then stained with anti-CD4 PerCP, anti-CD8 FITC, anti–GR-1 (Ly-6G + Ly-6C) APC, anti-CD19 PE, anti-CD11b APC, anti-CD45 PerCP, anti–Ly-6G FITC (BD Biosciences), and anti-F4/80 APC (eBioscience). After antibody incubation, red blood cells were lysed using Lyse/Fix (BD phosflow) for 10 minutes at 37°C. Cells were then washed and analyzed using a FACSCanto II Cytometer (BD Biosciences). To determine total cell counts, AccuCheck counting beads (Invitrogen) were used.
DTH
Mice were depleted of doxycycline 4 weeks in advance. Epicutaneous application of 2.4 dinitrofluorobenzene (DNFB; Sigma-Aldrich) was used to induce delayed-type hypersensitivity (DTH) reaction.19 Mice were sensitized on day 1 by painting bare abdominal skin with 0.5% DNFB (20 μL, in acetone:oliveoil, 4:1). Challenge occurred on day 7 by administration of 0.25% DNFB (20 μL) on the left ear. Right ears served as internal control and were left untreated. Mice were killed 48 hours later, and ears were formalin fixed, processed for cryosections, and stained with H&E. Mean ear diameter was measured using a Nikon 80i microscope and NIS Elements AR3.10 software.
Peritonitis experiments
Mice were depleted of doxycycline 4 weeks in advance. Peritonitis experiments were performed by intraperitoneal injection of 1 mL sterile thioglycollate solution (Merck; 3% in PBS) or 1 mL sterile PBS, respectively. Accumulated neutrophils were collected by peritoneal lavage 4 hours after injection. Cells were stained with anti–mouse Gr-1 (Ly-6G + Ly-6C) APC and anti–mouse Ly-6G FITC (BD Biosciences PharMingen) and counted by FACS using AccuCheck counting beads (Invitrogen). Tie2+ macrophages in the peritoneal lavage were identified by flow cytometry. Gates were set on F4/80 APC (eBioscience)–positive macrophages, and Tie2 PE (eBioscience) expression was determined.
Intravital fluorescence video microscopy
We used Ang-2 double-transgenic (DT) and WT littermates (> 25 g of weight, 5 mice of WT genotype, 4 mice of Ang-2 DT) for fluorescence intravital video microscopy. Mice were depleted of doxycycline 4 weeks in advance. Microsurgical techniques for the implantation of the dorsal skinfold chambers have been previously described.6,20 Briefly, we anesthetized mice with ketamine-xylazine. Using microsurgical techniques, we implanted 2 symmetric titanium frames into the dorsal skinfold so as to sandwich the extended double layer of skin. One layer was completely removed in a circular area 15 mm in diameter. The remaining layer, consisting of the striated muscle, subcutaneous tissue, and epidermis, was covered with a glass cover slip incorporated into one of the frames. This observation window allows for repeated intravital microscopic observations of the microvasculature. To eliminate the effects of surgical trauma, we implanted chambers 2 days before the experiment. The mice tolerated the skinfold chamber well and showed no signs of discomfort. Mice were transferred and fixed to a microscope stage, and fluorescence intravital video microscopy was performed by epi-illumination techniques using a modified Axiotech vario microscope (Attoarc; Zeiss) using 10× long-distance and 20× water-immersion objectives (Zeiss). Microscopic images were recorded through a charge-coupled device video camera with an optional image intensifier for weak fluorescence (κ) and transferred to a S-VHS video system (Panasonic) for offline analysis. Microvessels were visualized by contrast enhancement with 2% FITC-conjugated dextran (0.1 mL, intravenous; molecular weight 150 000; Sigma-Aldrich) and the use of blue-light epi-illumination. Simultaneous in vivo staining of leukocytes with 0.2% rhodamine-6G (0.1 mL, intravenous; Sigma-Aldrich) and the use of green-light epi-illumination allowed for sequential analysis of leukocytes within the microcirculation. Vessel diameters were assessed using computer-assisted image analysis. Three regions of interest per animal were chosen, and 1-minute recordings of one to 3 postcapillary venules were taken (20-60 μm diameter, 200-400 μm length) to assess baseline rolling, leukocyte sticking, and leukocyte velocities. Subsequently, 50 μL of TNF-α (10 μg/mL) was superfused onto the skin, and identical venules were recorded 2 hours after superfusion. The rolling leukocyte flux was determined by counting the number of leukocytes passing through a stationary plane perpendicular to the axis of each venule over a given period. Sticking leukocytes were defined as cells that remained stationary for at least 30 seconds and are given as number of cells per vessel surface (millimeters squared). Rolling leukocytes are reported as the number of nonadherent cells passing through the observed vessel segment within 60 seconds.
Integrin activation
To assess β2-integrin activation on the surface of human monocytes, cells were stimulated with 500 ng/mL recombinant human Ang-2 (R&D Systems) or 1mM MnCl2 for 10 minutes after stain for active CD11b with the mAb24 antibody (Hycult) on ice. Cells were analyzed by flow cytometry and mean fluorescence was acquired.
Statistics
For statistical comparison of 2 samples, a 2-tailed Student t test was used when applicable. Data are represented as mean ± SEM if not indicated different.
Image acquisition
Brightfield pictures were taken on a Nikon Eclipse 80i microscope with a 20×/0.50 objective running Nis Elements AR3.10 software. For fluorescent images a confocal microscope Nikon Eclipse TE-2000E with a 20×/0.75 objective running EZ-C1 3.9 software was used. All images were contrast enhanced using ImageJ 1.4.03 software.
Results
Ang-2 expression in human inflammatory diseases
Because inflammation is often accompanied by angiogenic sprouting, we investigated a series of human inflamed tissues (for a complete list, see Table 1) with regard to the up-regulation of angiogenic growth factors. Intriguingly, we made the observation that biopsy specimens of inflamed tissue that are dominated by monocyte/macrophage (CD68+) cell infiltration, exhibit very robust Ang-2 expression in surrounding vessels (Figure 1A-B; abscess with granulation tissue). At the same time, we detected only few CD3+ lymphocytes (Figure 1C). In contrast, in biopsy specimen with predominant T-cell infiltration (eg, vasculitis in peripheral nerve, which stained positive for CD3 and negative for CD68; Figure 1E-F), we were not able to detect Ang-2 expression in the vasculature (Figure 1D). The strong correlation between Ang-2 expression and myeloid cell infiltration was confirmed in a total series of 13 patient biopsies (Table 1).
Ang-2 expression in human inflammatory disease
| Diagnosis . | Tissue . | Ang-2 expression* . |
|---|---|---|
| Myeloid dominated | ||
| Abscess with granulation tissue | Brain | +++ |
| Abscess with granulation tissue | Connective tissue | +++ |
| Abscess with granulation tissue | Intervertebral disc | ++ |
| Granulation tissue | Dura mater | + |
| Granulation tissue | Dura mater | ++ |
| Granulation tissue | Intervertebral disc | + |
| Granulation tissue | Intervertebral disc | ++ |
| Granulation tissue | Intervertebral disc | + |
| Granulation tissue | Intervertebral disc | ++ |
| Granulation tissue | Nervous cyst | + |
| Granulation tissue | Skin | + |
| Granulation tissue | Synovial cyst | ++ |
| Spondylodiscitis with granulation tissue | Fibrocartilage | ++ |
| Lymphoid dominated | ||
| Acute vasculitis | Nerve | − |
| Arteritis temporalis | Artery | − |
| Arteritis temporalis | Artery | − |
| Arteritis temporalis | Artery | − |
| Encephalitis | Brain | − |
| Vasculitis | Nerve | − |
| Diagnosis . | Tissue . | Ang-2 expression* . |
|---|---|---|
| Myeloid dominated | ||
| Abscess with granulation tissue | Brain | +++ |
| Abscess with granulation tissue | Connective tissue | +++ |
| Abscess with granulation tissue | Intervertebral disc | ++ |
| Granulation tissue | Dura mater | + |
| Granulation tissue | Dura mater | ++ |
| Granulation tissue | Intervertebral disc | + |
| Granulation tissue | Intervertebral disc | ++ |
| Granulation tissue | Intervertebral disc | + |
| Granulation tissue | Intervertebral disc | ++ |
| Granulation tissue | Nervous cyst | + |
| Granulation tissue | Skin | + |
| Granulation tissue | Synovial cyst | ++ |
| Spondylodiscitis with granulation tissue | Fibrocartilage | ++ |
| Lymphoid dominated | ||
| Acute vasculitis | Nerve | − |
| Arteritis temporalis | Artery | − |
| Arteritis temporalis | Artery | − |
| Arteritis temporalis | Artery | − |
| Encephalitis | Brain | − |
| Vasculitis | Nerve | − |
Data represent type, tissue, and Ang-2 expression from inflamed specimens of 19 patients. Ang-2 expression strongly correlates with myeloid but is absent in lymphoid-dominated inflammation.
Ang-2 expression score: +++ indicates strong; ++, moderate; +, weak; and −, none.
Ang-2 expression is strongly associated with myeloid cell infiltration. Tissue sections derived from human inflammatory diseases were separated into myeloid dominated (A-C, abscess with granulation tissue) and lymphocyte dominated specimen (D-F, vasculitis/peripheral nerve). Specimen with high numbers of CD68+ macrophages (B) and low numbers of CD3+ T cells (C) show strong vascular staining for Ang-2 (A). In contrast, specimens with high numbers of infiltrating T cells (F) and low numbers of macrophages (E) were negative for Ang-2 (D). Scale bar represents 100 μm.
Ang-2 expression is strongly associated with myeloid cell infiltration. Tissue sections derived from human inflammatory diseases were separated into myeloid dominated (A-C, abscess with granulation tissue) and lymphocyte dominated specimen (D-F, vasculitis/peripheral nerve). Specimen with high numbers of CD68+ macrophages (B) and low numbers of CD3+ T cells (C) show strong vascular staining for Ang-2 (A). In contrast, specimens with high numbers of infiltrating T cells (F) and low numbers of macrophages (E) were negative for Ang-2 (D). Scale bar represents 100 μm.
Generation of transgenic mice with endothelial cell-specific expression of Ang-2
We next asked whether endothelial Ang-2 may be directly responsible for the recruitment of myeloid cells. To mechanistically dissect the functional role of Ang-2 in mediating monocyte/macrophage cell infiltration in (inflamed) tissues, we applied a doxycycline-inducible mouse model.18 To direct Ang-2 transgene expression in endothelial cells, we crossed Tie1 tTA driver mice with Ang-2 TetOS responder mice as described before.18 Mice were fed with doxycycline until adulthood, and doxycycline was omitted 4 weeks before inflammation experiments, such as DTH or peritonitis. Alternatively, for kinetic experiments designed to directly assess the role of Ang-2 in mediating myeloid cell recruitment in vivo, doxycycline was removed at 8 weeks (up to 12 months).
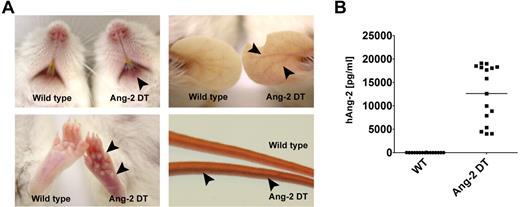
Although Ang-2 DT mice appeared generally healthy, they displayed signs of erythema starting after 8 weeks of transgene expression (Figure 2A). The skin of transgenic mice became progressively redder, most prominent at 6-12 months of age as shown in Figure 2A. Severity of erythema was most prominent in transgenic animals with high Ang-2 serum levels (data not shown). Most likely, increased densities of immature vessel (ie, devoid of pericytes) contribute to intense redness of the skin in Ang-2 DT mice (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Ang-2 protein levels of mice used for the study described here are displayed in Figure 2B.
Macroscopic phenotype of Ang-2 transgenic mice. Ang-2 DT mice with high Ang-2 protein level in the serum display signs of skin erythema starting after 6 months of transgene expression (arrowheads). (A) Arrowheads in lower right panel point at dilated tail vein of an Ang-2 DT mouse. (B) Human Ang-2 serum levels secreted in the peripheral blood of Ang-2 DT mice after doxycycline depletion were determined by ELISA.
Macroscopic phenotype of Ang-2 transgenic mice. Ang-2 DT mice with high Ang-2 protein level in the serum display signs of skin erythema starting after 6 months of transgene expression (arrowheads). (A) Arrowheads in lower right panel point at dilated tail vein of an Ang-2 DT mouse. (B) Human Ang-2 serum levels secreted in the peripheral blood of Ang-2 DT mice after doxycycline depletion were determined by ELISA.
Transgenic mice expressing Ang-2 are prone to inflammation
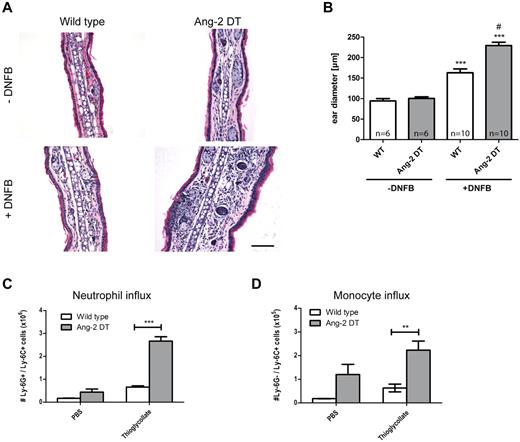
We next wanted to test the hypothesis whether conditional Ang-2 expression in the vasculature of transgenic mice promotes cell infiltration and/or interferes with the onset of inflammatory reactions. Consequently, we performed short-term inflammation experiments, such as DTH and peritonitis (Figure 3). After challenge with DNFB, ear diameters of transgenic mice were significantly increased after 48 hours of stimulation because of edema and elevated numbers of infiltrating cells (Figure 3A-B; compared with WT controls). The right ear served as an internal control and was left untreated. In a model of thioglycollate-induced peritonitis, we made similar observations and detected increased responsiveness in Ang-2 DT mice after intraperitoneal injection of thioglycollate (Figure 3C). We choose to apply anti-Ly-6G/Ly-6C (Gr-1) to discriminate between neutrophil granulocytes and monocytes in the peritoneal cavity. As evidenced by FACS analysis, the number of infiltrating neutrophils (Ly-6G+/Ly-6C+) and monocytes (Ly-6G−/Ly-6C+) was significantly increased in the peritoneum of transgenic mice 4 hours after initial challenge (Figure 3C-D). In contrast, baseline neutrophil numbers were not altered after PBS treatment (Figure 3C). However, the baseline influx of monocytes (expressing Ly6C) were elevated. These findings indicate that endothelial expression of Ang-2 (> 4 weeks) facilitates the onset of inflammatory reactions.
Ang-2 augments DTH and peritonitis in transgenic mice. After induction of DTH, leukocyte infiltration was measured in DNFB-treated ears (A). Severely increased ear swelling was evident in Ang-2 DT as shown by H&E staining (A). Quantification revealed significantly increased ear diameters in Ang-2 DT compared with WT mice (B). ***P < .005, compared with untreated ears. #P < .001, compared with treated WT ears. In a model of thioglycollate-induced peritonitis, peritoneal neutrophils were identified according to the expression of Ly-6G and Ly-6C and counted by FACS (C). After thioglycollate challenge, transgenic animals showed significantly increased numbers of myeloid cells in the peritoneal cavity (C-D). Among those, neutrophils (Ly-6G+/Ly-6C+) and monocytes (Ly-6G−/Ly-6C+) were prominent (C-D). Data are mean ± SEM; n = 4. *P < .05. ***P < .005.
Ang-2 augments DTH and peritonitis in transgenic mice. After induction of DTH, leukocyte infiltration was measured in DNFB-treated ears (A). Severely increased ear swelling was evident in Ang-2 DT as shown by H&E staining (A). Quantification revealed significantly increased ear diameters in Ang-2 DT compared with WT mice (B). ***P < .005, compared with untreated ears. #P < .001, compared with treated WT ears. In a model of thioglycollate-induced peritonitis, peritoneal neutrophils were identified according to the expression of Ly-6G and Ly-6C and counted by FACS (C). After thioglycollate challenge, transgenic animals showed significantly increased numbers of myeloid cells in the peritoneal cavity (C-D). Among those, neutrophils (Ly-6G+/Ly-6C+) and monocytes (Ly-6G−/Ly-6C+) were prominent (C-D). Data are mean ± SEM; n = 4. *P < .05. ***P < .005.
Elevated numbers of infiltrating cells in organs of Ang-2 transgenic mice
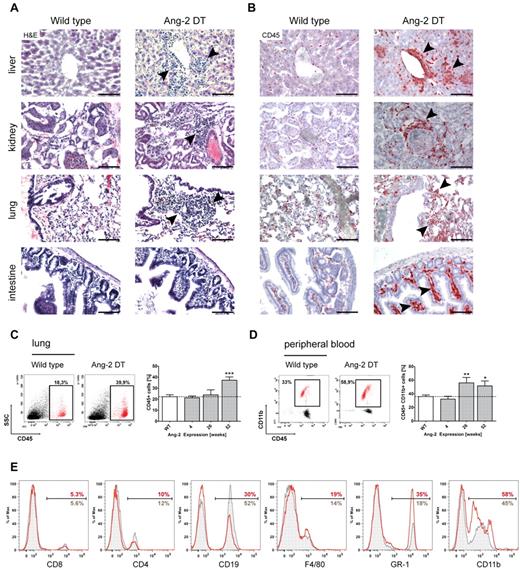
We next aimed to decipher the nature of infiltrating cells in more detail and analyzed transgenic mice on long-term expression of Ang-2. Strikingly, we observed prominent cell infiltrates in numerous organs (eg, liver, kidney, lung, and intestine) of Ang-2 DT compared with WT littermates (Figure 4A arrowheads). To determine the origin of tissue-infiltrating cells, we first applied the pan-hematopoietic marker CD45 (Figure 4B). CD45-positive cells in organs of Ang-2 DT mice (examples here are liver, kidney, lung, and intestine) were numerous and often localized next to vessels (Figure 4B arrowheads). As shown in Figure 4C, the number of infiltrating CD45+ hematopoietic cells in the lung significantly increased over time and peaked at 52 weeks of Ang-2 expression. When further analyzing the composition of blood leukocytes in Ang-2 DT mice, we noticed elevated numbers of mononuclear cells in forward/side scatter blots (data not shown). Those consisted mainly of CD45+CD11b+ myeloid cells that accumulated in Ang-2 DT mice after 26 weeks of Ang-2 expression (Figure 4D). We applied a series of additional marker against blood leukocyte populations, such as T and B cells, monocytes/macrophages, and neutrophil granulocytes (Figure 4D). We observed a strong shift toward cells of the myeloid lineage (monocytes and neutrophils; Figure 4E). This was also evident when determining absolute blood counts using FACS counting beads (supplemental Figure 2C), although the increase in monocyte (F4/80) and neutrophil (Ly-6G) numbers was not significant.
Leukocyte infiltration in multiple organs. Large infiltrates of hematopoietic cells were detected in liver, kidney, lung, and intestine sections of Ang-2 transgenic animals after 6 months of Ang-2 expression, as shown in H&E staining (A) and anti-CD45 immunohistochemistry (B). FACS analysis of dissociated lung samples showed a time-dependent increase of CD45+ leukocytes on prolonged Ang-2 expression (C), which was accompanied by a gain of CD45+ CD11b+ myeloid cells in the peripheral blood (D). Further analysis of leukocyte subsets of either WT (gray filled lines) or Ang-2 DT mice (red lines) showed a shift toward the myeloid lineage in the peripheral blood of transgenic mice at the expense of lymphocytes (E). Data are mean ± SEM. (C) n = 4. (D) n = 5. Scale bar represents 100 μm. *P < .05. **P < .01. ***P < .005.
Leukocyte infiltration in multiple organs. Large infiltrates of hematopoietic cells were detected in liver, kidney, lung, and intestine sections of Ang-2 transgenic animals after 6 months of Ang-2 expression, as shown in H&E staining (A) and anti-CD45 immunohistochemistry (B). FACS analysis of dissociated lung samples showed a time-dependent increase of CD45+ leukocytes on prolonged Ang-2 expression (C), which was accompanied by a gain of CD45+ CD11b+ myeloid cells in the peripheral blood (D). Further analysis of leukocyte subsets of either WT (gray filled lines) or Ang-2 DT mice (red lines) showed a shift toward the myeloid lineage in the peripheral blood of transgenic mice at the expense of lymphocytes (E). Data are mean ± SEM. (C) n = 4. (D) n = 5. Scale bar represents 100 μm. *P < .05. **P < .01. ***P < .005.
Continuous Ang-2 signaling potentiates the recruitment of monocytes in transgenic mice
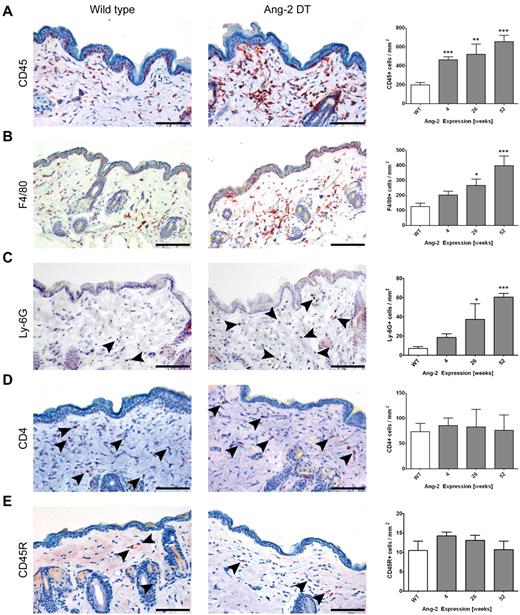
To investigate whether Ang-2 promotes the infiltration of distinct leukocyte populations (as indicated in human biopsies; Figure 1) and whether this effect is dependent on the kinetics or duration of Ang-2 expression, we performed immunohistochemistry on skin sections (Figure 5) as well as FACS analyses of infiltrating cells dissected from the lung (supplemental Figure 2) of transgenic mice after prolonged expression of Ang-2. As shown in Figure 4B, anti-CD45 immunohistochemistry revealed strong signals in a number of organs of Ang-2 DT mice. In addition, the frequency of CD45-positive cells in Ang-2 DT skin was significantly increased at 4 weeks of Ang-2 transgene expression (463 cells/mm3 in DT compared with 199 cells/mm3 in WT mice) and further increased at 26 and 52 weeks (Figure 5A). We also observed significantly elevated numbers of myeloid cells consisting of both F4/80-positive macrophages and neutrophils expressing Ly-6G starting at 26 weeks of transgene expression (Figure 5B; F4/80: 266 cells/mm3 in DT vs 126 cells/mm3 in WT; Ly-6G: 37.27 cells/mm3 in DT vs 7 cells/mm3 in WT). However, other leukocyte populations, such as CD4-positive T cells or B220 (CD45R)–positive B cells were not elevated in the skin of Ang-2 DT mice (Figure 5C-D). The increased infiltration of F4/80+ and Ly-6G+ cells in the skin and the elevated numbers of CD11b+ cells in the blood of transgenic mice (Figure 4D) suggest that Ang-2 expression in endothelial cells is directly responsible for the predominant recruitment of myeloid cells.
Ang-2 induced skin infiltration of macrophages. Immunostaining of WT and Ang-2 transgenic skin sections using various leukocyte markers. Representative images after 52 weeks of transgene expression are displayed, and the number of CD45+, F4/80+, Ly-6G+, CD4+, and CD45R+ B cells per millimeter squared were determined. CD45+ leukocytes accumulate progressively on prolonged Ang-2 expression (A). Among these, F4/80+ macrophages (B) and Ly-6G+ neutrophil granulocytes (C) are enriched, whereas CD4+ T cells and CD45R+ B cells compose only a minor fraction (indicated by arrowheads) that remains constant over time (C-D). Data are mean ± SEM of 5 animals. P values represent significance over WT cell count. Scale bar represents 100 μm. *P < .05. **P < .01. ***P < .005.
Ang-2 induced skin infiltration of macrophages. Immunostaining of WT and Ang-2 transgenic skin sections using various leukocyte markers. Representative images after 52 weeks of transgene expression are displayed, and the number of CD45+, F4/80+, Ly-6G+, CD4+, and CD45R+ B cells per millimeter squared were determined. CD45+ leukocytes accumulate progressively on prolonged Ang-2 expression (A). Among these, F4/80+ macrophages (B) and Ly-6G+ neutrophil granulocytes (C) are enriched, whereas CD4+ T cells and CD45R+ B cells compose only a minor fraction (indicated by arrowheads) that remains constant over time (C-D). Data are mean ± SEM of 5 animals. P values represent significance over WT cell count. Scale bar represents 100 μm. *P < .05. **P < .01. ***P < .005.
Augmented adhesion in Ang-2 transgenic mice in vivo and in the flow chamber assay in vitro
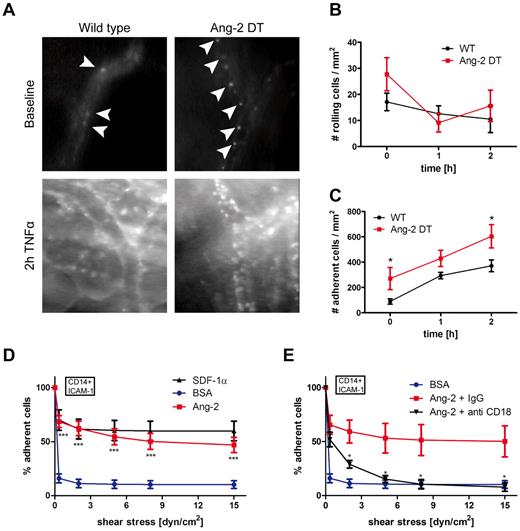
To dissect the mechanism of myeloid cell infiltration mediated by Ang-2, we performed intravital fluorescence video microscopy (Figure 6). We applied the dorsal skinfold chamber model to assess interactions of blood leukocytes with the skin microvasculature in Ang-2 and WT mice (Figure 6). Surgical procedures have been performed as previously described.6,20 Blood leukocytes were visualized with rhodamine-6G fluorescence. We analyzed skin vessels at baseline levels and after superfusion with TNF-α. As demonstrated in Figure 5A, baseline rolling did not significantly differ among Ang-2 DT and WT mice. However, after stimulation with TNF-α (at 1 and 2 hours), the fraction of rolling cells decreased, indicative of subsequent adhesion of previously rolling cells (Figure 6A-B). Accordingly, after superfusion with TNF-α, the number of adhering cells was significantly increased after 2 hours (Figure 6A,C). Moreover, we already observed increased baseline adhesion of blood leukocytes in Ang-2 DT mice (Figure 6C). These findings indicate that continuous expression of Ang-2 positively affects leukocyte-endothelial interactions in Ang2 DT mice in vivo.
Ang-2-induced leukocyte adhesion. Leukocyte-endothelial interactions of rhodamine-6G–labeled leukocytes in Ang-2 DT and WT littermates were assessed by fluorescence intravital microscopy using the skinfold chamber model (top). (A) Inflammatory activation was induced by superfusion with TNF-α for 2 hours (bottom). (B) The number of rolling cells was not significantly altered by Ang-2 expression. Quantification of adherent leukocytes (arrowheads) revealed significantly increased baseline adhesion in Ang-2 DT mice (n = 4) compared with WT (n = 5). After 2 hours of TNF-α stimulation, the number of adhesive leukocytes further increased above adhesion levels determined in WT mice (C). Data are mean ± SD of 4 animals. Adhesion under shear of CD14+ human monocytes to ICAM-1 was significantly increased when cells were additionally exposed to Ang-2 (cocoating with ICAM-1) compared with BSA control (D). SDF-1α served as a positive control (D). This effect was completely abolished on incubation with an inhibitory antibody against β2-integrin (anti-CD18) but not with control IgG (E). Data are mean ± SEM of 10 independent experiments (using anti-β2 antibody: n = 4). **P < .01. ***P < .005.
Ang-2-induced leukocyte adhesion. Leukocyte-endothelial interactions of rhodamine-6G–labeled leukocytes in Ang-2 DT and WT littermates were assessed by fluorescence intravital microscopy using the skinfold chamber model (top). (A) Inflammatory activation was induced by superfusion with TNF-α for 2 hours (bottom). (B) The number of rolling cells was not significantly altered by Ang-2 expression. Quantification of adherent leukocytes (arrowheads) revealed significantly increased baseline adhesion in Ang-2 DT mice (n = 4) compared with WT (n = 5). After 2 hours of TNF-α stimulation, the number of adhesive leukocytes further increased above adhesion levels determined in WT mice (C). Data are mean ± SD of 4 animals. Adhesion under shear of CD14+ human monocytes to ICAM-1 was significantly increased when cells were additionally exposed to Ang-2 (cocoating with ICAM-1) compared with BSA control (D). SDF-1α served as a positive control (D). This effect was completely abolished on incubation with an inhibitory antibody against β2-integrin (anti-CD18) but not with control IgG (E). Data are mean ± SEM of 10 independent experiments (using anti-β2 antibody: n = 4). **P < .01. ***P < .005.
To more precisely investigate which step of the adhesion cascade was affected by prolonged Ang-2 expression and whether adhesion promotion by Ang-2 involved vascular adhesion molecules and/or leukocyte integrins, we performed adhesion assays under shear in vitro (Figure 6D). CD14+ monocytes isolated from human blood (for purity and technical details, see “Isolation of human monocytes” and supplemental Figure 3A) were processed for adhesion on ICAM-1, co-coated with either SDF-1α as a positive control, or with Ang-2 under various shear stresses (Figure 6D). As demonstrated in Figure 6D, Ang-2 protein was able to promote strong adhesion of CD14+ monocytes on ICAM-1 (but not CD14- cells including T- and B-lymphocytes; supplemental Figure 3B). This finding appeared to be independent of integrin affinity changes (β2-integrin respectively; supplemental Figure 3C) or G-protein coupled receptor signaling since treatment with pertussis toxin did not affect Ang-2 mediated adhesion of CD14+ cells (supplemental Figure 3D-E).
To identify whether integrin signaling is involved in the Ang-2-mediated monocyte adhesion, we performed antibody inhibition experiments in the flow chamber model. We initially aimed to determine the involvement of CD18 and CD11a (LFA-1)/CD11b (Mac-1; supplemental Figure 3F-G). Blocking antibodies directed against β2-integrin (CD18) strongly reduced Ang-2–mediated monocyte adhesion almost down to baseline levels (Figure 6E). To further dissect the different β2-integrins involved in Ang-2-mediated adhesion, we applied functional blocking antibodies against CD11a (αL) and CD11b (αM). Both were able to significantly block monocyte adhesion by ∼ 50% (supplemental Figure 3F). However, they did not exhibit an additive effect as blocking with a combination of both antibodies did not result in further inhibition of Ang-2–mediated adhesion (supplementalFigure 3G), suggesting the possible involvement of other β2-integrins. Ang-2 was additionally able to induce monocyte adhesion on VCAM-1, but this binding was not blocked by anti-β1 (CD29; supplemental Figure 3H; compare with I (SDF-1α control)). Importantly, the Ang-2 antagonizing molecule Ang-1 was not involved in mediating CD14+ monocyte adhesion (supplemental Figure 4A) or the recruitment of myeloid cells in the inflammation models (ie, peritonitis in Ang-2 DT mice; supplemental Figure 4B-C).
We conclude from our results that Ang-2 promoted the adhesion of monocytes on ICAM-1 in a β2-integrin–dependent manner. In summary, by using a genetic gain-of-function mouse model, we identified Ang-2 as a novel, endothelial-derived regulator of myeloid cell infiltration.
Discussion
The study was based on our observation that Ang-2 is strongly up-regulated in human inflammatory diseases. Ang-2 was predominantly detected in the vasculature of biopsy specimens that were accompanied by strong infiltration of myeloid cells. As such, high numbers of monocytes/macrophages were found nearby Ang-2–expressing vessels. With the aid of a genetic mouse model, we furthermore demonstrated that Ang-2 mediates the onset of inflammation. As in human inflammatory diseases, we observed that Ang-2 expression in the vasculature of transgenic mice18 correlated with susceptibility to inflammatory reactions in models of contact hypersensitivity and peritonitis. Under physiologic conditions without external insults, prolonged, autocrine expression of Ang-2 in endothelial cells led to an increased accumulation of hematopoietic cells in numerous organs. Our analyses revealed the preferential infiltration of myeloid cells. Mechanistically, using fluorescence intravital video microscopy and in vitro flow assays, we provide evidence that the increased cell adhesion mediated by β2-integrins is responsible for the observed effects.
We initially aimed to generate an inducible mouse model to study physiologic consequences of autocrine Ang-2 signaling in pathologic models, such as ischemia18 and in experimental tumors.21 Endothelial cells are known to be the predominant source of Ang-2.12 In the literature, Ang-2 signaling in vivo has been studied intensively, however, mainly by overexpression in tumor cells22 or using adenoviruses leading to widespread cellular expression.23-27 In the genetic model used within this study, Ang-2 expression is predominant in the vasculature, the physiologic site of Ang-2 expression, and as such provides an excellent tool to investigate angiopoietin signaling in vivo.
To mechanistically dissect the relative contribution of endothelial Ang-2 expression for (myeloid) cell recruitment in vivo, we exposed transgenic animals to prolonged expression of Ang-2. We observed a strong phenotype associated with skin erythema starting at 6 months of transgene expression. Investigation of the vasculature in Ang-2 transgenic mice suggested that the skin erythema may be caused by increased numbers of immature vessels. Thus, Ang-2 signaling in endothelial cells in vivo resulted in the anticipated vascular phenotype induced by Ang-2.1,22 Interestingly, elevated numbers of infiltrating CD45+ hematopoietic cells were prominent in numerous organs of Ang-2 transgenic mice. Our analyses revealed a predominant infiltration of monocytes/macrophages. This novel finding suggests that Ang-2 expression in endothelial cells led to paracrine activation of myeloid cells. Importantly, vascular Ang-2 expression was also strongly associated with human inflammatory diseases that are dominated by monocytes/macrophage. As such, our observations in the genetic mouse model prove clinical relevance and suggest Ang-2 as a potential target for therapeutic intervention.
Angiopoietin signaling has previously been linked to inflammation.7 In detail, Ang-1 has been shown to act anti-inflammatory in transgenic mice using skin inflammatory models.13,14 Our own previous studies performed in Ang-2–deficient mice demonstrated a delayed onset of inflammation.6 Similarly, Ang-2 blockade using 2 different antibodies decreased the influx of inflammatory cells in a model of airway inflammation.28 However, until now, there was no physiologic evidence that Ang-2 will actually promote inflammation. Within the study described here, we provide clear evidence that enduring endothelial expression of Ang-2 in transgenic mice led to profound recruitment of inflammatory cells. Moreover, the recruitment was restricted to cells from the myeloid lineage and occurred in all mouse organs investigated.
The recruitment of hematopoietic or “accessory” cells induced by growth factors has been previously described.5 In the study by Grunewald et al, organ-specific expression of VEGF in mice induced the release of SDF-α from endothelial cells, which then in turn attracted hematopoietic cells to localize next to vessels.5 Those cells contributed to angiogenesis by the secretion of additional amounts of VEGF. Although we performed numerous protein arrays and quantitative PCR analyses (data not shown), we did not detect altered growth factor/cytokine levels or changes in adhesion receptors. Furthermore, our flow chamber assays represent an endothelial cell–depleted system that clearly demonstrate a direct paracrine effect of Ang-2 on the adhesion of monocytes. We previously reported a possible regulatory effect of Ang-2 on endothelial cell adhesion molecule expression (ICAM-1) in vitro on TNF-α stimulation.6 In vivo, paracrine and autocrine signals will presumably act in concert and, as a consequence, lead to a profound inflammatory response.
The mechanism of integrin activation mediated by Ang-2 is currently not resolved. We excluded the possibility of integrin-affinity changes mediated by Ang-2, as an antibody recognizing the intermediate affinity state of β2-integrin (mAb24;29 ) did not significantly bind CD14+ monocytes after stimulation with Ang-2 (compared with MnCl2 control; data not shown). In addition, chemotactic cytokines (chemokines) appeared not to be involved in the Ang-2–mediated monocyte adhesion. In the flow chamber model, adhesion of CD14+ cells could not be blocked by pretreatment with pertussis toxin. Pertussis toxin inhibits G-protein coupled (chemokine) receptor signaling, which results in changes of integrin affinity. Whether the binding of Ang-2 to integrins leads to a regulation of integrin avidity (receptor clustering) remains to be investigated.
Besides, it has previously been reported that Ang-1 and Ang-2 are able to promote cell adhesion via binding to integrins.30-35 The highly conserved fibrinogen-like domain of angiopoietins thereby implies a functional association with the integrin receptor family.36 Binding can occur in Tie2 receptor-dependent and -independent manners and has been attributed to various cell types (ie, endothelial cells, tumor cells, fibroblasts, myocytes, and monocytes30-35 ). Given that the frequency of Tie2-positive cells is not increased in Ang-2 transgenic mice during inflammation (peritonitis, supplemental Figure 2C) and that the abundance of Tie2 expression on human CD14+ monocytes is low,37 integrins may function as potential binding partners of Ang-2 during monocyte adhesion in our system. A differential binding of Ang-2 to specific integrins subunits may also represent a possible explanation for the specific recruitment of myeloid cells. In the course of this study, we identified β2-integrin (but no β1-integrin) to be essential for Ang-2–mediated monocyte/macrophage adhesion on ICAM-1. Although α-integrins (αL, αM, both expressed on monocytes) partially mediated cell adhesion, we excluded the involvement of lymphocyte-derived (αL) in the adhesion event as CD14− cells (including lymphocytes) did not readily bind to ICAM-1 in the presence of Ang-2. Similarly, Ang-1 had no effect on adhesion triggering. This finding is in line with the antagonizing role of Ang-1 on Ang-2.
In addition, the preferential recruitment of myeloid cells may simply reflect the increased frequency of myeloid cells that we detected in the peripheral blood of Ang-2 transgenic mice. Ang-1 has been reported to promote the retention of hematopoietic cells in the bone marrow.38 In our genetic mouse model, it is therefore possible that Ang-2 promotes increased mobilization of hematopoietic cells from the bone marrow into the blood. In conclusion, we provide evidence that continuous, endothelial expression of Ang-2 provokes the preferential recruitment of myeloid cells. This finding applies to inflammatory diseases in humans that are predominated by myeloid cells and to inflammation models (ie, DTH, peritonitis) conducted in Ang-2 transgenic mice. Mechanistically, the effect is attributed to increased monocyte/macrophage adhesion mediated via β2-integrins presumably in a Tie2-independent manner. Consequently, we defined Ang-2 as a novel, endogenous growth factor that is involved in the onset of inflammation.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank S. Jesgarek for her excellent technical support.
K.H.P., Y.R., and E.C. were supported by the German Research Council (Sonderforschungsbereich/Transregio 23, projects C1 and A2) and the Excellence Cluster Cardiopulmonary Systems. R.H. was supported by the Sonderforschungsbereich 834 of the German Research Council.
Authorship
Contribution: A.S. performed research and analyzed data; A.S., V.L., and R.H. performed flow chamber experiments; M.C. and P.V. performed and analyzed intravital microscopy experiments; E.C. provided support with experiments to assess integrin activation; J.D. provided support with immunohistochemistry of human material; P.N.H. and M.M. provided support with analysis and interpretation of human data; D.J.D. provided the mouse model; Y.R. and K.H.P. designed the study; and A.S., Y.R., and K.H.P. interpreted data and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yvonne Reiss, Edinger Institute/Institute of Neurology, Heinrich-Hoffmann-Strasse 7, 60528 Frankfurt, Germany; e-mail: yvonne.reiss@kgu.de.
References
Author notes
K.H.P. and Y.R. contributed equally to this study.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal