Abstract
During development, natural killer (NK) cells exit the BM to reach the blood. CXCR4 retains NK cells in the BM, whereas the sphingosine-1 phosphate receptor 5 (S1P5) promotes their exit from this organ. However, how the action of these receptors is coordinated to preserve NK-cell development in the BM parenchyma while providing mature NK cells at the periphery is unclear. The role of CXCR4 and S1P5 in NK-cell recirculation at the periphery is also unknown. In the present study, we show that, during NK-cell differentiation, CXCR4 expression decreases whereas S1P5 expression increases, thus favoring the exit of mature NK cells via BM sinusoids. Using S1P5−/− mice and a new knockin mouse model in which CXCR4 cannot be desensitized (a mouse model of warts, hypogammaglobulinemia, infections, and myelokathexis [WHIM] syndrome), we demonstrate that NK-cell exit from the BM requires both CXCR4 desensitization and S1P5 engagement. These 2 signals occur independently of each other: CXCR4 desensitization is not induced by S1P5 engagement and vice versa. Once in the blood, the S1P concentration increases and S1P5 responsiveness decreases. This responsiveness is recovered in the lymph nodes to allow NK-cell exit via lymphatics in a CXCR4-independent manner. Therefore, coordinated changes in CXCR4 and S1P5 responsiveness govern NK-cell trafficking.
Introduction
Natural killer (NK) cells are lymphocytes of the innate immune system that are involved in the early control of infections by viruses and intracellular bacteria or parasites.1 They can kill cells recognized as targets through a battery of surface receptors2 and also produce large amounts of cytokines such as IFN-γ upon activation.1 Several NK-cell subsets have been described on the basis of surface expression of the TNF superfamily member CD27 and the integrin CD11b: CD11blowCD27low (thereafter called double negative or “DN”), CD11blowCD27high NK cells (“CD11blow”), CD11bhighCD27high (double positive or “DP”), and CD11bhighCD27low (“CD27low”).3,4 We previously found that NK-cell maturation is a 4-stage process that starts at the DN stage and follows the pathway DN → CD11blow → DP → CD27low.3 DN NK cells are very rare and immature and could arguably correspond to precursors. All 3 other subsets are quite abundant in lymphoid organs, but their distribution is very different: CD11blow NK cells are mostly located within the BM and lymph nodes (LNs); CD27low NK cells are mostly found in the blood, the spleen, and nonlymphoid organs such as liver and lung; and DP NK cells are more evenly distributed in these organs.5 NK cells develop mainly in the BM. Like other lymphocytes, they are thought to reach the blood circulation via venous sinusoids.6 Once in the periphery, they can also enter the LNs through a CD62L-dependent process.7 Exit from the LNs presumably occurs via medullar sinusoids that connect to efferent lymphatics.6 The chemotactic receptors that control the differential distribution of NK-cell subsets are poorly defined but could involve sphingosine-1 phosphate receptor 5 (S1P5) and CXCR4.
We found previously that S1P5, 1 of the 5 receptors used for the chemoattracting lipid S1P, was involved in the trafficking of NK cells among the blood, lymphoid organs (eg, spleen), and peripheral tissues such as liver and lung.5 S1P5 expression is progressively up-regulated during NK-cell maturation.5 Accordingly, we found that the release of CD27low NK cells to the blood was altered more by S1P5 deficiency compared with the other subsets.5 S1P5 is closely related to S1P1, another S1P receptor expressed by T and B cells that promotes egress of these cells from lymphoid organs.6,8,9 Based on this similarity, the expression pattern of S1P5, and the phenotype of S1P5-deficient mice, we hypothesized that S1P5 was involved in the egress of NK cells from lymphoid organs. A recent study by Jenne et al confirmed this hypothesis by comparing NK cells present in the thoracic duct of wild-type (WT) and S1P5-deficient mice.10 However, among other open questions, it is still unclear which NK cells are affected by S1P5 deficiency, whether S1P5 is important for exit from both the BM and LNs, and, if so, what is the precise mechanism by which S1P5 promotes NK-cell exit with respect to other receptors regulating this process.
The administration of AMD3100, a selective CXCR4 antagonist, to mice has been shown to increase the number of blood and spleen NK cells, presumably by recruiting them from the BM.11 Other NK-cell reservoirs could also be involved, because CXCL12, the CXCR4 ligand, is expressed in peripheral organs such as the LNs.12 This initial study suggested that CXCR4-mediated retention has to be overcome for NK cells to exit BM,11 and therefore opened the possibility that CXCR4 and S1P5 regulate NK-cell exit from the BM in opposite directions (ie, retention in the BM vs exit to the blood). Several nonmutually exclusive models can be considered for the molecular control of NK-cell exit: (1) CXCR4 desensitization occurs in the BM because of high local concentrations of CXCL12 and another exit signal is provided by S1P5 engagement; (2) CXCR4 is not desensitized but S1P5 transduces unique signals that overcome CXCL12/CXCR4-dependent signaling to promote exit; and (3) S1P5 engagement by S1P induces heterologous desensitization of CXCR4 to promote NK-cell exit. Heterologous desensitization of G protein–coupled receptors has been described in various settings.13 In the present study, we set up several experimental models to discriminate between these possibilities. We demonstrate that NK-cell exit from the BM requires 2 signals, CXCR4 desensitization and S1P5 engagement. These events are coordinated but not dependent on each other. Indeed, S1P5 engagement had no effect on CXCR4 responsiveness and vice versa. The egress capacity of NK cells increased with the maturation status in both the BM and LNs. CD27low NK cells were more affected by S1P5 deficiency than the other subsets. Reciprocally, immature NK cells were more efficiently retained by CXCR4 in the BM. Our results support a model in which CXCR4 and S1P5 responsiveness are cyclically modulated during NK-cell trafficking and maturation to promote exit from the BM or entry into lymphoid organs.
Methods
Mice and reagents
C57BL/6 mice were purchased from Charles River Laboratories. S1P5−/− mice (12 generations backcrossed to C57BL/6) have been described previously14 and were bred in our pathogen-free breeding facility. The generation of Cxcr4+/1013 mice has been described elsewhere (K.B., E. Brotin, V.B., L. Bouchet-Delbos, E. Lainey, O. Fenneteau, L. Fiette, D. Emilie, and F. Bachelerie, unpublished data, June 2011). Briefly, heterozygous mice were engineered following a gene knockin strategy: the homologous recombination of a mouse mutated Cxcr4 gene (CT > GA) reproducing the CXCR41013 mutation we described in a warts, hypogammaglobulinemia, infections, and myelokathexis (WHIM) syndrome (WS) pedigree,15 which leads to the truncation of the last 15 residues of the C-tail domain. Generated Cxcr4+/1013 mice were backcrossed for > 10 generations to a C57BL6/J background (> 98%). All mice used in this study were between 6 and 10 weeks old. AMD3100 (Sigma-Aldrich) was injected intraperitoneally (150 μg/mouse). Experimental procedures and mice housing were approved by the Inserm Ethics Committee for Animals and carried out according to French and European laws.
In vivo labeling of sinusoidal lymphocytes
Immunofluorescence
Immunofluorescence was carried out on 10-μm-thick serial frozen sections. Sections were fixed with acetone and stained with anti-LYVE1 (rat monoclonal; R&D Systems) and anti-NKp46 (goat Ab; R&D Systems) Abs, followed by staining with the appropriate Alexa Fluor 488– and Alexa Fluor 647–coupled secondary Abs (Invitrogen). Slides were analyzed by confocal microscopy (LSM 510; Zeiss) using a 40× objective at room temperature.
Abs and flow cytometry
BM, LNs, spleen, and blood cells were isolated and stained as described previously.5 The following mAbs from eBioscience were used: anti-CD27 (LG.3A10), anti-CD3 (2C11), anti-NK1.1 (PK136), anti-CD11b (M1/70), anti-CXCR4 (2B11), and relevant isotype controls. Flow cytometry was carried out on a FACSCanto or FACS LSR II analyzer (BD Biosciences).
Chemotaxis assays
Spleen, BM, or blood cells were suspended in RPMI 1640 medium supplemented with 4 mg/mL of fatty acid-free bovine albumin (Sigma-Aldrich). The same medium was used to prepare S1P (Sigma-Aldrich) at 10−8M or CCL5 at 50 ng/mL (R&D Systems) or CXCL12 at 50 ng/mL (kindly provided by Dr F. Baleux, Institut Pasteur) unless otherwise specified. Cell migration was analyzed in Transwell chambers (Costar) with 5-μm pore-width polycarbonate filters. Transmigrated cells were stained for CD3, NK1.1, CD27, and CD11b and counted by flow cytometry as described previously.5 In some experiments, BM cells were cultured at 5 × 106 cells/mL for 2 hours in RPMI 1640 medium supplemented with 4 mg/mL of fatty acid-free bovine albumin in the absence or presence of either CXCL12 (50 ng/mL) or S1P (10−8M), before the migration assay.
Internalization assays
CXCR4 internalization was studied as described previously15 with some minor modifications. Briefly, 1 × 106 BM cells were incubated at 37°C for 45 minutes with various concentrations of CXCL12. After one wash in acidic glycine buffer (pH = 4.3), levels of CXCR4 cell-surface expression were determined using the PE-conjugated 2B11 mAb in combination with fluorescent mAbs specific for CD3, CD11b, CD27, and NK1.1 antigens to delineate NK-cell subsets. Background fluorescence was evaluated using the corresponding PE-conjugated, Ig-isotype control Ab. No receptor internalization was found when BM cells were incubated at 4°C in the presence of 250nM CXCL12. CXCR4 expression in stimulated cells was calculated as follows: (CXCR4 geometric MFI of treated cells/CXCR4 geometric MFI of unstimulated cells) × 100; 100% correspond to receptor expression at the surface of cells incubated in medium alone.
Statistical analyses
Statistical analyses were performed using 2-tailed t tests run on Excel Version 12.3.1 (Microsoft) or Prism Version 5 (GraphPad) software. Levels of significance are expressed as follows: *P < .05; **P < .01; and ***P < .001.
Results
In vivo analysis of NK-cell exit from BM and LNs
Lymphocytes exit central and peripheral lymphoid organs through sinusoids that connect either to the blood circulation (for BM) or to efferent lymphatics (for LNs).6 To study the exit of NK cells from lymphoid organs, we used a recently described procedure that allows the discrimination of parenchymal and sinusoidal cells in the BM.16,17 In this procedure, IV injection of anti-CD45 Ab labels circulating cells, including BM sinusoidal cells. Mice are rapidly killed before the Ab can diffuse to the parenchyma of lymphoid organs. As shown in Figure 1A, a significant number of BM NK cells were labeled after anti-CD45 injection. We also observed that a similar fraction of NK cells was labeled in the LNs (Figure 1A), suggesting that the anti-CD45 Ab also stained lymphocytes in the process of leaving the LNs. Lymphocyte egress from LNs is thought to occur at the medullary sinuses that connect to the efferent lymphatics. Moreover, lymphocytes can initiate exit from the LNs via cortical sinuses present at the border between T- and B-cell areas that will eventually lead them to the medullary sinuses.18 Both medullary and cortical sinuses are positive for the lymphatic endothelial cell marker LYVE-1.18 To identify the LN compartment labeled with the anti-CD45 Ab during the in vivo procedure, we stained LN sections from anti-CD45–treated mice for LYVE-1 and NKp46 (a NK-cell marker) expression. As shown in supplemental Figure 1A (available on the Blood Web site; see the Supplemental Materials link at the top of the online article), most anti-CD45–labeled cells are present in the LYVE-1 positive medullar region of the LNs in the same area as NK cells. Moreover, many of the anti-CD45–stained cells are located within vessel-like structures that could correspond to efferent lymphatics (white arrows on supplemental Figure 1A-B). In these structures, NK cells doubly stained with anti-CD45 and anti-NKp46 are visible (supplemental Figure 1B). We also observed that some anti-CD45–labeled cells were present in the T-cell cortex around LYVE-1–positive structures that are likely cortical sinuses (supplemental Figure 1A,C). These results show that IV-injected anti-CD45 Ab mainly labels lymphocytes exiting LN via medullar and cortical sinusoids, allowing us to analyze NK-cell exit from both the BM and LNs using this technique.
Phenotypic analysis of parenchymal and sinusoidal NK cells. Flow cytometric analysis of BM and LN NK cells isolated from WT mice injected with anti-CD45 and then stained in vitro with Abs for CD3, NK1.1, CD27, and CD11b. Numbers above bracketed lines or in quadrants indicate the percentage of cells in each area. (A) CD45 labeling of gated NK cells (NK1.1+ CD3−). (B-C) Analysis of CD45 staining in NK-cell subsets defined by CD27 and CD11b expression. (B) Representative FACS plot of CD27/CD11b expression in gated CD45− (top panels) and CD45+ (bottom panels) NK cells. (C) Mean ± SD percentage of CD45− (parenchymal, black bars) and CD45+ (sinusoidal, white bars) cells among CD11blow, DP, and CD27low NK cells calculated from the analysis of 10 mice.
Phenotypic analysis of parenchymal and sinusoidal NK cells. Flow cytometric analysis of BM and LN NK cells isolated from WT mice injected with anti-CD45 and then stained in vitro with Abs for CD3, NK1.1, CD27, and CD11b. Numbers above bracketed lines or in quadrants indicate the percentage of cells in each area. (A) CD45 labeling of gated NK cells (NK1.1+ CD3−). (B-C) Analysis of CD45 staining in NK-cell subsets defined by CD27 and CD11b expression. (B) Representative FACS plot of CD27/CD11b expression in gated CD45− (top panels) and CD45+ (bottom panels) NK cells. (C) Mean ± SD percentage of CD45− (parenchymal, black bars) and CD45+ (sinusoidal, white bars) cells among CD11blow, DP, and CD27low NK cells calculated from the analysis of 10 mice.
We analyzed the CD11b/CD27 phenotype of sinusoidal versus parenchymal NK cells. As described previously3,4 and as depicted in Figure 1B, the 4 NK-cell subsets could be discriminated based on CD11b/CD27 surface levels. We focused our attention on the 3 main subsets, CD11blow, DP, and CD27low NK cells, as defined at top left panel in Figure 1B. We observed that sinusoidal NK cells were strongly enriched for the CD27low subset compared with parenchymal NK cells in both organs (Figure 1B-C), indicating that CD27low NK cells had an improved capacity to exit lymphoid organs. These findings are consistent with our previous data5 showing that only CD27low NK cells are capable of responding efficiently to S1P, an exit signal for lymphocytes. Interestingly, a significant number of CD11blow NK cells was capable of exiting the BM, suggesting that immature NK cells can complete their maturation program in the periphery.
S1P5 is more important for mature NK-cell exit from the LNs than from the BM
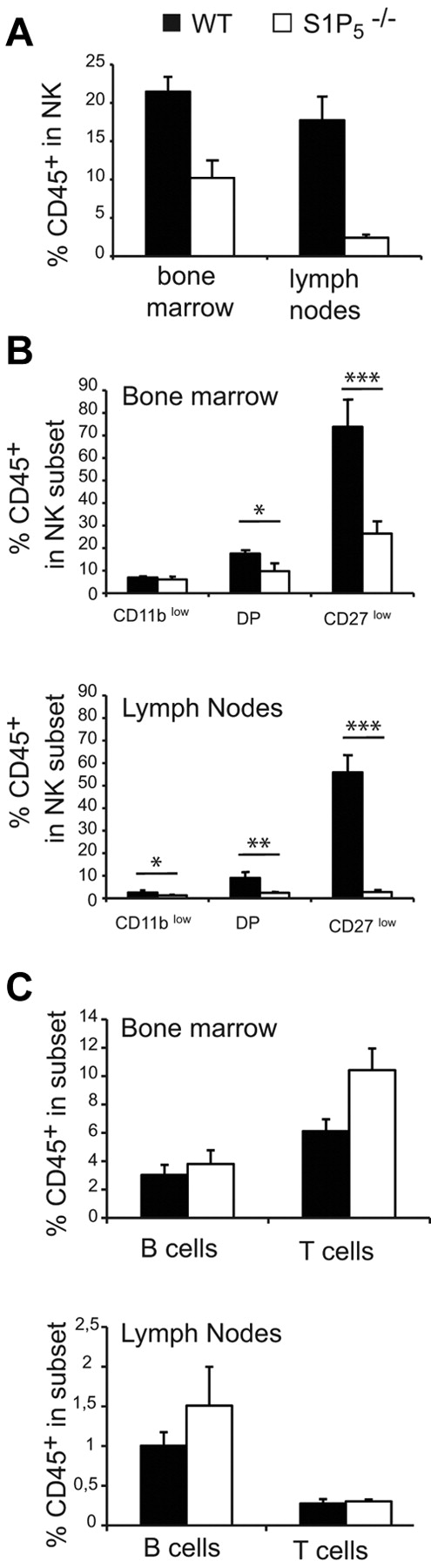
To address the role of S1P5 in NK-cell exit from the BM and LNs, we compared the percentage of sinusoidal NK cells in the BM and LNs of WT and S1P5−/− mice. We observed a decreased percentage of CD45+ cells (ie, sinusoidal) among NK cells in S1P5−/− mice for both BM and LNs (Figure 2A). The percentage of CD45+ NK cells was close to zero in S1P5−/− LNs, showing that S1P5 is likely more important for NK-cell exit from the LNs than for their exit from the BM. All NK-cell subsets were affected in their egress capacity by S1P5 mutation (Figure 2B). However, the dependency on S1P5 increased with NK-cell maturation, which we showed previously was correlated with the level of S1P5 expression.5 This was particularly true for CD27low NK cells, which were almost undetectable in the LN sinusoids of S1P5−/− mice. The role of S1P5 was NK specific, because no significant difference was observed between WT and S1P5−/− mice in the percentage of sinusoidal B or T cells either in the BM or the LNs (Figure 2C).
S1P5 is required for NK-cell trafficking to BM and LN sinusoids. Flow cytometry analysis of BM and LN NK cells isolated from WT and S1P5−/− mice injected with anti-CD45 and then stained in vitro with Abs for CD19, CD3, NK1.1, CD27, and CD11b. The percentage of CD45+ cells among gated NK cells (A), NK-cell subsets (B), or B and T cells (C) was measured in WT (black bars) and S1P5−/− mice (white bars). Data show the mean ± SD of 5-10 mice in each group.
S1P5 is required for NK-cell trafficking to BM and LN sinusoids. Flow cytometry analysis of BM and LN NK cells isolated from WT and S1P5−/− mice injected with anti-CD45 and then stained in vitro with Abs for CD19, CD3, NK1.1, CD27, and CD11b. The percentage of CD45+ cells among gated NK cells (A), NK-cell subsets (B), or B and T cells (C) was measured in WT (black bars) and S1P5−/− mice (white bars). Data show the mean ± SD of 5-10 mice in each group.
These results confirm the role of S1P5 in NK-cell egress from lymphoid organs and further demonstrate that: (1) S1P5 is more important for the egress of mature NK cells and (2) S1P5 is more important for NK-cell egress from the LNs than it is for their egress from the BM.
CXCR4 strongly retains immature NK cells in the BM
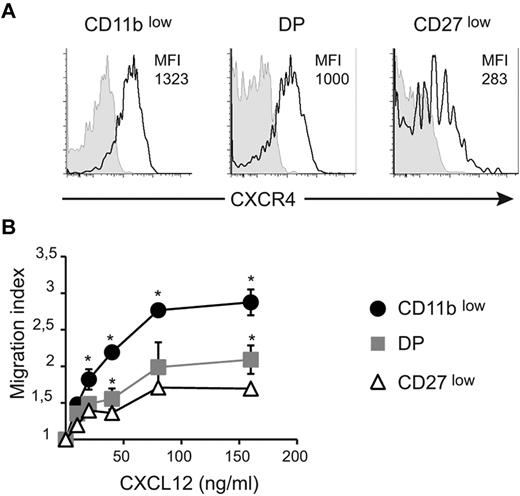
We next sought to investigate the role of CXCR4 in the regulation of NK-cell exit from the BM and LNs. Previous reports showed that all NK cells responded to CXCL12 and that CXCR4 was involved in NK-cell retention in the BM.4,11 CXCL12 is also expressed in LN parenchyma12 and could thus potentially mediate NK-cell retention. We first compared the surface level of CXCR4 in BM NK-cell subsets. Results in Figure 3A show that the level of CXCR4 was lower on CD27low NK cells than on the other NK-cell subsets, whereas DP NK cells displayed an intermediate level. Similar results were obtained for NK cells from other organs (ie, spleen and blood; supplemental Figure 2). To determine whether this difference in CXCR4 level was functionally relevant, we next measured the ex vivo chemotactic response of NK-cell subsets to graded doses of CXCL12. As shown in Figure 3B, CD11blow NK cells displayed increased migratory responses at all concentrations tested relative to the other NK-cell subsets, indicating a higher efficiency of chemotaxis to CXCL12. These findings are consistent with the increased cell-surface expression of CXCR4 observed on these cells. DP NK cells tended to respond better than CD27low NK cells. Therefore, responsiveness to CXCL12 is inversely correlated with NK-cell maturation.
CXCR4 surface level and responsiveness to CXCL12 decrease during NK-cell maturation. (A) BM cells were stained for NK1.1, CD3, CD27, CD11b, and CXCR4 or isotype control. Expression of CXCR4 (black line) or isotype (gray histogram) by gated NK-cell subsets is shown, as indicated. Numbers above histograms indicate mean fluorescence intensity (MFI) of CXCR4 staining minus MFI of isotype control. Data show representative results of 3 independent experiments. (B) Transwell assay of the migration of NK-cell subsets assessing movement toward different concentrations of CXCL12, as indicated. The migration index is calculated as the ratio between the number of cells migrating in the chemokine and in the control (without chemokine) condition. Data are the mean ± SD of 3 independent experiments with duplicates.
CXCR4 surface level and responsiveness to CXCL12 decrease during NK-cell maturation. (A) BM cells were stained for NK1.1, CD3, CD27, CD11b, and CXCR4 or isotype control. Expression of CXCR4 (black line) or isotype (gray histogram) by gated NK-cell subsets is shown, as indicated. Numbers above histograms indicate mean fluorescence intensity (MFI) of CXCR4 staining minus MFI of isotype control. Data show representative results of 3 independent experiments. (B) Transwell assay of the migration of NK-cell subsets assessing movement toward different concentrations of CXCL12, as indicated. The migration index is calculated as the ratio between the number of cells migrating in the chemokine and in the control (without chemokine) condition. Data are the mean ± SD of 3 independent experiments with duplicates.
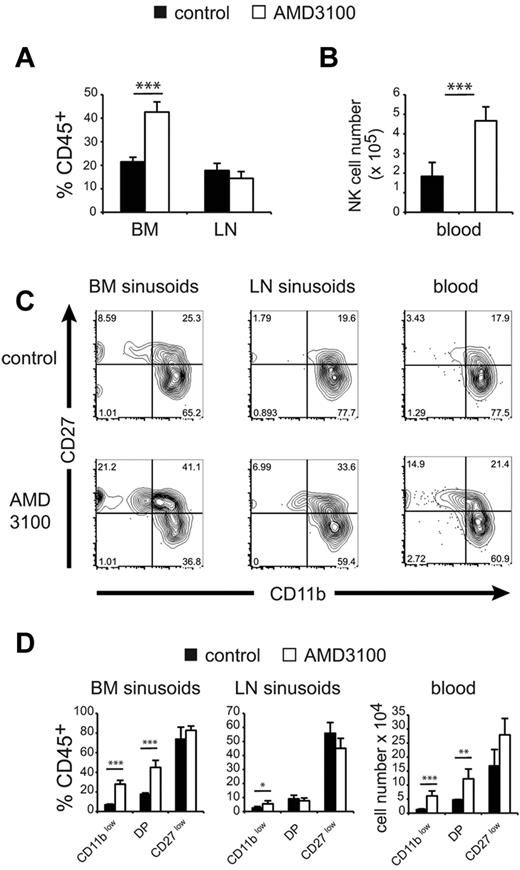
To investigate the in vivo role of CXCR4 in the retention of NK-cell subsets in the BM and LN parenchymas, we used the selective CXCR4 inhibitor AMD3100.19 We treated mice with 150 μg of AMD3100 and compared the number of NK cells of each subset in the different compartments 1 hour after treatment. AMD3100 treatment induced the recruitment of BM parenchymal NK cells to the sinusoids (Figure 4A) and the blood (Figure 4B). No change was observed for total NK cells in LN sinusoids (Figure 4A). When we looked at the CD11b/CD27 phenotype of NK cells in BM and LN sinusoids and in the blood (Figure 4C), we observed that AMD3100 changed the representation of NK-cell subsets so that the percentage of immature NK cells increased in all of these compartments. The effect of AMD3100 was the most pronounced on CD11blow NK cells in the BM, and to a lesser extent in the LNs. DP NK cells were only affected in BM sinusoids and in the blood, whereas CD27low NK cells were not significantly modified by AMD3100 treatment (Figure 4C-D).
CXCR4 inhibition recruits immature NK cells in BM sinusoids and the peripheral blood. Flow cytometric analysis of BM, LNs, and blood NK cells in WT mice treated for 1 hour with saline (control) or AMD3100 and injected intravenously with PE-conjugated anti-CD45 mAb for the final 2 minutes. (A) Mean ± SD percentage of CD45+ cells in gated NK cells in each group, as indicated. (B) Mean ± SD number of NK cells among PBMC (for 1 mL of blood) in each group, as indicated (n = 5 mice in each group). (C) Representative FACS plot of CD27/CD11b expression in gated CD45+ (sinusoidal) NK cells from BM and LNs and in gated blood NK cells in mice of each group, as indicated. (D) Mean ± SD percentage of CD45+ sinusoidal cells within each NK-cell subset and in mice of each group, as indicated (n = 5 mice in each group).
CXCR4 inhibition recruits immature NK cells in BM sinusoids and the peripheral blood. Flow cytometric analysis of BM, LNs, and blood NK cells in WT mice treated for 1 hour with saline (control) or AMD3100 and injected intravenously with PE-conjugated anti-CD45 mAb for the final 2 minutes. (A) Mean ± SD percentage of CD45+ cells in gated NK cells in each group, as indicated. (B) Mean ± SD number of NK cells among PBMC (for 1 mL of blood) in each group, as indicated (n = 5 mice in each group). (C) Representative FACS plot of CD27/CD11b expression in gated CD45+ (sinusoidal) NK cells from BM and LNs and in gated blood NK cells in mice of each group, as indicated. (D) Mean ± SD percentage of CD45+ sinusoidal cells within each NK-cell subset and in mice of each group, as indicated (n = 5 mice in each group).
These data show that, in BM, CXCR4-induced retention of NK cells decreases on maturation, which presumably favors S1P5 responsiveness and thus the exit of more mature NK cells. In the LNs, only the most immature NK-cell subset is affected by inhibition of CXCR4 signaling.
CXCR4 desensitization is required for NK-cell exit from the BM but not the LNs
Experiments using AMD3100 showed that CXCR4 retained NK cells in the BM parenchyma. We next sought to understand how NK cells alleviated this retention to leave the BM. We hypothesized that for NK cells to exit the BM, CXCR4 had to be inactivated—that is, uncoupled from G proteins (or desensitized) and internalized. To test this hypothesis, we used a new mouse model in which CXCR4 desensitization is impaired. Heterozygous Cxcr4+/1013 mice were generated following a gene knockin strategy and harbor the CXCR41013 punctual mutation we described previously in a WS pedigree.15 WS is a rare combined immunodeficiency disorder characterized by disseminated HPV infection–induced warts, hypogammaglobulinemia, recurrent bacterial infections, and myelokathexis. Many cases have been linked to inherited heterozygous autosomal-dominant mutations in CXCR4 that result in distal truncations of the receptor's C-tail.20 In WS patient leukocytes, truncated CXCR4 displays an enhanced and sustained activation of G proteins upon CXCL12 stimulation, which is associated with its inability to be both desensitized and internalized and is not a result of increased CXCR4 expression at the membrane. In keeping with this, membrane expression levels of CXCR4 were found to be in the same range between BM NK cells from Cxcr4+/1013 and WT mice (supplemental Figure 3A). However, Cxcr4+/1013 NK cells displayed an increased migration to CXCL12 gradients at all doses tested and irrespective of the NK-cell subset (supplemental Figure 3B-C). Such enhanced responsiveness of Cxcr4+/1013 NK cells to CXCL12 was associated with an impairment of CXCR4 to be internalized after CXCL12 stimulation (supplemental Figure 3D-E). Therefore, knockin Cxcr4+/1013 mice recapitulate the pattern of CXCR4 dysfunctions observed in WS patients.
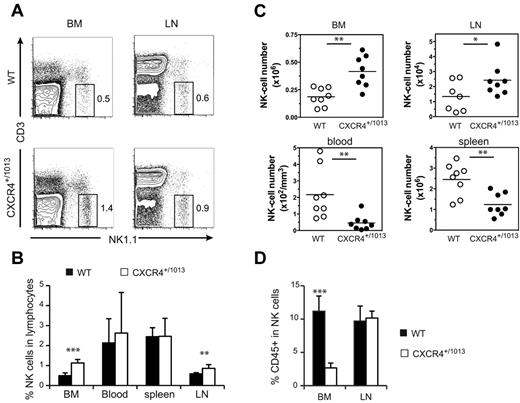
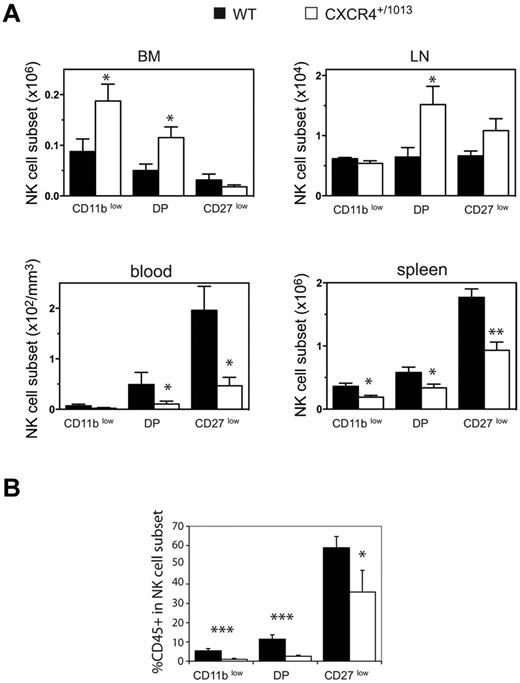
We compared the distribution of NK cells in WT and Cxcr4+/1013 mice, and detected an increased frequency of NK cells among lymphocytes in both the BM and LNs of Cxcr4+/1013 mice (Figure 5A-B). Moreover, the number of NK cells was also increased in these organs, whereas it was strongly reduced in the blood and spleen (Figure 5C). The fraction of sinusoidal CD45+ NK cells was also significantly reduced in the BM of Cxcr4+/1013 mice, but it was preserved in the LNs (Figure 5D). These results indicate that CXCR4 desensitization is required for NK-cell exit from the BM but not from the LNs. Focusing on NK-cell subsets, we found that CD11blow and DP NK cells accumulated in the BM, whereas CD27low NK cells were not affected (Figure 6A). Accordingly, the sinusoidal fraction of CD11blow and DP NK cells was strongly reduced and that of CD27low NK cells was less affected (Figure 6B). In the blood, the number of all NK-cell subsets was reduced, including CD27low NK cells (Figure 6A). These results show that CXCR4 desensitization was required for the exit from the BM of all NK-cell subsets, but particularly for the most immature ones. In the LNs, CXCR4 appears to be dispensable for NK-cell trafficking.
NK cells accumulate in the BM and LNs of Cxcr4+/1013 mice. Flow cytometric analysis of NK cells in the blood, spleen, LNs, and BM from WT and Cxcr4+/1013 mice stained for CD3 and NK1.1 expression. (A) Representative dot plots of CD3/NK1.1 expression. (B-C) Mean frequency (B) and number of gated NK cells (C; NK1.1+ CD3−) in the different organs. (D) Flow cytometric analysis of NK cells in the BM and LNs from WT and Cxcr4+/1013 mice injected with anti-CD45 mAb and then stained in vitro with Abs for CD3 and NK1.1. The percentage of CD45+ cells among gated NK cells was measured. Results represent the means ± SD of 7-8 mice in each group (B) with 1 dot-plot representing 1 mouse (A), all analyzed littermates (C, lines indicate the mean and each symbol represents an individual mouse), or are from 4 independent experiments (D).
NK cells accumulate in the BM and LNs of Cxcr4+/1013 mice. Flow cytometric analysis of NK cells in the blood, spleen, LNs, and BM from WT and Cxcr4+/1013 mice stained for CD3 and NK1.1 expression. (A) Representative dot plots of CD3/NK1.1 expression. (B-C) Mean frequency (B) and number of gated NK cells (C; NK1.1+ CD3−) in the different organs. (D) Flow cytometric analysis of NK cells in the BM and LNs from WT and Cxcr4+/1013 mice injected with anti-CD45 mAb and then stained in vitro with Abs for CD3 and NK1.1. The percentage of CD45+ cells among gated NK cells was measured. Results represent the means ± SD of 7-8 mice in each group (B) with 1 dot-plot representing 1 mouse (A), all analyzed littermates (C, lines indicate the mean and each symbol represents an individual mouse), or are from 4 independent experiments (D).
NK-cell subsets accumulate in the BM parenchyma area of Cxcr4+/1013 mice. (A-B) Flow cytometric analysis of NK-cell subsets in the blood, spleen, LNs, and BM from WT and Cxcr4+/1013 mice stained for CD3, NK1.1, CD11b, and CD27. (A) Number of gated NK cells of each subset in the different organs. (B) WT and Cxcr4+/1013 mice were injected with anti-CD45 and then stained in vitro for CD3, NK1.1, CD11b, and CD27. The percentage of CD45+ cells among gated NK cells of each subset was assessed in the BM from WT (black bars) and Cxcr4+/1013 mice (white bars). Results represent the means ± SD of 4-5 mice in each group.
NK-cell subsets accumulate in the BM parenchyma area of Cxcr4+/1013 mice. (A-B) Flow cytometric analysis of NK-cell subsets in the blood, spleen, LNs, and BM from WT and Cxcr4+/1013 mice stained for CD3, NK1.1, CD11b, and CD27. (A) Number of gated NK cells of each subset in the different organs. (B) WT and Cxcr4+/1013 mice were injected with anti-CD45 and then stained in vitro for CD3, NK1.1, CD11b, and CD27. The percentage of CD45+ cells among gated NK cells of each subset was assessed in the BM from WT (black bars) and Cxcr4+/1013 mice (white bars). Results represent the means ± SD of 4-5 mice in each group.
No cross-inhibition between S1P5 and CXCR4 during NK-cell exit from the BM
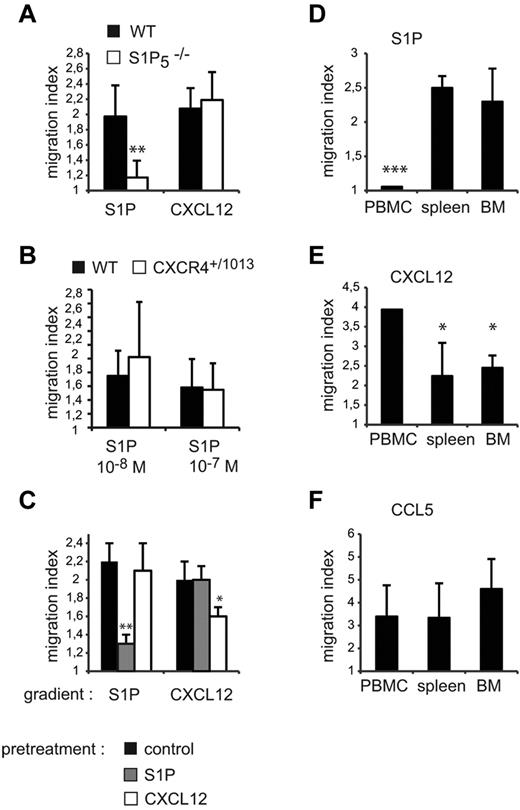
Our results show that CXCR4 and S1P5 have opposite activities in NK cells, promoting either retention or exit from the BM, and that CXCR4 needs to be desensitized to allow egress. We therefore investigated whether one of the effects of S1P5 engagement was to desensitize CXCR4 in a heterologous manner. To address this question, we performed a series of complementary experiments. First, we compared the capacity of WT and S1P5−/− BM NK cells to migrate in response to CXCL12 gradients, reasoning that if S1P5 inhibited CXCR4 response, this response should be increased in S1P5−/− mice. However, as shown in Figure 7A, WT and S1P5−/− NK cells displayed similar migration to CXCL12. Reciprocally, we compared the capacity of BM NK cells from WT and Cxcr4+/1013 mice to migrate toward S1P gradients, and our results showed no difference in migration (Figure 7B). No difference in the S1P-promoted chemotaxis of WT NK cells isolated from control or AMD3100-treated mice was detected either (supplemental Figure 4). We then pre-incubated WT NK cells with either S1P or CXCL12 at doses that would induce maximal NK-cell migration in vitro for 2 hours before measuring S1P- or CXCL12-induced migration. Figure 7C shows that S1P and CXCL12 induced homologous desensitization of their cognate receptors S1P5 and CXCR4, but did not induce heterologous desensitization (ie, S1P on CXCR4 and CXCL12 on S1P5). Therefore, CXCR4 engagement has no effect on S1P5 response and vice versa, indicating that CXCR4 and S1P5 act in a competitive but not cross-inhibitory manner in vivo.
CXCR4 and S1P5 responsiveness are modulated on mature NK cells. (A-B) BM cells were isolated from WT and S1P5−/− (A) or Cxcr4+/1013 (B) mice and migration of CD27low NK cells toward S1P or CXCL12 gradients was measured. (C) Spleen cells from WT mice were incubated for 2 hours in serum-free medium supplemented or not with S1P (10−8M) or CXCL12 (50 ng/mL). Migration of CD27low NK cells toward S1P or CXCL12 gradients was then assessed. (D-F) Lymphocytes were isolated from blood, spleen, and BM. Migration of mature NK cells toward S1P (D), CXCL12 (E), and CCL5 (F) gradients was assayed. The migration index is calculated as the ratio between the number of cells migrating in the chemokine and in the control (without chemokine) condition. Data are the mean ± SD of 4-5 independent experiments with duplicates.
CXCR4 and S1P5 responsiveness are modulated on mature NK cells. (A-B) BM cells were isolated from WT and S1P5−/− (A) or Cxcr4+/1013 (B) mice and migration of CD27low NK cells toward S1P or CXCL12 gradients was measured. (C) Spleen cells from WT mice were incubated for 2 hours in serum-free medium supplemented or not with S1P (10−8M) or CXCL12 (50 ng/mL). Migration of CD27low NK cells toward S1P or CXCL12 gradients was then assessed. (D-F) Lymphocytes were isolated from blood, spleen, and BM. Migration of mature NK cells toward S1P (D), CXCL12 (E), and CCL5 (F) gradients was assayed. The migration index is calculated as the ratio between the number of cells migrating in the chemokine and in the control (without chemokine) condition. Data are the mean ± SD of 4-5 independent experiments with duplicates.
Sequential desensitization of CXCR4 and S1P5 during NK-cell trafficking
Mature NK cells have been shown to recirculate to the BM when intravenously injected,21 suggesting that they could reacquire responsiveness to CXCL12 after leaving the BM. Therefore, we compared the responsiveness of NK cells to S1P and CXCL12 in different compartments (ie, peripheral blood, BM, and spleen). Figure 7D-E shows that the responsiveness of NK cells to CXCL12 and S1P was inversely correlated across lymphoid organs. In particular, NK-cell responsiveness to CXCL12 was higher in the blood, whereas S1P responsiveness was higher in the BM and the spleen but almost null in the blood. Therefore, S1P5 and CXCR4 responsiveness in NK cells is inversely correlated with the concentrations of their respective ligands, S1P and CXCL1, which are known to be high in the blood22 and in the BM,23 respectively. In comparison, responsiveness to CCL5, a pro-inflammatory chemokine not expressed under steady-state conditions, did not vary significantly among the spleen, BM, and blood (Figure 7F). These results show that, during NK-cell trafficking, CXCR4 and S1P5 are sequentially desensitized. The transient decrease in responsiveness to CXCL12 may favor response to S1P and NK-cell exit from the BM. Reciprocally, during recirculation in the blood, NK-cell entry to BM may be favored by a transient decreased responsiveness to S1P together with an increased sensitivity to CXCL12.
Discussion
In the present study, we combined in vivo labeling of circulating lymphocytes with relevant mouse models to investigate the molecular mechanisms involved in NK-cell exit from lymphoid organs. We demonstrated that: (1) NK-cell exit from the BM requires 2 signals, CXCR4 desensitization and S1P5 engagement; (2) CXCR4 desensitization is not induced by S1P5 engagement and reciprocally—rather, we propose that both S1P5 and CXCR4 are sequentially desensitized by their cognate ligands to promote NK-cell trafficking; (3) coordinated changes in CXCR4 and S1P5 expression favor the exit of mature NK cells from the BM to the periphery; and (4) NK-cell exit from the LNs is not regulated by CXCR4 but is completely dependent on S1P5 engagement.
We used in vivo labeling of circulating cells to study NK-cell exit. This procedure was originally described to study lymphocyte exit from the BM16 and more recently from the thymus.17 However, several lines of evidence showed that the injected anti-CD45 mAb also labels lymphocytes exiting the LNs. First, the percentage of lymphocytes labeled in the BM and LNs was comparable. Second, the composition of this labeled fraction was very similar in both organs. In particular, in both organs, CD45 staining was restricted to the most mature NK cells. Third, very few NK cells were CD45+ in the LNs of S1P5−/− mice, which fits very well with previous results showing that S1P5−/− NK cells had a defective capacity to reach the thoracic duct.10 Fourth, visualization of CD45+ cells on LN sections showed that most of them are contained within lymphatic LYVE-1+ structures corresponding to cortical or medullary sinuses. Therefore, although we cannot rule out that CD45+ LN cells also include a few cells entering the LNs via the blood or afferent lymph, our data suggest that most CD45+ LN cells are lymphocytes exiting the LNs via lymphatic sinuses.
Mature CD27low NK cells have an increased capacity to exit lymphoid organs (Figure 1). In fact, almost all CD27low NK cells are found in the circulation or in sinusoids about to enter the blood circulation. This suggests that CD27low NK cells are sentinels constantly patrolling the blood circulation and ready to respond to arising infections. Interestingly, compared with other NK-cell subsets, they are uniquely equipped with CX3CR1,21 which could allow them to interact with endothelial cells upon inflammation24 and to rapidly extravasate within tissues upon infection, similar to monocytes.25 A very recent study confirmed our findings by showing that CX3CR1-positive NK cells are preferentially localized in BM sinusoids.26 Other NK-cell subsets may be recruited from the BM or spleen to inflamed sites at later phases of the response. In particular, previous results showed that CCL3 could overcome CXCL12-induced retention of CD11blow NK cells in the BM and recruit these cells to the blood.11
A previous study showed that S1P5 was required for NK-cell exit from the BM.10 Our present data confirm these findings and further indicate that mature NK cells are more dependent on S1P5 than immature NK cells for their exit. Indeed, CD27low NK cells are very infrequent in BM sinusoids and are virtually absent from LN sinusoids in S1P5−/− mice. This fits well with our previous data showing that S1P5 expression is up-regulated upon NK-cell maturation.5 The lack of NK cells in the BM and LN sinusoids is not because of a defect in their differentiation, but rather results from their abnormal accumulation in BM and LN parenchyma (present results and Walzer et al5 ). The difference between the BM and LNs in terms of S1P5 dependency for exit is unclear at the time, but could be linked to the nature of the exit sinusoids, which are venous for BM and lymphatic for the LNs. Endothelial or other stromal cells in the BM may provide additional exit signals. Moreover, the local S1P concentration could be different in BM and LN sinusoids, as suggested by a previous study showing that separate sources provide S1P to plasma and lymph.27 How does S1P5 function to promote NK-cell exit from lymphoid organs? Several lines of evidence suggest that S1P5 could function similarly to S1P1. In particular, as in the case of S1P1,22 cyclical internalization of S1P5 is likely to occur in vivo during NK-cell trafficking. In agreement with this, we found that NK cells located in blood that contains high S1P concentration are poorly responsive to S1P, unlike BM NK cells, which may favor reentry into lymphoid organs. Like S1P1, S1P5 contains a stretch of serines in the C-terminus region that could be important for receptor internalization.28 However, there are also several important differences between these 2 receptors. For example, S1P1 but not S1P5 contains 2 sites of tyrosine sulfation in its extracellular region that increase affinity for S1P.29 Moreover, S1P1 but not S1P5 is internalized in response to FTY720 treatment,10 which may explain why FTY720 sequesters T cells in lymphoid organs but has no effect on NK-cell trafficking.5
In the BM, a significant number of immature NK cells were capable of reaching sinusoids in an S1P5-independent fashion. These results suggest that receptors other than S1P5 may induce NK-cell exit from the BM. Candidate receptors include S1P1, which has been shown to be expressed at low levels in NK cells and to contribute to NK-cell exit from the BM.10 NK cells also express many receptors for pro-inflammatory chemokines (eg, CCR5 and CXCR3). Expression of low levels of these chemokines may participate in the recruitment of NK cells at the periphery, similar to what has been described for inflammatory monocytes.30
We found that CXCR4 expression progressively decreased from the CD11blow to the CD27low maturation stage. In particular, CD27low NK cells expressed much lower surface levels of CXCR4 (present study) and lower CXCR4 mRNA levels compared with the other subsets.3 The different CXCR4 surface levels in NK-cell subsets was well correlated with their responsiveness to CXCL12, which was found to decrease upon maturation. When we treated mice with AMD3100, a selective CXCR4 antagonist, CD11blow, and to a lesser extent DP but not CD27low, NK cells were recruited to BM sinusoids and peripheral blood. This indicates that CXCR4 retains immature NK cells in the BM parenchyma, presumably to ensure full development of these cells in an optimal environment23 before their release into the periphery. One key question regarding the control of NK-cell exit was the mechanism by which NK cells overcome CXCR4-mediated retention. There were 2 possibilities: either the S1P5 signal was sufficient to induce NK-cell exit or CXCR4 also had to be desensitized. To discriminate between these hypotheses, we used a novel mouse model (Cxcr4+/1013 mice), in which one of the 2 CXCR4 alleles encodes a truncated CXCR4 receptor that cannot be inactivated. The CXCR41013 mutation has been originally described in patients with WS.15 Its failure to desensitize and internalize is thought to result from the distal truncation of the C-tail that removes serine/threonine residues, thus preventing site-specific phosphorylation by GPCR kinases.31 Our results show that CXCR4 desensitization is required for the exit of all NK-cell subsets from the BM. In agreement with experiments using AMD3100, the effect of the CXCR41013 mutation was more pronounced for immature NK cells. Interestingly, the reduction in mature CD27low NK cells was also much more severe in the blood than in the BM sinusoids in these mice. This could be indirectly because of the important reduction in the exit of immature NK cells from the BM. Indeed, NK cells can also mature at the periphery, as shown by adoptive transfer experiments of immature NK cells.3,32 Therefore, a reduction in immature NK cells at the periphery is expected to result in a decrease in mature NK cells. Homeostatic effects could also explain the increase in LN NK cells observed in Cxcr4+/1013 mice that occurs even though NK-cell exit from the LNs is not decreased in these mice. This increase is mostly because of an increase in DP NK cells, which are known to accumulate in conditions of NK-cell proliferation.3,5 Increased CXCR4 signaling could therefore lead to an enhanced NK-cell proliferation in Cxcr4+/1013 LNs. In agreement with this, a recent study showed that CXCL12 increased the number of NK cells obtained in cultures of BM progenitors with IL-15.23
How is CXCR4 desensitized in BM NK cells? Our in vitro and ex vivo results indicate that S1P5 engagement is not the cause of CXCR4 desensitization, but rather is a concomitant and independent event. Therefore, CXCR4 is likely to be desensitized by CXCL12, which is produced at high concentrations in the BM microenvironment next to clusters of developing NK cells.23 A recent study modeling the behavior of cells exposed to competing chemokine gradients concluded that receptor desensitization is in fact crucial for cells to integrate signals from different chemoattractants.33 Therefore, transient CXCR4 desensitization within the BM may facilitate NK-cell exit induced by S1P via S1P5. Reciprocally, S1P5 level could be cyclically modulated during NK-cell trafficking, depending on the local concentration of S1P, as suggested by our findings that responsiveness to S1P is higher for BM NK cells than for blood ones. Therefore, modulation of responsiveness to CXCL12 and S1P may favor recirculation of NK cells across the BM and blood. CXCR4 and S1P5 responsiveness are also regulated at the gene-expression level, because we observed a coordinated switch between CXCR4 and S1P5 expression during NK-cell maturation. Such a change in responsiveness to chemoattracting factors during differentiation has already been described for B cells.12 This switch between CXCR4 and S1P5 is likely to maintain the differential distribution of immature and mature NK cells.
The number of peripheral NK cells is also decreased in some patients with WS.34,35 Moreover, CXCR4 has been shown to retain human NK cells to the BM and spleen in NOD/SCID mice reconstituted with human immune system.36 We reported previously that S1P5 was up-regulated during human NK-cell differentiation.5 Therefore, the mechanism controlling NK-cell exit from the human BM is likely to be similar to the one we describe here in the mouse counterpart. The paucity of peripheral NK cells in WS patients could render them more susceptible to viral infections and, indeed, WS patients suffer from severe HPV-induced diseases.37 Although the role of NK cells in HPV infections is still poorly documented, recent evidence suggests their involvement. In particular, an association between the lack of KIR3DS1 and KIR2DS1 activating NK-cell receptors and the susceptibility to recurrent respiratory papillomatosis has been reported.38 Moreover, cases of disseminated Mycobacterium avium complex (MAC) infection have been reported in patients with a new immunodeficiency syndrome related to CXCR4 dysfunction.39 Because these patients display a severe defect in circulating NK cells, and because MAC infections have been associated with reduced NK cell activity,40 one can speculate that a reduction in peripheral NK-cell numbers contributes to the susceptibility to HPV and MAC infections in such leukopenic patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Plateau de Biologie Expérimentale de la Souris; the flow cytometry facility of IFR128; A. Calver (GlaxoSmithKline) for providing S1P5 knockout mice; L. Bouchet-Delbos and A. Bignon (Université Paris-Sud, Laboratoire “Cytokines, Chemokines and Immunopathology,” Inserm UMR_S996, Clamart, France) for their technical help; Dr F. Baleux (Unité de Chimie Organique, Institut Pasteur, Paris) for providing us with CXCL12 proteins; and Drs F. Bachelerie (Université Paris-Sud, Laboratoire “Cytokines, Chemokines and Immunopathology,” Inserm UMR_S996, Clamart), T. Henry, M. C. Michallet, and Y. Leverrier for critical reading of the manuscript.
The Walzer laboratory is supported by the FINOVI foundation, Agence Nationale de la Recherche (ANR), Inserm, Ligue contre le Cancer (Comité du Rhône), and Université de Lyon. The Balabanian laboratory is supported by the ANR (grant number 2010 JCJC 1104 01), the Université Paris-Sud, Inserm, and the Ligue Nationale Contre le Cancer (Comité Val d'Oise), and is a member of the Laboratory of Excellence LERMIT supported by a grant from ANR (ANR-10-LABX-33).
Authorship
Contribution: T.W., K.B., and J.M. designed the experiments and wrote the manuscript and T.W., V.B., and K.M. performed all of the experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Thierry Walzer, Inserm U851, 21 avenue Tony Garnier, 69007 Lyon, France; e-mail: thierry.walzer@inserm.fr; or Karl Balabanian, Inserm UMR_S 996, 32 rue des Carnets, 92140 Clamart, France; e-mail: karl.balabanian@u-psud.fr.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal