Abstract
Regulatory T cells (Tregs) may impede cancer vaccine efficacy in hematologic malignancies and cancer. CCR4 antagonists, an emergent class of Treg inhibitor, have been shown to block recruitment of Tregs mediated by CCL22 and CCL17. Our aim was to demonstrate the ability of a CCR4 antagonist (a small chemical molecule identified in silico) when combined with vaccines to break peripheral tolerance controlled by Tregs, a prerequisite for the induction of CD8+ T cells against self Ags. Immunization of transgenic or normal mice expressing tumor-associated self Ags (Her2/neu, OVA, gp100) with a CCR4 antagonist combined with various vaccines led to the induction of effector CD8+ T cells and partial inhibition of tumor growth expressing self Ags in both prophylactic and therapeutic settings. The CCR4 antagonist was more efficient than cyclophosphamide to elicit anti-self CD8+ T cells. We also showed that the population of Tregs expressing CCR4 corresponded to memory (CD44high) and activated (ICOS+) Tregs, an important population to be targeted to modulate Treg activity. CCR4 antagonist represents a competitive class of Treg inhibitor able to induce functional anti-self CD8+ T cells and tumor growth inhibition when combined with vaccines. High expression of CCR4 on human Tregs also supports the clinical development of this strategy.
Introduction
Most high-avidity autoreactive T cells are deleted in the thymus during T-cell development, reducing both the frequency and avidity of autoreactive T cells in the peripheral repertoire. However, it is clearly established that some self-reactive T cells escape negative selection and leave the thymus. Maintenance of peripheral immune tolerance via the control of these cells is therefore important to dampen potentially damaging immune reactions in peripheral tissues and to prevent autoimmune disease.1 Among key mechanisms of peripheral self-tolerance, CD4+CD25+Foxp3+ regulatory T cells (Tregs) have emerged as the dominant T-cell population inhibiting self-reactive effector T cells.2,3
Some tumor-associated Ags in hematologic malignancies are self proteins that elicit weak T-cell responses as a consequence of immune tolerance, anergy, or exhaustion.4 It has been shown that Tregs are able to recognize tumor-associated self Ags and to control natural T-cell responses against various cancer Ags. For example, tyrosinase and NY-ESO1–specific CD4+ T cells can expand and become detectable by in vitro antigenic stimulation of peripheral CD4+ T cells only after depletion of Tregs.5 In addition, a therapeutic cancer vaccine could induce tumor-specific Tregs blunting the expansion and function of antitumor T cells.6 In line with these results, Treg depletion or blockade has been shown to enhance tumor immunity elicited by vaccination.7
Thus, to improve vaccination efficacy against foreign Ags and to break tolerance against self-tumor Ags, various approaches have been developed to delete or inhibit the activity of Tregs. However, specific elimination of Tregs is difficult because current markers of these cells (Foxp3, CD25, GITR, OX40, CTLA-4, Lag3, etc) are also shared by activated T cells.2 In addition, because many mechanisms (ie, inhibitory signal on APCs, lysis, or inhibition of effector T cells) underlie the suppressive activity of Tregs,8 it is probable that the relative importance of each inhibitory mechanism is context dependent, which makes the implementation of a general strategy to inactivate Tregs a difficult task.
CCR4 is a receptor for 2 chemokines CCL17 and CCL22, both of which are secreted by activated mature dendritic cells (DCs) in lymphoid and nonlymphoid tissues.9 It has been shown that Tregs preferentially express CCR4 compared with conventional T cells in both mice and humans.10,11 The binding of CCL17 and CCL22 to CCR4 helps to guide Tregs toward DCs. This interaction can suppress DC-mediated immune responses by inhibition of DC maturation and the expression of costimulatory molecules required for effector T-cell activation, as well as by inhibition of stable contact between DCs and effector cells.12,13
Preliminary work showed that small molecule antagonists to CCR4, predicted in silico, occupy a cavity within the transmembrane region of the receptor that corresponds to a typical ligand-binding site and thus prevent the interaction of chemokine with its receptor. In vitro experiments in humans showed that these CCR4 antagonists inhibit the recruitment of Tregs mediated by CCL22 and CCL17 and, when administered in combination with vaccines, increased humoral responses against foreign Ags.14,15
The present study was designed to address whether a CCR4 antagonist (a small chemical molecule with a molecular weight of 565.93; contain six 5- or 6-membered aromatic rings; and 3 nitrogen atoms) previously described14 was efficient to elicit CD8+ T cells directed against various self Ags. Tregs are known to exert a stringent peripheral control on these anti-self CD8+ T cells. The clinical relevance of this study was supported by the fact that most tumor Ags are self Ags and CD8+ T cells are considered as main effectors against cancer.16 Immunosuppression in part mediated by Tregs may explain the failure of cancer vaccines.
Methods
Mice
Female C57BL/6 (H-2b) mice were purchased from Charles River Laboratories. The generation K14 type-16 human papillomavirus (HPV-16) E6-E7 transgenic (TG) mice congenic for H2b17 is described in supplemental Figure 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Mice expressing the neuOT-I/OT-II transgene in mammary epithelium under the control of the mouse mammary tumor virus promoter and a dominant-negative mutant of P53 under the control of the whey acid protein promoter were obtained from the laboratory of B.H.N. The activated rat neu oncogene was tagged at its COOH terminus with CD8+ (OVA257-264) and CD4+ (OVA323-339) T-cell epitopes from OVA, resulting in neuOT-I/OT-II TG mice.18 Details are given in supplemental Methods for HLA-A*0201 and green fluorescent protein (GFP)–Foxp3 TG mice.19
Chemical reagents and vaccines
Purified chicken OVA (grade 5) was purchased from Sigma-Aldrich. Synthetic OVA-derived peptide OVA257-264 (SIINFEKL) and HPV-16–derived peptide E749-57 (RAHYNIVTF) or murine HER2/neu-derived peptide Her2435-443 (ILHDGAYSL), or gp10025-33 (EGSRNQDWL) were obtained from Polypeptide Laboratories.
STxB-OVA was obtained by chemical coupling as previously described.20 STxB-Her2435-443 and STxB-gp10025-33 were produced with a chemical coupling between the N-bromoacetylated Her2435-443 peptide and the sulfhydryl group of the STxB-Cys recombinant protein as previously described.21 Contaminating lipopolysaccharide was removed by EndoTrap affinity chromatography (Hyglos GmbH). After purification, endotoxin concentrations were < 0.5 EU/mg as determined by the Limulus assay (Lonza).
The DNA vaccine (pgDE7) encoding the HPV-16 E7 oncoprotein was genetically fused with the HSV-1 glycoprotein D as previously described.22 The endotoxin-free plasmid DNA was obtained after purification with disposable columns (QIAGEN).
The invariant natural killer T-cell ligand α-GalCer (KRN7000) was purchased from Funakoshi.
The CCR4 antagonist (AF399/420/18 025) provided by J Bayry (Inserm U872) was dissolved in 10% DMSO and mixed with each vaccine as previously described.16 Mice were injected with 1.5 μg of CCR4 antagonist, a dose that has been optimized in previous experiments.14,15
Cyclophosphamide was used at 200 mg/kg (4 mg/mouse) as previously described.23 IFA was emulsified with the vaccine (v/v). CpG 1826 (Sigma-Aldrich) was used at 50 μg/mouse.
Anti-CD25–purified mAb (clone PC61, rat IgG1) was administered once intraperitoneally (500 μg/mouse), 3 days before immunization as previously described.24
CD3/CD28 MACSiBead (Miltenyi Biotec) were used to activate human PBMCs.
Immunization
Mice were immunized twice (day 0 and day 14) with various vaccines combined or not with strategies to inhibit Tregs (cyclophosphamide, anti-CD25, CCR4 antagonist). Splenocytes were harvested 7 days after the last injection. CD8+ T cells were purified with anti-CD8–coated magnetic beads (Miltenyi Biotec), and their specificity was analyzed by tetramer staining or ELISPOT.
For DNA immunizations, 50 μL of cardiotoxin (6-8 μg; Latoxan) were injected into each tibialis anterior muscles 5 days before DNA immunization.
All animal experiments were conducted in accordance with the guidelines of the Ecole Nationale Veterinaire d'Alfort and were approved by the Université Paris Descartes Ethics Committee.
Tumor cell lines
The mouse thymoma cell lines EL4 (H-2b) and EG7, which is a chicken egg OVA-transfected subclone of EL4, were kindly provided by B. Combadiere (Université Pierre et Marie Curie).
Flow cytometry
To detect CD8+ T cells specific for anti-OVA257-264/Kb or anti-E749-57/Db, cells were stained with OVA257-264/Kb or E749-57/Db tetramer according to the manufacturer's recommendations (Beckman-Coulter Immunomics). Briefly, cells were incubated with PE-labeled tetramer (45 minutes at 4°C in the dark). After incubation and washes, labeled anti-CD8 mAbs (eBioscience) were applied. Irrelevant tetramers recognizing a vesicular stomatitis virus (VSV)–derived peptide in the context of Kb or Db were used in each experiment. Naive nonimmunized mice were also included as controls for these experiments.
All reagents used for the characterization of Tregs are detailed in supplemental Methods.
ELISPOT
The functionality of specific CD8+ T cells was determined by ELISPOT according to the manufacturer's recommendations (Gen-Probe Diaclone) and as previously described.21 A response was considered positive if the number of spots in the wells stimulated with specific peptides was 2-fold higher than the number of spots in the wells without peptide with a cutoff of 10 spot-forming cells per 2.105 cells as previously detailed.25
In vitro expansion and transfer of Tregs
CD4+ T cells were purified with anti-CD4–coated magnetic beads (Miltenyi Biotec) from lymph nodes (LNs) of OTII Ly5.2 mice TG for a specific TCR recognizing the I-Ab OVA323-339 complex.
Six-well plates were coated for 2 hours at 37°C with 4 μg of mouse anti-CD3 (eBioscience) in 1 mL of PBS. After incubation, 3.5 million Ly5.2+ CD4+ T cells were added and stimulated with TGFβ (5 ng/mL) and IL-2 (100 IU/mL) for 72 hours at 37°C. As previously described, this protocol generated 60%-70% Tregs.26
Ten million in vitro–expanded Tregs either alone or mixed with the CCR4 antagonist (2 μg) were injected intravenously in each C57BL6 Ly5.1 mouse, immunized 2 hours previously with STxB-OVA (30 μg) and αGalCer (1 μg) to induce local specific immune response against OVA protein.
Twenty-four hours after Treg administration, vaccine-draining LNs of C57Bl6 Ly5.1 mice were harvested, and cells were stained with PE-Cy5 anti–mouse CD4 (rat IgG2b; eBioscience), Alexa Fluor 700 anti-Ly5.2 (CD45.2; mouse IgG2a; eBioscience), and APC anti–mouse Foxp3 (rat IgG2a; eBioscience) in cells previously fixed and permeabilized. Isotype controls were included in each experiment.
Therapeutic B16 tumor model
B16 cells (5 × 105) expressing the self-melanocyte Ag gp100 were injected subcutaneously in the right flank of C57BL/6 mice as previously described.27 Mice were then either unimmunized or immunized by intraperitoneal injection at day 12 and day 19 after tumor graft with the STxB-gp100 (30 μg) vaccine alone or combined with the CCR4 antagonist (1.5 μg) every 4 days. A group was treated by the CCR4 antagonist alone. Mice were monitored every 3-4 days for tumor growth.
Statistical analysis
Statistical analysis was performed with the Mann-Whitney U test and the Kruskal-Wallis test. Significance was defined as P < .05.
Results
A CCR4 antagonist breaks tolerance to OVA in neuOT-I/OT-II TG mice
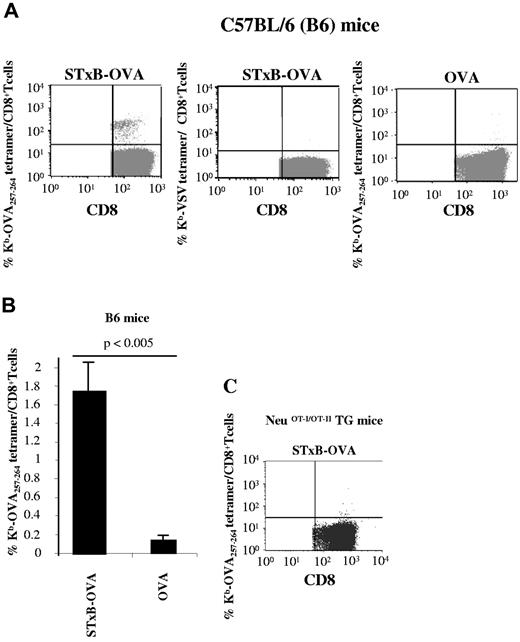
Wild-type B6 mice immunized with free OVA protein (0.25 nmol = 10 μg) combined with αGalCer elicited a weak induction of OVA257-264 CD8+ T cells corresponding to 0.1% of CD8+ T cells (Figure 1A-B). In contrast, when STxB, a vector targeting DCs because of the preferential expression of its glycolipid Gb3 receptor on these cells,28 was coupled to OVA (STxB-OVA, 0.25 nmol), a significant increase in anti-OVA257-264 CD8+ T cells was observed, reaching 1.7% of CD8+ T cells (Figure 1A-B). As controls, staining with Kb-VSV tetramer led to the labeling of < 0.02% CD8+ T cells (Figure 1A). In the absence of adjuvant, no induction of anti-OVA257-264 CD8+ T cells was recorded after immunization with free OVA, and STxB-OVA alone induced only 0.4% anti-OVA257-264 CD8+ T cells (data not shown).
NeuOT-I/OT-II TG mice are tolerant to OVA. C57BL/6 mice (A) or neuOT-I/OT-II TG mice (C) were immunized twice (day 0 and day 14) intraperitoneally with STxB-OVA (0.25 nmol) or OVA (0.25 nmol) both mixed with αGalCer (1 μg) at day 0. One week later, splenocytes were harvested, semipurified with anti-CD8–coated magnetic beads, and stained with Kb-OVA257-264 tetramer gated on CD8+ T cells or irrelevant Kb-VSV tetramer. (B) Mean tetramer levels in B6 mice immunized with STxB-OVA or OVA. These results are representative of 3 experiments with 4 mice per group. Values of P were calculated by the Mann-Whitney U test.
NeuOT-I/OT-II TG mice are tolerant to OVA. C57BL/6 mice (A) or neuOT-I/OT-II TG mice (C) were immunized twice (day 0 and day 14) intraperitoneally with STxB-OVA (0.25 nmol) or OVA (0.25 nmol) both mixed with αGalCer (1 μg) at day 0. One week later, splenocytes were harvested, semipurified with anti-CD8–coated magnetic beads, and stained with Kb-OVA257-264 tetramer gated on CD8+ T cells or irrelevant Kb-VSV tetramer. (B) Mean tetramer levels in B6 mice immunized with STxB-OVA or OVA. These results are representative of 3 experiments with 4 mice per group. Values of P were calculated by the Mann-Whitney U test.
These results confirmed previous reports on the potency of STxB as a vaccine vector that synergizes with αGalCer.21
In contrast to the results obtained in wild-type B6 mice, neither OVA nor STxB-OVA with or without αGalCer could induce anti-OVA257-264 CD8+ T cells in neuOT-I/OT-II TG mice (Figure 1C; data not shown).
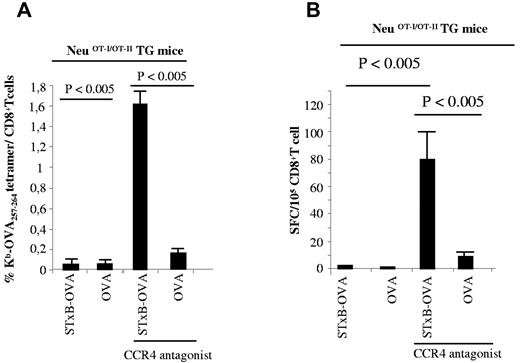
Because Tregs have been reported to play a role in tolerance against Her2/neu in Her2/neu TG mice,29 we checked whether a CCR4 antagonist that blocks the migration of Tregs toward DC-secreting CCR4 ligands could break tolerance in neuOT-I/OT-II TG mice. Indeed, CCR4 antagonist mixed with the STxB-OVA/αGalCer vaccine reproducibly induced similar levels of anti-OVA257-264 CD8+ T cells (1.6% of CD8+ T cells; Figure 2A) than the levels observed in wild-type B6 mice (Figure 1A). These results were confirmed with an ELISPOT assay, which showed a large number of CD8+ T cells producing IFNγ (mean, 80/105 cells) after vaccination with STxB-OVA/αGalCer combined with the CCR4 antagonist (Figure 2B). Free OVA combined with the CCR4 antagonist was less efficient at breaking tolerance in neuOT-I/OT-II TG mice (Figure 2A-B).
A CCR4 antagonist combined with STxB-OVA breaks CD8+ T-cell tolerance against OVA in neuOT-I/OT-II TG mice. NeuOT-I/OT-II TG mice were immunized twice (day 0 and day 14) intraperitoneally with STxB-OVA (0.25 nmol) or OVA (0.25 nmol) mixed with αGalCer at day 0 and combined or not with a CCR4 antagonist (1.5 μg). One week later, splenocytes were harvested and semipurified with anti-CD8–coated magnetic beads. Detection of anti-OVA257-264 CD8+ T cells was performed either with Kb-OVA257-264 tetramer (as described in Figure 1A) or by IFNγ ELISPOT (B). These results are representative of 3 experiments with 4 mice per group. Values of P were calculated by the Mann-Whitney U test.
A CCR4 antagonist combined with STxB-OVA breaks CD8+ T-cell tolerance against OVA in neuOT-I/OT-II TG mice. NeuOT-I/OT-II TG mice were immunized twice (day 0 and day 14) intraperitoneally with STxB-OVA (0.25 nmol) or OVA (0.25 nmol) mixed with αGalCer at day 0 and combined or not with a CCR4 antagonist (1.5 μg). One week later, splenocytes were harvested and semipurified with anti-CD8–coated magnetic beads. Detection of anti-OVA257-264 CD8+ T cells was performed either with Kb-OVA257-264 tetramer (as described in Figure 1A) or by IFNγ ELISPOT (B). These results are representative of 3 experiments with 4 mice per group. Values of P were calculated by the Mann-Whitney U test.
CCR4 antagonist combined with various vaccines efficiently elicits CD8+ T cells directed against self Ags
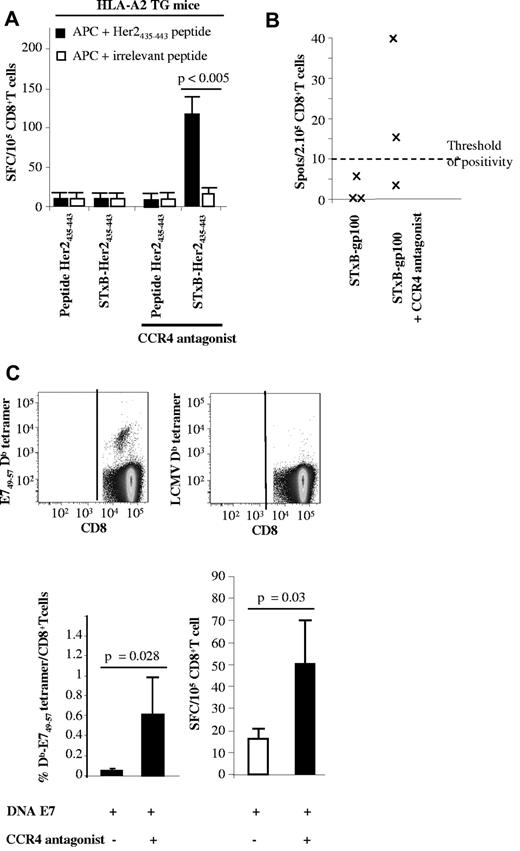
As previously reported for other Her2/neu-based vaccines,30 neuOT-I/OT-II TG mice on a B6 genetic background responded poorly to vaccination against the murine Her2/neu435-443 coupled or not with STxB even after Treg depletion (data not shown). It may be that the repertoire of anti-Her2/neu435-443 CD8+ T cells restricted by H-2 Kb is absent in Her2/neu TG mice on a B6 genetic background. Because this Her2/neu435-443 peptide binds to both H-2 Kb and HLA-A2,31 we tested the ability of the CCR4 antagonist to break tolerance against this peptide in HLA-A2 TG mice. Because H-2 class I knockout, HLA-A*0201 mice do not express CD1d, the ligand of αGalCer, the combination of CpG and IFA, replaced αGalCer as adjuvants in all these experiments. As shown in Figure 3, HLA-A2 TG mice immunized with STxB-Her2435-443 (0.5 nmol)/CpG (50 μg) plus IFA (v/v) or Her2435-443 peptide (0.5 nmol) failed to elicit anti-Her2/neu435-443 CD8+ T cells (Figure 3A). In contrast, vaccination with adjuvanted STxB-Her2/neu435-443 combined with the CCR4 antagonist induced specific CD8+ T cells that produced IFNγ (102 spots/105 CD8+ T cells) after in vitro sensitization with Her2/neu435-443 peptide (Figure 3A). Immunization with free Her2/neu435-443 peptide combined with CCR4 antagonist failed to elicit specific CD8+ T cells (Figure 3A).
A CCR4 antagonist combined with STxB-Her2/neu or a DNA-E7 (pgDE7) vaccine enhances Ag-specific CD8+ T cells in HLA-A2 or E7 TG mice. (A) HLA-A2 TG mice were immunized twice (day 0 and day 14) subcutaneously with STxB-Her435-443 or the Her2435-443 peptide in combination or not with the CCR4 antagonist. One week later, splenocytes were harvested and purified with anti-CD8–coated magnetic beads. Detection of anti-Her2435-443 CD8+ T cells was performed by ELISPOT with the use of CD8− cells because APCs were sensitized with the Her435-443 or an irrelevant peptide. (B) Mice were immunized at day 0 and day 14 with STxB-gp10025-33 (30 μg) alone or combined with the CCR4 antagonist (1.5 μg). One week later, splenocytes were harvested, and anti-gp10025-33 CD8+ T cells were detected by IFNγ ELISPOT. The number of spots was calculated after subtracting background (APCs incubated with medium alone). Threshold of positivity was defined at 10 spot-forming cells/2.105 cells. (C) E7 TG mice were immunized twice intramuscularly with DNA-E7 (100 μg) in combination or not with the CCR4 antagonist. One week later, splenocytes were harvested and semipurified with anti-CD8–coated magnetic beads. Detection of anti-E749-57 CD8+ T cells was performed with E749-57 Db tetramer (top and bottom left) gated on CD8+ T cells or by ELISPOT (bottom right). An irrelevant tetramer was used in all experiments (top right), and the number of spots was calculated after subtracting background (APCs incubated with medium alone). Results represent the mean of 4 mice per group and has been reproduced twice. P values were calculated by the Mann-Whitney U test.
A CCR4 antagonist combined with STxB-Her2/neu or a DNA-E7 (pgDE7) vaccine enhances Ag-specific CD8+ T cells in HLA-A2 or E7 TG mice. (A) HLA-A2 TG mice were immunized twice (day 0 and day 14) subcutaneously with STxB-Her435-443 or the Her2435-443 peptide in combination or not with the CCR4 antagonist. One week later, splenocytes were harvested and purified with anti-CD8–coated magnetic beads. Detection of anti-Her2435-443 CD8+ T cells was performed by ELISPOT with the use of CD8− cells because APCs were sensitized with the Her435-443 or an irrelevant peptide. (B) Mice were immunized at day 0 and day 14 with STxB-gp10025-33 (30 μg) alone or combined with the CCR4 antagonist (1.5 μg). One week later, splenocytes were harvested, and anti-gp10025-33 CD8+ T cells were detected by IFNγ ELISPOT. The number of spots was calculated after subtracting background (APCs incubated with medium alone). Threshold of positivity was defined at 10 spot-forming cells/2.105 cells. (C) E7 TG mice were immunized twice intramuscularly with DNA-E7 (100 μg) in combination or not with the CCR4 antagonist. One week later, splenocytes were harvested and semipurified with anti-CD8–coated magnetic beads. Detection of anti-E749-57 CD8+ T cells was performed with E749-57 Db tetramer (top and bottom left) gated on CD8+ T cells or by ELISPOT (bottom right). An irrelevant tetramer was used in all experiments (top right), and the number of spots was calculated after subtracting background (APCs incubated with medium alone). Results represent the mean of 4 mice per group and has been reproduced twice. P values were calculated by the Mann-Whitney U test.
We confirmed these results with the melanocytic self Ag gp100. Indeed, no anti-gp100 CD8+ T cells were detected in mice immunized with STxB-gp100 alone, whereas anti-gp100 CD8+ T cells were detected by ELISPOT in 2 of 3 mice vaccinated with the STxB-gp100 vaccine combined with the CCR4 antagonist (Figure 3B)
To test if the CCR4 antagonist could break tolerance when combined with vaccine delivery other than STxB, we immunized K14 HPV-16 TG mice expressing the E7 oncoprotein in squamous epithelia with a DNA vaccine encoding the E7 oncoprotein genetically fused with the HSV-1 gD protein (pgDE7, 100 μg) with or without CCR4 antagonist. pgD-E7 alone induced an ex vivo anti-E749-57 CD8+T-cell response detectable by ELISPOT (16 spots/105 cells) but not by Db-E74957 tetramer (Figure 3C). In contrast when mixed with a CCR4 antagonist, a clear induction of anti-E749-57 CD8+ T cells was observed both by tetramer (0.7% of CD8+ T cells) and ELISPOT (50 spots/105 CD8+ T cells; Figure 3C).
Comparative analysis of various approaches to block regulatory T-cell activity
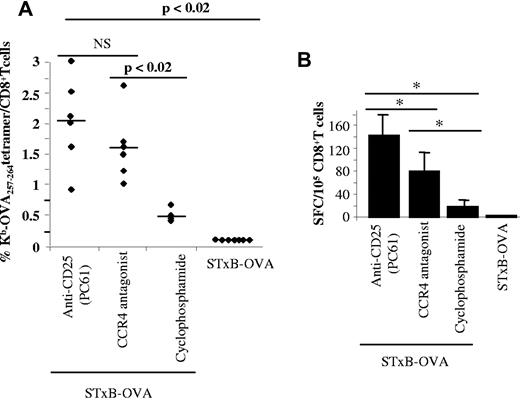
NeuOT-I/OT-II TG mice were immunized with STxB-OVA/αGalCer in combination with various optimized approaches to deplete Tregs.23,24
Anti-CD25 (0.5 mg intraperitoneally once) and cyclophosphamide (200 mg/kg intraperitoneally once) were administered 3 days before the vaccine, whereas the CCR4 antagonist (1.5 μg/mouse) was administered at the same time as the vaccine.
All 3 approaches of Treg blockade combined with STxB-OVA were able to break tolerance and to elicit anti-OVA257-264 CD8+ T cells (Figure 4A-B). As a control, an anti-PD1 Ab mixed with the vaccine failed to induce specific anti-OVA257-264 CD8+ T cells (data not shown)
Comparative analysis of various Treg blockade strategies on the induction of specific anti-self CD8+ T cells. NeuOT-I/OT-II TG mice were immunized intraperitoneally twice (day 0 and day 14) with STxB-OVA/αGalCer alone or combined with anti-CD25 (500 μg at day −3) or cyclophosphamide (4 mg at day −3) or the antagonist of CCR4 (1.5 μg) mixed with the vaccine at day 0. One week later, splenocytes were harvested, purified with anti-CD8–coated magnetic beads, and stained with Kb-OVA257-264 tetramer or irrelevant Kb-VSV tetramer (A) or cocultured for 18 hours with CD8− cells as APCs sensitized or not with the OVA257-264 and IFNγ shown by ELISPOT (B). Percentages shown in panel A correspond to specific values after subtracting the percentage observed with irrelevant tetramer (always < 0.1%). Each symbol corresponds to one mouse. The number of spots (B) was calculated after subtracting background (APCs incubated with medium alone). Values of P were calculated by the Mann-Whitney U test.
Comparative analysis of various Treg blockade strategies on the induction of specific anti-self CD8+ T cells. NeuOT-I/OT-II TG mice were immunized intraperitoneally twice (day 0 and day 14) with STxB-OVA/αGalCer alone or combined with anti-CD25 (500 μg at day −3) or cyclophosphamide (4 mg at day −3) or the antagonist of CCR4 (1.5 μg) mixed with the vaccine at day 0. One week later, splenocytes were harvested, purified with anti-CD8–coated magnetic beads, and stained with Kb-OVA257-264 tetramer or irrelevant Kb-VSV tetramer (A) or cocultured for 18 hours with CD8− cells as APCs sensitized or not with the OVA257-264 and IFNγ shown by ELISPOT (B). Percentages shown in panel A correspond to specific values after subtracting the percentage observed with irrelevant tetramer (always < 0.1%). Each symbol corresponds to one mouse. The number of spots (B) was calculated after subtracting background (APCs incubated with medium alone). Values of P were calculated by the Mann-Whitney U test.
However, the levels of specific CD8+ T cells varied according to the methods used to inhibit or deplete Tregs (mean, 2.1%; range, 1.4%-2.8% of CD8+ T cells with anti-CD25; mean, 1.6%; range, 1.1%-2.1% of CD8+ T cells with CCR4 antagonist; and mean, 0.45%; range, 0.32%-0.58% with cyclophosphamide; Figure 4A). Strategies that used anti-CD25 mAb or CCR4 antagonist were significantly more efficient than the use of cyclophosphamide (P < .02; Figure 4A). The number of IFNγ ELISPOT was significantly higher with the use of anti-CD25 than when the vaccine was combined with the CCR4 antagonist (Figure 4B). In line with the tetramer results, the CCR4 antagonist combined with the vaccine led to a greater number of spots than that observed with the use of cyclophosphamide (Figure 4B).
We then compared the influence of the 3 methods for Treg blockade on the percentage and absolute number of Foxp3+CD4+ T cells, as well as on the number of CD8+ T cells.
Anti-CD25 and cyclophosphamide significantly reduced both the percentage and the absolute number of Tregs (CD4+Foxp3+ T cells), as early as day 2 after administration, and this decrease persisted for ≥ 7 days (supplemental Figure 2A-B). In contrast, as expected for an agent that influences cell trafficking, the CCR4 antagonist did not change either the level or absolute number of peripheral regulatory CD4+Foxp3+ T cells.
Cyclophosphamide but not anti-CD25 or the CCR4 antagonist partially depleted CD8+ T cells (supplemental Figure 2C). Indeed, after cyclophosphamide administration, the number of CD8+ T cells decreased from 600 000/mm3 to 280 000/mm3 at day 2 and rebounded to 390 000/mm3 at day 7 (supplemental Figure 2C).
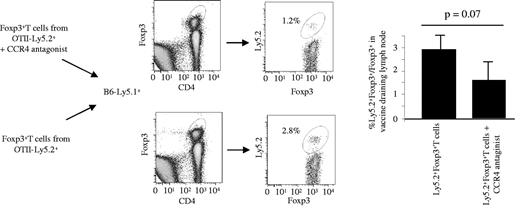
To further define the mechanism of action of CCR4 antagonist, we transferred in vitro–expanded anti–OVA-Tregs from OTII TG Ly5.2 mice to B6 Ly5.1 mice 2 hours after STxB-OVA vaccination. Twenty-four hours after transfer, we observed, in the vaccine-draining LN, a partial inhibition of transferred anti-OVA Tregs in the presence of CCR4 antagonist at the limit of statistical significance (P = .07; Figure 5).
Role of the CCR4 antagonist in migration of Tregs to the vaccine-draining LN. Ten million in vitro–generated Tregs from OTII Ly5.2 mice, alone or mixed with the CCR4 antagonist (2 μg), were administered to Ly5.1 B6 mice and immunized 2 hours previously with STxB-OVA (30μg) and αGalcer (1 μg). Twenty-four hours later, vaccine draining LNs were harvested, and cells were stained with anti-CD4 and anti-Foxp3 mAbs. Cells were than gated on CD4+Foxp3+ cells and stained with anti-Ly5.2 mAb. Isotype controls were included in each experiment. Double-positive Foxp3-Ly5.2 cells correspond to the transferred Tregs. Four mice per group were injected with Tregs with or without the CCR4 antagonist. Experiments shown are representative of 3 series of experiments with similar results. The Mann-Whitney test was used for statistical analysis.
Role of the CCR4 antagonist in migration of Tregs to the vaccine-draining LN. Ten million in vitro–generated Tregs from OTII Ly5.2 mice, alone or mixed with the CCR4 antagonist (2 μg), were administered to Ly5.1 B6 mice and immunized 2 hours previously with STxB-OVA (30μg) and αGalcer (1 μg). Twenty-four hours later, vaccine draining LNs were harvested, and cells were stained with anti-CD4 and anti-Foxp3 mAbs. Cells were than gated on CD4+Foxp3+ cells and stained with anti-Ly5.2 mAb. Isotype controls were included in each experiment. Double-positive Foxp3-Ly5.2 cells correspond to the transferred Tregs. Four mice per group were injected with Tregs with or without the CCR4 antagonist. Experiments shown are representative of 3 series of experiments with similar results. The Mann-Whitney test was used for statistical analysis.
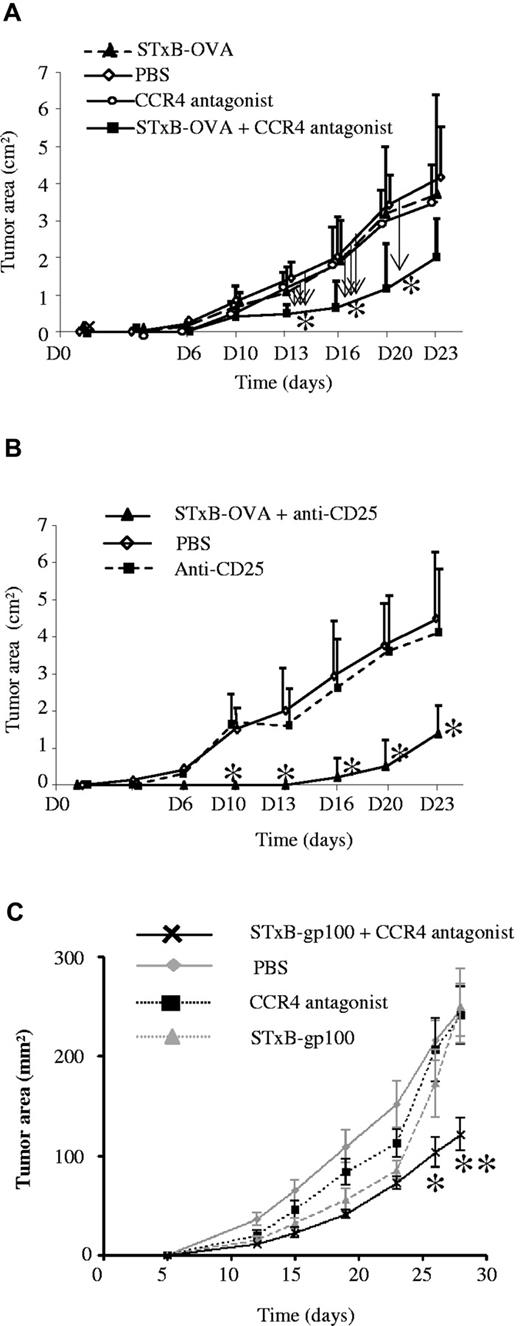
CCR4 antagonist combined with STxB-based vaccines partially protect against the development of tumor-expressing self Ags
Immunization of neuOT-I/OT-II TG mice with STxB-OVA mixed with αGalCer did not prevent the development of OVA-expressing tumor (EG7) grafted in these mice (Figure 6A). In contrast, when neuOT-I/OT-II TG mice were previously immunized with STxB-OVA combined with αGalCer and mixed with the CCR4 antagonist, a partial protection against the development of EG7 was observed (Figure 6A). As control, administration of CCR4 alone before the graft of EG7 or immunization of neuOT-I/OT-II TG mice with STxB-OVA combined with αGalCer and the antagonist of CCR4 before the graft of EL-4, the parent tumor cell line not expressing OVA, were inefficient to inhibit tumor development (Figure 6A; data not shown). When neuOT-I/OT-II TG mice were immunized with STxB-OVA/αGalCer combined with anti-CD25, a partial protection against EG7 tumor development was also observed (Figure 6B). The delay of tumor onset appears to be longer, when the STxB-OVA vaccine was mixed with anti-CD25 than after the combination with the CCR4 antagonist (Figure 6A-B).
STxB-OVA or STxB-gp100 combined with the CCR4 antagonist partially protect against the growth of tumors in neuOT-I/OT-II TG mice or B6 mice, respectively, in prophylactic or therapeutic settings. NeuOT-I/OT-II TG mice were immunized with STxB-OVA mixed with αGalCer alone or combined with the CCR4 antagonist at day 0 and day 14 (A). The CCR4 antagonist alone was also administered in another group of mice at day 0 and day 14 without the vaccine (A). Another group of NeuOT-I/OT-II TG mice was not treated but injected with PBS (A-B). In another experiment, NeuOT-I/OT-II TG mice were immunized intraperitoneally twice (day 0 and day 14) with STxB-OVA/αGalCer combined with anti-CD25 (500 μg at day −3) or treated with anti-CD25 alone (B). At day 21, the EG7 tumor cell line (106 cells) were subcutaneously grafted on the left flank (A-B). Six to 8 mice per group were used in each experiment, which was reproduced twice. (A) Arrow indicated the comparison side-by-side of the various groups of mice. (B) Statistical comparison was performed between the STxB-OVA + anti-CD25 and the anti-CD25 alone groups. *P < .05. (C) B16 melanoma cells (5 ×105) were injected subcutaneously in the right flank of C57BL6 mice. Mice were then immunized by the intraperitoneal route at day 12 and day 19 after tumor graft, with the STxB-gp10025-33 (30 μg) vaccine alone or combined with the CCR4 antagonist (1.5 μg). An other group was treated with the CCR4 antagonist alone, and a last group was not treated. In the 2 groups receiving the CCR4 antagonist (alone or combined with STxB-gp10025-33), the CCR4 antagonist was administered intraperitoneally twice a week. Mice were monitored every 3-4 days for tumor growth. Five mice per group were used in each experiment. A representative experiment of 2 similar experiments is shown. Statistical analysis shown compared the 2 vaccinated groups (STxB-gp100 vs STxB-gp100 + the CCR4 antagonist). *P < .05, **P < .01.
STxB-OVA or STxB-gp100 combined with the CCR4 antagonist partially protect against the growth of tumors in neuOT-I/OT-II TG mice or B6 mice, respectively, in prophylactic or therapeutic settings. NeuOT-I/OT-II TG mice were immunized with STxB-OVA mixed with αGalCer alone or combined with the CCR4 antagonist at day 0 and day 14 (A). The CCR4 antagonist alone was also administered in another group of mice at day 0 and day 14 without the vaccine (A). Another group of NeuOT-I/OT-II TG mice was not treated but injected with PBS (A-B). In another experiment, NeuOT-I/OT-II TG mice were immunized intraperitoneally twice (day 0 and day 14) with STxB-OVA/αGalCer combined with anti-CD25 (500 μg at day −3) or treated with anti-CD25 alone (B). At day 21, the EG7 tumor cell line (106 cells) were subcutaneously grafted on the left flank (A-B). Six to 8 mice per group were used in each experiment, which was reproduced twice. (A) Arrow indicated the comparison side-by-side of the various groups of mice. (B) Statistical comparison was performed between the STxB-OVA + anti-CD25 and the anti-CD25 alone groups. *P < .05. (C) B16 melanoma cells (5 ×105) were injected subcutaneously in the right flank of C57BL6 mice. Mice were then immunized by the intraperitoneal route at day 12 and day 19 after tumor graft, with the STxB-gp10025-33 (30 μg) vaccine alone or combined with the CCR4 antagonist (1.5 μg). An other group was treated with the CCR4 antagonist alone, and a last group was not treated. In the 2 groups receiving the CCR4 antagonist (alone or combined with STxB-gp10025-33), the CCR4 antagonist was administered intraperitoneally twice a week. Mice were monitored every 3-4 days for tumor growth. Five mice per group were used in each experiment. A representative experiment of 2 similar experiments is shown. Statistical analysis shown compared the 2 vaccinated groups (STxB-gp100 vs STxB-gp100 + the CCR4 antagonist). *P < .05, **P < .01.
We further tested the efficiency of the CCR4 antagonist in wild-type mice with the use of the gp100 melanocytic self Ag, which is overexpressed in the B16 tumor model as previously described.27 In a therapeutic setting, we observed a significant inhibition of the growth of tumor in mice treated with STxB-gp100 combined with the CCR4 antagonist compared with those treated by STxB-gp100 alone (P < .01 at day 26; Figure 6C).
CCR4+ Tregs have an activated, memory cell surface phenotype
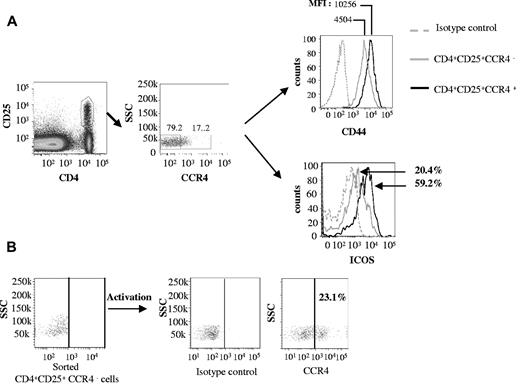
Because the CCR4 antagonist targets CCR4+ regulatory T cells, we checked for the expression of this marker on blood-, spleen-, and LN-derived Tregs. Approximately 15%-20% of CD4+CD25+ cells express CCR4, compared with < 5% of CD25−CD4+ T cells or CD8+ T cells (Figure 7A; data not shown). We further characterized the CCR4+ Treg subpopulation.
CCR4+ Tregs have hallmarks of activated memory cells. (A) Splenocytes from B6 mice were stained with CD4, CD25, CCR4, CD44, and ICOS mAbs. Expression of CD44 and ICOS was compared among CD4+CD25+CCR4+ and CD4+CD25+CCR4− cells. Isotype controls were included in each experiment. (B) CD4+CD25+CCR4− cells were sorted and activated by anti-CD3 + anti-CD28 mAbs and IL-2 for 36 hours. Expression of CCR4 was then detected on activated cells by flow cytometry. These experiments were reproduced twice.
CCR4+ Tregs have hallmarks of activated memory cells. (A) Splenocytes from B6 mice were stained with CD4, CD25, CCR4, CD44, and ICOS mAbs. Expression of CD44 and ICOS was compared among CD4+CD25+CCR4+ and CD4+CD25+CCR4− cells. Isotype controls were included in each experiment. (B) CD4+CD25+CCR4− cells were sorted and activated by anti-CD3 + anti-CD28 mAbs and IL-2 for 36 hours. Expression of CCR4 was then detected on activated cells by flow cytometry. These experiments were reproduced twice.
Memory Tregs have been shown to express high levels of CD44 (CD44hi), and the marker ICOS characterizes a subpopulation of activated Tregs.32 When we compared the expression of these markers on CD4+CD25+CCR4+ and CD4+CD25+CCR4− populations derived from splenocytes, we found that the CCR4+ Treg subpopulation predominantly expressed ICOS (59.2%) and CD44high compared with the CCR4− population (ICOS, 20.4% and CD44low; Figure 7A).
When CCR4− Tregs were activated in vitro, up-regulation of CCR4 was clearly observed (Figure 7B), which confirmed the activation status associated with CCR4+ regulatory T cells.
Foxp3 expression was not analyzed because this required intracellular staining that conflicts with cell sorting with the use of membrane markers. In addition, as already reported,33 a high background for CCR4 appeared when combined with Foxp3. However, to confirm these results, Tregs were analyzed from Foxp3-GFP mice. High levels of CD44 and an increased expression of ICOS were again observed in the CCR4+ GFP cells (supplemental Figure 3).
Human Tregs express high levels of CCR4
To address potential clinical relevance of these results, we analyzed the expression of CCR4 on human Tregs defined as CD4+CD25high Foxp3+CD127− T-cell population.
In contrast to mice, 61% (+14%) of peripheral blood Tregs from healthy subjects express CCR4 (supplemental Figure 4A). These CCR4+ Tregs were memory T cells, because they were CD45RA− (data not shown). Activation of Tregs up-regulated CCR4 expression, as observed in mice (supplemental Figure 4C). In addition, 46% (+17%) of Foxp3+ Tregs in normal human LNs coexpressed Foxp3 and CCR4 (supplemental Figure 4C).
These results therefore establish that CCR4 is expressed at higher levels in Tregs derived from human than from mice.
Discussion
This study showed that a CCR4 antagonist combined with protein- or DNA-based vaccines elicited potent CD8+ T cells against various tumor relevant self Ags. This CCR4 antagonist, belonging to an emergent class of Treg inhibitors, is thus efficient to break tolerance mediated by Tregs. Indeed, in neuOT-I/OT-II or HLA-A2 TG mice, vaccines alone were inefficient to induce CD8+ T cells against the self Ag, OVA, or murine Her2/neu. However, when the CCR4 antagonist was combined with OVA or Her2/neu Ags delivered by the B subunit of Shiga toxin, a clear induction of functional CD8+ T cells could be observed. The advantage to vectorize Ags by the B subunit of Shiga toxin, a vector that targets DCs, has already been shown for the induction of CD8+ T-cell responses.20 However, in another model of OVA TG mice, the ability of STxB alone to break tolerance was transient and inconsistent21 compared with the robust and reproducible induction of specific CD8+ T cells detected directly ex vivo when combined with the CCR4 antagonist in this study.
In addition, a synergy was observed for the induction of specific CD8+ T cells when the CCR4 antagonist was combined with a DNA vaccine encoding the E7 protein derived from HPV-16. DNA-based vaccines represent a promising strategy to fight against cancer.34 However, its efficiency to induce immune responses against self Ags is low, supporting the addition of immunosuppression blockade, as achieved with the CCR4 antagonist.
This CCR4 antagonist is thus a versatile approach to block Treg activity when combined with various vaccine strategies. Induction of antitumor CD8+ T cells was associated with a partial tumor protection, when the CCR4 antagonist was combined with a vaccine targeting self-tumor Ag.
This CCR4 antagonist was previously shown to block in vitro the migration of human Tregs mediated by CCL22 or CCL17 and to increase humoral and cellular responses against various bacterial and viral Ags.14,15 This study confirms and extends these results by showing the ability of this CCR4 antagonist to break tolerance in models, where Tregs play a main role in the control of anti-self CD8+ T cells. Compared with conventional approaches to block Tregs (anti-CD25 mAb, cyclophosphamide), we show that this CCR4 antagonist appears to be a competitive strategy. One of the main advantages of this CCR4 antagonist is its short life time (24 hours),14 allowing transient inhibition of Tregs only during the priming phase and avoiding the potential autoimmune complications caused by long-term blockade or depletion of Tregs by mAbs (eg, anti-CD25, anti-OX40, anti-GITR, etc) with longer half-lives (2-3 weeks).35 We show that the administration of the CCR4 antagonist did not lead to the induction of biologic mark of autoimmunity such as the presence of antinuclear Abs, rheumatoid factors, or antineutrophil cytoplasmic Abs (data not shown). In contrast to anti-CD25 or cyclophosphamide, the administration of this CCR4 antagonist did not deplete Tregs (supplemental Figure 2) and had no effect on the number of CD8+ T cells. Injection of cyclophosphamide transiently decreased the number of CD8+ T cells (supplemental Figure 2), as previously reported,23 which may explain the weaker potency of this approach for the induction of CD8+ T cells compared with anti-CD25 and CCR4 antagonist strategies. That said, in humans metronomic administration of low doses of cyclophosphamide may be more selective in the reduction of Tregs.36
Another method of Treg depletion based on the use of a fusion protein combining IL-2 and diphtheria toxin (denileukin diftitox) has shown some efficacy in humans to reduce Tregs and to potentiate cancer vaccines.37,38 However, these results were not reproduced by all groups, and the role of genetic background in the variability of the denileukin diftitox activity has to be more thoroughly analyzed.39,40
The mechanism of action of this CCR4 antagonist is supposed to rely on its already shown ability to inhibit the recruitment of Tregs to secondary lymphoid organs mediated by CCL22 and CCL17 produced by activated DCs.14 In this report, we observed a partial inhibition (at the limit of statistical significance; P = .07) of the migration of Ag-specific Tregs in the vaccine-draining LN, when these cells were transferred in the presence of CCR4 antagonist. This partial inhibition may be explained by the role of other chemokine receptors (such as CCR7) in the migration of Tregs to LN.41 Because in vivo imaging studies have shown that within LNs Tregs interact with DCs and limit priming of naive T cells,13,42 the CCR4 antagonist may also inhibit the contact between Tregs and DCs within the LN. The role of CCR4 in the migration of Tregs toward the LN is also reinforced by studies showing that CCR4-deficient Tregs failed to traffic to LNs to inhibit pathogenic T cells.43 In addition, tolerance mediated by Tregs in a cardiac allograft model could not be achieved in CCR4-deficient recipients.44 Other chemokine receptors (CCR5, CXCR3, etc) play a role in the recruitment of Tregs in non–lymphoid-inflamed peripheral tissue.43-45
Although various experimental results from previous studies (in vitro inhibition of the migration of Tregs by the CCR4 antagonist,14 role of CCR4 in the migration of Tregs in the LN43 ) and from our present work (the CCR4 antagonist has the same ability than other strategies targeting Tregs (anti-CD25, cyclophosphamide) to break tolerance against self Ags, the CCR4 antagonist partially inhibits the migration of Tregs to LN) argue for a role of CCR4 in Treg blockade, we cannot exclude that other mechanisms may also participate in the ability of the CCR4 antagonist to break tolerance, allowing the induction of anti-self CD8+ T cells.
One limitation of this strategy is that CCR4 may be expressed by Th2 T cells, which are required for Ab production. Therefore, repeated vaccination with CCR4 antagonist may inhibit humoral response to vaccine Ags. Because naive T cells do not express CCR4, primary humoral responses are not expected to be reduced by CCR4 antagonists. In fact, mice depleted of CCR4-expressing cells by systemic injection of CCL17 fused to a fragment of pseudomonas exotoxin were able to mount Ag-specific humoral responses, and administration of our CCR4 antagonist combined with vaccine previously enhanced humoral responses.14
With respect to the expression of CCR4 by effector T cells, as already reported by other groups,45,46 we found that expression of CCR4 by naive CD8+ T cells was low (< 5%) in mice and human (data not shown). In a model of colitis, it was shown that transfer of CCR4−/− pathogenic T cells induced colitis similar to wild-type naive T cells, whereas CCR4-deficient Tregs could not control disease.43 This example strongly suggests that CCR4 has specificity with respect to the migration of Tregs compared with effector T cells.
In mice, CCR4 is expressed at high frequency in Tregs derived from nonlymphoid tissues, but its expression in Tregs located in the spleen, LN, and peripheral blood is ∼ 20%47 (Figure 7; data not shown). Because all Tregs do not express CCR4, we hypothesized that CCR4+ Tregs are an important regulatory subpopulations, at least in the context of vaccination. Indeed, in line with previous studies,33,48 we have shown that CCR4+ Tregs have the phenotype of activated, memory cells (Figure 7), and this population may be preferentially recruited by CCL22- and CCL17-producing DCs during priming in the LNs. In addition, although CCR4-deficient and wild-type Tregs exhibit similar suppressive activity in vitro, CCR4− Tregs require anti-CD3 Ab-mediated activation to acquire regulatory activity, whereas CCR4+ Tregs appear to be already primed to suppress the proliferation of CD8+ T cells.33 Our results may have clinical applications because many arguments support a predominant, although not exclusive, role of CD8+ T cells in the control of tumor development. However, in many cases, Tregs may impede the activity of antitumor CD8+ effector cells.32 Our results from neuOT-I/OT-II TG mice indicate that this CCR4 antagonist may help to shift the balance between Tregs and effector T cells and favor the expansion of anti-Her2/neu CD8+ T cells.
Our vaccine combining an efficient Ag delivery system, the B subunit of Shiga toxin, to a CCR4 antagonist able to break tolerance mediated by Tregs during the priming phase may represent a novel strategy to elicit potent effector CD8+ T cells in the context of immunosuppression mediated by Tregs in vivo.
The previous demonstration that the CCR4 antagonist used in this study is efficient to block the migration and function of human Tregs in vitro together with the high expression of CCR4 in human Tregs (supplemental Figure 4)11,33 also provides the rationale to develop this new class of Treg inhibitor for use in humans.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by Canceropole Ile de France, ANR (Agence Nationale de la Recherche), Ligue contre le Cancer, Association pour la Recherche sur le Cancer, Pole de compétitivité Medicen (Immucan) Center d'investigation Clinique en Biothérapie (CIC-BT505), Labex Immuno-Oncology, Institut National du Cancer (INCA), European Community's Seventh Framework Program (FP7/2007-2013; under grant agreement no. 260338 ALLFUN, J.B).
Authorship
Contribution: H.P., Y.M., J.B., L.J., and E.T. designed research; H.P., Y.M., N.M., E.D., C.B., A.G., E.M., F.B., F.S., O.A., C.C., S.G., and Y.-C.L. performed research; P.R. provided statistical support; L.C.F., B.H.N., and D.H. contributed vital new reagents; H.P., P.R., Y.M., J.B., B.C., F.B., F.S., N.M., O.A., C.C., S.G., W.H.F., L.J., E.T., and C.T. analyzed data; and E.T., H.P., F.Q.-C., and L.J. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Eric Tartour, Hôpital Européen Georges Pompidou, Service d'immunologie biologique, 20 Rue Leblanc 75015 Paris, France; e-mail: eric.tartour@egp.aphp.fr.
References
Author notes
H.P. and Y.M. contributed equally to this study.
L.J. and E.T. were principal investigators.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal