Abstract
Patients referred to tertiary care centers occasionally may have their diagnostic procedures repeated and have a final diagnosis that differs from that of the referring center. The aim of this study was to evaluate discordance rates and their clinical implications in the diagnosis of patients with myelodysplastic syndrome (MDS) referred to a tertiary center. We analyzed 915 patients with MDS who were referred to M. D. Anderson Cancer Center between September 2005 and December 2009. Discordance in the diagnosis was documented in 109 (12%) patients, with a majority reclassified as having higher-risk disease by French-American-British (67%) or by International Prognostic Scoring System (77%) with implications for therapy selection and prognosis calculation. These results demonstrate the complexity of the diagnosis of MDS and highlight the need for confirmation of diagnosis when in doubt.
Introduction
Myelodysplastic syndromes (MDSs) are a group of clonal stem cell disorders characterized by ineffective hematopoiesis and peripheral cytopenias.1 MDS is very heterogeneous, at the morphologic, clinical, and molecular levels.1 An accurate diagnosis is needed to predict survival, risk of transformation, and need to initiate therapy.2 Most patients with MDS are diagnosed at community/university hospitals. It is our experience that morphologic diagnosis can be difficult. Previous studies have shown a discordance rate of 18% in the diagnoses of leukemia.3 The aim of this study was to analyze the rate and implications of diagnostic discordance in patients with MDS referred to a tertiary care center.
Methods
We reviewed the medical records of 915 patients referred to M. D. Anderson Cancer Center (MDACC) between September 2005 and December 2009 with an outside diagnosis of MDS. We reviewed all the available outside bone marrow slides. Outside results were compared with the pathology report on the bone marrow aspirate and biopsy performed at our institution. Three independent pathologists were involved. Pathologist 1 released in the laboratory information system a report on the bone marrow differential from a 500-cell count of cellular bone marrow aspirates and/or touch imprints on day 1. Pathologist 2 released a final bone marrow pathology report on day 2, including peripheral blood film, core biopsy, clot section, and aspirate smears. He/she also reviewed outside specimens. Pathologist 3 reviewed all new leukemia cases with clinicians at the weekly leukemia planning conference and integrated molecular and genetic data (day 4).
Diagnoses were coded according to WHO4 and French-American-British5 MDS classification systems; that was helpful to enroll patients in clinical trials using different classifications and having different inclusion criteria. An extensive workup was done to exclude nonclonal causes for the dysplasia, including comprehensive review of peripheral blood film, flow immunophenotypic MDS panel, paroxysmal nocturnal hemoglobinuria (PNH) panel, infectious disease panel, clinical chemistry panel, and immunohistochemical stains on biopsy or clot section for CD34, CD61, CD117, glycophorin A, and mast cell tryptase. In addition to demographic data that included age and gender, we also collected information on the International Prognostic Scoring System (IPSS),6 cytogenetics, complete blood count, percentage of bone marrow blasts, and transformation to acute myelogenous leukemia. Overall survival was calculated in months from the date of presentation to MDACC until death from any cause. Persons were censored if alive at the time of last observation. The median survival was calculated using Kaplan-Meier method.
Results and discussion
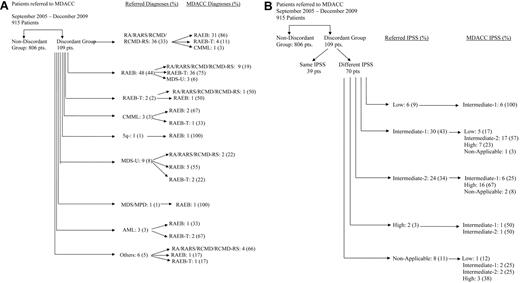
A total of 915 patients were reviewed. Discordance, defined as a difference in morphologic diagnoses between the referring center and MDACC, was documented in 109 of the 915 (12%) patients (Figure 1A). Referred cases were diagnosed at community hospitals in 69 (63%) patients, commercial laboratory in 30 (28%), and university hospitals in 9 (9%). Of the 109 patients, 36 (33%) had an outside diagnoses of refractory anemia (RA)/refractory anemia with ring sideroblasts (RARS)/refractory cytopenia with multilineage dysplasia (RCMD)/refractory cytopenia with multilineage dysplasia with ringed sideroblasts (RCMD-RS, marrow blast < 5%). Thirty-one (86%) of these were diagnosed with refractory anemia with excess blasts (RAEB; marrow blasts 5%-20%), 4 (11%) with RAEB-T (marrow blasts > 20%), and 1 (3%) with chronic myelomonocytic leukemia at MDACC. In contrast, 48 of the 109 patients (44%) had an outside diagnosis of RAEB. Of these, 9 (19%) patients were diagnosed with RA/RARS/RCMD/RCMD-RS and 36 (75%) patients were diagnosed with RAEB-T. Only 2 patients were diagnosed with RAEB-T (marrow blast > 20%) on the outside but were diagnosed with RA and RAEB, one of each at MDACC. Three patients were diagnosed with chronic myelomonocytic leukemia, 9 with unclassified MDS, 1 with MDS/MPD, and 1 with 5q- syndrome. No discrepancies were noted between RA/RARS and RCMD, or between RA and RARS.
Discordance between referral diagnoses and final diagnoses at MDACC. (A) By French-American-British. RA indicates refractory anemia; RARS, refractory anemia with ring sideroblasts; RCMD, refractory cytopenia with multilineage dysplasia; RCMD-RS, refractory cytopenia with multilineage dysplasia with ringed sideroblasts; CMML, chronic myelomonocytic leukemia; MDS-U, myelodysplastic syndrome unclassifiable; AML, acute myelogenous leukemia; others, chronic myeloid leukemia, myelofibrosis, normal bone marrow; and pts, patients. (B) By International Prognostic Scoring System (IPSS). Nonapplicable includes referral diagnosis of AML, chronic lymphocytic leukemia, CMML, myelofibrosis, or normal bone marrow examination. Pts indicates patients.
Discordance between referral diagnoses and final diagnoses at MDACC. (A) By French-American-British. RA indicates refractory anemia; RARS, refractory anemia with ring sideroblasts; RCMD, refractory cytopenia with multilineage dysplasia; RCMD-RS, refractory cytopenia with multilineage dysplasia with ringed sideroblasts; CMML, chronic myelomonocytic leukemia; MDS-U, myelodysplastic syndrome unclassifiable; AML, acute myelogenous leukemia; others, chronic myeloid leukemia, myelofibrosis, normal bone marrow; and pts, patients. (B) By International Prognostic Scoring System (IPSS). Nonapplicable includes referral diagnosis of AML, chronic lymphocytic leukemia, CMML, myelofibrosis, or normal bone marrow examination. Pts indicates patients.
Because treatment decision is based on the IPSS in the majority of the cases, we assessed the impact of discrepancy by applying the IPSS at the time of referral to MDACC and compared with the IPSS calculated based on local report information. Of the 109 patients reported in the preceding paragraph, 70 (64%) patients were found to have discordance in their IPSS staging (Figure 1B). All the 6 (100%) patients with low-risk disease from outside were reclassified as intermediate-1. Of the 30 patients with intermediate-1 risk disease, 24 (80%) were reclassified as intermediate-2 (n = 17) or high-risk (n = 7) disease. Of the 24 patients with intermediate-2 disease, 6 (25%) had lower-risk disease. In 8 patients where IPSS was not applicable on outside diagnosis, 63% of them were either intermediate-2 (n = 2) or high-risk (n = 3) disease. Discrepancies in cytogenetic data were noted in 10 of 66 (15%) evaluable patients; of those 10 patients with cytogenetic discrepancy, 7 (70%) considered to be diploid were found to harbor complex cytogenetics. Table 1 highlights the discrepancy by blast percentage.
Comparison of bone marrow blasts percentage between referral center and MDACC in 76 patients in whom outside slides were available
| Referral BM blasts, % . | MDACC BM blasts, % . | ||||
|---|---|---|---|---|---|
| < 5 . | 5-10 . | 11-20 . | > 20 . | Total . | |
| < 5 | 0 | 22 | 8 | 2 | 32 |
| 5-10 | 8 | 0 | 3 | 3 | 14 |
| 11-20 | 2 | 1 | 0 | 18 | 21 |
| > 20 | 0 | 0 | 3 | 0 | 3 |
| NA | 3 | 2 | 1 | 0 | 6 |
| Total | 13 | 25 | 15 | 23 | 76 |
| Referral BM blasts, % . | MDACC BM blasts, % . | ||||
|---|---|---|---|---|---|
| < 5 . | 5-10 . | 11-20 . | > 20 . | Total . | |
| < 5 | 0 | 22 | 8 | 2 | 32 |
| 5-10 | 8 | 0 | 3 | 3 | 14 |
| 11-20 | 2 | 1 | 0 | 18 | 21 |
| > 20 | 0 | 0 | 3 | 0 | 3 |
| NA | 3 | 2 | 1 | 0 | 6 |
| Total | 13 | 25 | 15 | 23 | 76 |
Blast differences were based on counting the same slides when available.
BM indicates bone marrow; and NA, not applicable.
To rule out disease progression as a possible cause of discordance between the 2 diagnoses, we calculated the number of patients with outside diagnostic marrow slides/hematopathology reports of ≥ 6 months from diagnosis to referral to MDACC. We chose 6 months, considering it to be an appropriate duration for disease progression. Only 19 (17%) patients in the discordant group were noted to have an outside slide/report of ≥ 6 months; 6 (6%) patients of these had a higher marrow blast percentage than outside, indicating possible disease progression.
The characteristics of patients with diagnostic discordance were compared with the 806 patients without discordance (Table 2). As expected, MDS was more common in the elderly: 68% in discordant versus 74% in the nondiscordant group were older than 60 years (P = .17). The majority of the patients in both groups had diploid cytogenetics (P = .87). Patients in the discordant group had a lower platelet count (P = .05), a lower hemoglobin level (P = .004), and a higher bone marrow blast percentage (P < .001); therefore, patients in the discordant group were noted to have a higher percentage of RAEB-T (42% vs 11%, P < .0001) and were of higher-risk IPSS (P < .0001). No difference was noted in transformation to acute myelogenous leukemia between the 2 groups (P = .96). Finally, of the 109 patients with discrepancy, 22 (20%) patients were therapy related.
Comparison of patient characteristics between the discordant and nondiscordant groups
| Characteristic . | Nondiscordant . | Discordant . | Total . | P . | ||
|---|---|---|---|---|---|---|
| n . | % . | n . | % . | |||
| Age | ||||||
| < 60 y | 209 | 26 | 35 | 32 | 244 | .17 |
| ≥ 60 y | 597 | 74 | 74 | 68 | 671 | |
| Sex | ||||||
| Female | 308 | 38 | 40 | 37 | 348 | .76 |
| Male | 498 | 62 | 69 | 63 | 567 | |
| Diagnosis | ||||||
| RA/RARS/RCMD/RCMD-RS | 355 | 44 | 16 | 14 | 371 | < .001* |
| RAEB | 316 | 39 | 43 | 39 | 359 | |
| RAEB-T | 86 | 11 | 46 | 42 | 132 | |
| MDS-U | 32 | 4 | 3 | 3 | 35 | |
| 5q− | 14 | 2 | 0 | 0 | 14 | |
| CMML | 2 | 0.2 | 1 | 1 | 3 | |
| AML | 1 | 0.1 | 0 | 0 | 1 | |
| IPSS | ||||||
| Low | 161 | 20 | 7 | 6 | 168 | < .001* |
| Intermediate-1 | 291 | 36 | 28 | 26 | 319 | |
| Intermediate-2 | 196 | 24 | 28 | 26 | 224 | |
| High | 102 | 13 | 40 | 37 | 142 | |
| Not applicable | 56 | 7 | 6 | 5 | 62 | |
| Cytogenetics | ||||||
| Diploid | 343 | 43 | 44 | 41 | 387 | .87 |
| −5/−7 | 231 | 29 | 35 | 32 | 266 | |
| Other | 162 | 20 | 22 | 20 | 184 | |
| Insufficient metaphases | 70 | 8 | 8 | 7 | 78 | |
| WBC | ||||||
| <10 | 740 | 92 | 98 | 90 | 838 | .47 |
| ≥ 10 | 61 | 7 | 11 | 10 | 72 | |
| Information not available | 5 | 1 | 0 | 0 | 5 | |
| Platelet | ||||||
| < 100 | 488 | 60 | 79 | 72 | 567 | .05* |
| ≥ 100 | 313 | 39 | 30 | 28 | 343 | |
| Information not available | 5 | 1 | 0 | 0 | 5 | |
| Hemoglobin | ||||||
| <10 | 428 | 53 | 76 | 70 | 504 | .004* |
| ≥ 10 | 373 | 46 | 33 | 30 | 406 | |
| Information not available | 5 | 1 | 0 | 0 | 5 | |
| Marrow blasts | ||||||
| 0-4 | 436 | 54 | 18 | 17 | 454 | < .001* |
| 5-10 | 162 | 20 | 32 | 29 | 194 | |
| 11-20 | 115 | 14 | 25 | 23 | 140 | |
| > 20 | 64 | 8 | 33 | 30 | 97 | |
| Not done/not applicable | 29 | 4 | 1 | 1 | 30 | |
| Transformation to AML | ||||||
| Yes | 75 | 9 | 10 | 9 | 85 | .96 |
| No | 731 | 91 | 99 | 91 | 830 | |
| Characteristic . | Nondiscordant . | Discordant . | Total . | P . | ||
|---|---|---|---|---|---|---|
| n . | % . | n . | % . | |||
| Age | ||||||
| < 60 y | 209 | 26 | 35 | 32 | 244 | .17 |
| ≥ 60 y | 597 | 74 | 74 | 68 | 671 | |
| Sex | ||||||
| Female | 308 | 38 | 40 | 37 | 348 | .76 |
| Male | 498 | 62 | 69 | 63 | 567 | |
| Diagnosis | ||||||
| RA/RARS/RCMD/RCMD-RS | 355 | 44 | 16 | 14 | 371 | < .001* |
| RAEB | 316 | 39 | 43 | 39 | 359 | |
| RAEB-T | 86 | 11 | 46 | 42 | 132 | |
| MDS-U | 32 | 4 | 3 | 3 | 35 | |
| 5q− | 14 | 2 | 0 | 0 | 14 | |
| CMML | 2 | 0.2 | 1 | 1 | 3 | |
| AML | 1 | 0.1 | 0 | 0 | 1 | |
| IPSS | ||||||
| Low | 161 | 20 | 7 | 6 | 168 | < .001* |
| Intermediate-1 | 291 | 36 | 28 | 26 | 319 | |
| Intermediate-2 | 196 | 24 | 28 | 26 | 224 | |
| High | 102 | 13 | 40 | 37 | 142 | |
| Not applicable | 56 | 7 | 6 | 5 | 62 | |
| Cytogenetics | ||||||
| Diploid | 343 | 43 | 44 | 41 | 387 | .87 |
| −5/−7 | 231 | 29 | 35 | 32 | 266 | |
| Other | 162 | 20 | 22 | 20 | 184 | |
| Insufficient metaphases | 70 | 8 | 8 | 7 | 78 | |
| WBC | ||||||
| <10 | 740 | 92 | 98 | 90 | 838 | .47 |
| ≥ 10 | 61 | 7 | 11 | 10 | 72 | |
| Information not available | 5 | 1 | 0 | 0 | 5 | |
| Platelet | ||||||
| < 100 | 488 | 60 | 79 | 72 | 567 | .05* |
| ≥ 100 | 313 | 39 | 30 | 28 | 343 | |
| Information not available | 5 | 1 | 0 | 0 | 5 | |
| Hemoglobin | ||||||
| <10 | 428 | 53 | 76 | 70 | 504 | .004* |
| ≥ 10 | 373 | 46 | 33 | 30 | 406 | |
| Information not available | 5 | 1 | 0 | 0 | 5 | |
| Marrow blasts | ||||||
| 0-4 | 436 | 54 | 18 | 17 | 454 | < .001* |
| 5-10 | 162 | 20 | 32 | 29 | 194 | |
| 11-20 | 115 | 14 | 25 | 23 | 140 | |
| > 20 | 64 | 8 | 33 | 30 | 97 | |
| Not done/not applicable | 29 | 4 | 1 | 1 | 30 | |
| Transformation to AML | ||||||
| Yes | 75 | 9 | 10 | 9 | 85 | .96 |
| No | 731 | 91 | 99 | 91 | 830 | |
Statistically significant (P = .05).
Few studies have been conducted to evaluate discrepancies in the diagnoses between the referring and tertiary care centers. DeLima et al reported major discrepancies in diagnoses of 18% of patients with leukemia that affected their treatment and/or prognosis.3 To the best of our knowledge, this is the first study to evaluate diagnostic discrepancies in patients with MDS, where treatment decision and prognosis are mainly based on morphologic diagnosis. The majority of the patients with diagnostic discordance were noted to have higher-risk disease at MDACC than originally calculated (77%); therefore, they were reclassified and treated as such. This has significant implications for calculation of prognosis and therapeutic decision making. New therapies that have changed the natural history of this disease are now available and could benefit these patients.7-9 Indeed, the overall survival between the nondiscordant and the discordant groups at our institution were similar (data not shown), probably because of the appropriate diagnosis and subsequent management of such patients resulting in prolonged survival in the latter group.
One major limitation of our study was the assumption that the diagnosis made at MDACC is accurate and correct. No third party reviewed the slides to confirm this assumption, but it should be noted that 3 independent pathologists reviewed each case referred. Moreover, we had no control on the time that lapsed between outside evaluation and presentation to our cancer center. However, we did a subset analysis, looking into patients with diagnostic slides ≥ 6 months. Only 19 (17%) patients had an outside evaluation ≥ 6 months, with only 6 (6%) patients with a more high-risk disease indicating possible progression.
In the absence of biologic markers to stratify patients with MDS, morphologic assessment determining the percentage of blasts as well as of ring sideroblasts is essential for defining risk, regardless of which risk system is used. To minimize discrepancies, Mufti et al have proposed a consensus for the definition and enumeration of myeloblasts and ring sideroblasts.10 These definitions used in conjunction with the WHO classification are critical to make the categorization of patients with MDS more precise. However, in certain cases, repeated bone marrow biopsies, including cytogenetic studies over a period of several months, may be necessary in difficult cases.
In conclusion, discordance in the diagnosis of MDS patients is frequent (12%) and can affect the treatment plan and overall prognosis of these patients. These results demonstrate the complexity of the diagnosis of MDS and the need for morphologic confirmation by experienced pathologists.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: K.N. analyzed data and wrote the manuscript; E.J. analyzed data, wrote the manuscript, and provided materials; C.B.-R. analyzed data and wrote and approved the manuscript; S.P. analyzed data; G.B., Z.E., F.R., S.F., and H.K. provided materials and approved the manuscript; and G.G.-M. designed the concept, analyzed data, provided materials, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Elias Jabbour, Department of Leukemia, University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Box 428, Houston, TX 77030; e-mail: ejabbour@mdanderson.org.
References
Author notes
K.N. and E.J. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal