Abstract
Apoptosis is crucial for immune system homeostasis, including selection and survival of long-lived antibody-forming cells and memory cells. The interactions between proapoptotic and pro-survival proteins of the Bcl-2 family are critical for this process. In this report, we show that expression of the proapoptotic BH3-only Bcl-2 family member Puma was selectively up-regulated on in vitro activation with antigens or mitogens of both human and mouse B cells. Puma expression coincided in vivo, with the prosurvival Bcl-2 family member Mcl-1 within the germinal centers and its expression correlates with the germinal center like phenotype of Burkitt lymphoma. Experiments performed in Puma-deficient mice revealed that Puma is essential for apoptosis of mitogen-activated B cells in vitro and for the control of memory B-cell survival. In conclusion, using both human and murine models, our data show that Puma has a major role in the T cell– dependent B-cell immune response. These data demonstrate that Puma is a major regulator of memory B lymphocyte survival and therefore a key molecule in the control of the immune response.
Introduction
Humoral immunity is a highly orchestrated process involving antigen-specific T-B cell interactions leading naive B cells to (1) rapidly become activated, proliferate and differentiate into short-lived plasma cells secreting low affinity antibodies, and (2) generate high-affinity antigen-specific antibody secreting B cells after somatic hypermutations and recombination of immuno-globulin genes in the germinal center.1 This cellular process allows for the formation of memory B cells and long-lived antibody-forming cells (AFCs).2 Generation and persistence of these cells are critical for the life-long production of high-affinity antibodies against the immunizing antigen which is an important component of immunologic memory. Apoptosis is indispensable for selection of high-affinity effector cells and for maintenance of self-tolerance. B cells expressing low affinity antibodies are deleted by apoptosis, whereas clones expressing BCR with enhanced affinity for the immunogen are positively selected.1,3,4 Apoptosis is also crucial for immune system homeostasis by inducing the death of the clonally expanded lymphocytes once the antigen has been eliminated.5
Proteins of the Bcl-2 family play a critical role in controlling the humoral immune response. Immunized transgenic mice over-expressing antiapoptotic Bcl-2 or Bcl-xL in their lymphocytes exhibit a profound increase in the numbers of antigen-specific B cells and antibody secreting plasma cells compared with wild type mice.6-9 The Bcl-2 proteins are key regulators of cell survival and are classified into 3 sub-groups.10 The pro-survival members (Bcl-2, Bcl-xL, Bcl-w, Mcl-1, and A1) are essential for cell survival. Bax, Bak are proapoptotic and required for activation of the downstream phases of apoptosis, including permeabilization of the outer mitochondrial membrane (MOMP) with consequent activation of the caspase cascade that elicits cellular demolition. The so-called BH3-only proteins (Bad, Bid, Bim/Bod, Bik/Blk/Nbk, Hrk/DP5, Bmf, Noxa, and Puma/Bbc3) share with each other and the wider Bcl-2 family only the BH3 region and are essential for initiation of apoptosis signaling.11
BH3-only proteins play an important role in the homeostasis of the immune system.5 For instance, Bim-deficient mice accumulate abnormally increased numbers of B cells and develop hyper-gammaglobulinemia, which, on a mixed C57BL/6 × 129SV background, progresses to fatal immune complex mediated systemic lupus erythematosus (SLE)–like autoimmune kidney disease.12 Moreover, immunized Bim−/− mice exhibited an abnormal excess of antigen-specific memory B cells and antibody-forming cells.13 However, the less marked phenotype of the Bim−/− mice compared with the Bcl-2 transgenic mice indicates that other BH3-only proteins may also contribute to the apoptosis of activated B cells during humoral immune responses. Analyses of Bid−/−, Bad−/−, Bik−/− single knock-out as well as Bim−/−Bid−/−, Bim−/−Bad−/− and Bim−/−Bik−/− mice have demonstrated that Bid, Bad and Bik are not critical for this process.13-17 In contrast, studies of mice deficient for both Bim and Puma showed that these 2 BH3-only proteins have overlapping functions in B-cell homeostasis.18 In culture, the Bim−/−Puma−/− B cells are more resistant to a range of apoptotic stimuli, including cytokine deprivation, compared with Bim−/− or Puma−/− B cells. Although early studies on Puma-deficient mice have demonstrated the essential role of Puma DNA damage-induced p53-mediated apoptosis,19,20 no abnormalities in B-cell immune responses of Puma−/− mice have been reported so far.
In this study, we investigated the role of the BH3-only protein Puma in the control of apoptosis during B-cell activation. We found that Puma is up-regulated in vitro on mitogenic activation in human as well as mouse B cells. Experiments performed on Puma-deficient mice revealed that Puma is essential for apoptosis of activated B cells both in vitro and in vivo. Immunized Puma-deficient mice exhibited an excess of antigen-specific memory B cells compared with wild-type animals. This indicates that Puma is a critical regulator of the survival of antigen-specific memory B cells.
Methods
Reagents and antibodies
Anti–human IgM (DA.44) and anti–human CD40 (G.28-5) mAbs (ATCC) were purified on protein A-Sepharose columns (Pharmacia Biotech). Anti–mouse IgM antibody was purchased from Jackson Immunoresearch. The c-Myc inhibitor 10058-F4 and the caspase inhibitor QVD-OPH were obtained from Calbiochem and R&D Systems. Lipopolysaccharide was from Sigma-Aldrich.
Anti–Mcl-1 (S-19), anti-Tubulin (TU-02), anti–Bcl-2 (C-2), anti-Bik (fl-160), and anti–Bcl-xL (S-18) antibodies were from Santa Cruz Biotechnology Inc. Anti-GAPDH and anti-actin antibodies were from Sigma-Aldrich and the anti-Puma, anti-A1/Bfl1, anti–Bcl-6, and anti-Bid antibodies were from Cell Signaling Technology. Anti-Bim, anti-Noxa, and anti-Bad antibodies were from Epitomics, Imgenex, and Delta Biolabs, respectively.
Mice, antigens, and immunization
Mice were bred and maintained at The Walter and Eliza Hall Institute (WEHI) and all experiments with mice had been approved by the WEHI Animal Ethics Committee. The generation and genotyping of Puma−/− mice has been described previously.19 Mice were immunized with a single intraperitoneal injection of NP (100 μg) conjugated to KLH (Calbiochem) at a ratio of 13:1 and precipitated onto alum.
Cells and cell culture
Resting B cells were purified from human tonsils as previously described,21 or from murine spleens using the CD19+ B-cell isolation kit (Miltenyi Biotec). The BJAB, Ramos, BL41 Burkitt lymphoma cell lines were obtained from ATCC and Dr G. Lenoir (Lyon). Resting B cells (2 × 106/mL) were cultured for various periods of time in RPMI 1640 medium (Life Technologies) supplemented with 2mM l-glutamine, 25mM HEPES, and 10% FCS (Life Technologies) in the presence of either SAC (1/10 000), LPS (10 μg/mL) or anti-μ Ab (10 μg/mL), and anti-CD40 Ab (5 μg/mL). Burkitt lymphoma–derived cell lines were cultured as described.22
Western blot analysis, immunoprecipitation, and RT-PCR
For Western blotting and immunoprecipitation, cells were lysed and SDS-PAGE performed as described previously.22 Antibody binding was detected by incubation with sheep anti-mouse IgG or sheep anti–rabbit IgG antibodies conjugated to HRP followed by chemiluminescence (West-pico, Pierce). Images were captured using a DDC camera (LAS-1000 Fuji). RT-PCR were performed as described23 using 5′- GTCCTCAGCCCTCGCTCT -3′ and 5′- CTAATTGGGCTCCATCTCG -3′ primers for Puma and 5′- CGAGATCCCTCCAAAATCAA -3′ and 5′- GTCTTCTGGGTGGCAGTGAT -3′ primers for GAPDH.
Immunofluorescent staining, flow cytometric analysis, cell sorting, and IgVH gene sequencing
Spleen, bone marrow, and blood were collected and processed to obtain single cell suspensions as described.24 Single cell suspensions or B220+ enriched cell populations using MACSORT columns (Miltenyi Biotec) were stained using the following rat monoclonal antibodies: RA3-6B2 (anti-B220), 331.12 (anti-IgM), 11-26C (anti-IgD), IgG1 × 56 (anti-IgG1; BD Pharmingen), MI/70 (anti–Mac-1), RB6-8C5 (anti–Gr-1), 281.2 (anti-CD138; BD Pharmingen), NIMR-5 (anti-CD38), 1D3 (anti-CD19), and F4/80. NP binding was detected as described previously25 by staining with APC-coupled NP. Stained cells were analyzed on a FACStar (BD Biosciences).
Single antigen-specific B cells were sorted and subjected to VH186.2 gene PCR amplification and nucleotide sequencing as described previously.7
For detection of apoptotic cells, dot-blot light scatter profiles were analyzed by flow cytometry using a FACScan (BD Biosciences). Apoptotic cells, having relatively high side scatter and low forward scatter properties, were expressed as a percentage of the total population.
ELISPOT
ELISPOT assays were performed by titrating spleen- or bone marrow–derived leukocytes into cellulose ester-based plates coated with NP20- or NP2-BSA and cultured overnight. Bound anti-NP antibodies were visualized by staining with goat anti–mouse IgG1 antibodies directly coupled to HRP as described.26 Spots were counted with an ELISPOT reader system (Autoimmun Diagnostika).
Immunostaining of histologic sections and frozen spleen
Neutral formalin fixed and paraffin embedded histologic sections of tonsils were used for immunostaining. After pretreatment in EDTA buffer (Dako), slides were incubated with anti-Puma antibody (Cell Signaling) at a dilution of 1/300 or with anti–Bcl-2 antibody (Dako), anti–Mcl-1 antibody (Santa Cruz Biotechnology) or anti–Bcl-xL antibody (Zymed) at a dilution of 1/100. Immunostaining was revealed by secondary staining with anti-IgG antibodies coupled to horseradish peroxidase with the EnVision + dual link System-HRP (Dako) and sections were counterstained with hematoxylin (Dako).
Spleens from 14 days immunized wild-type or Puma−/− mice were embedded, stored, sectioned, and stained as described previously.27 GL7 was detected with Alexa555-conjugated goat anti–rat antibodies (Invitrogen) and biotinylated anti-B220 antibodies with streptavidin-Cy5 (Southern Biotechnology Associates).
Results
Activated human B cells expressed higher levels of Puma compared with resting B cells
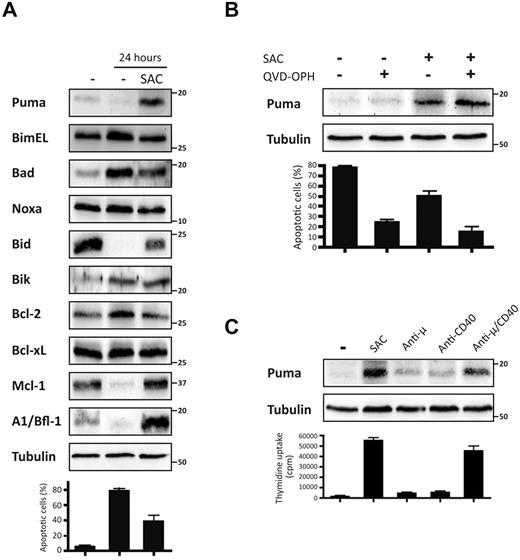
Expression of different Bcl-2 family proteins was investigated in human tonsillar purified B cells that had been left untreated or stimulated for 24 hours with the mitogen Staphylococcus aureus Cowan 1 strain (SAC). Resting B cells that had been left untreated for 24 hours were mostly apoptotic. In contrast, stimulation with SAC allowed cultured B cells to survive (39% vs 80% survival). Analysis of protein levels are presented in Figure 1A. Among the BH3-only proteins assessed, only Bim, Noxa and Bid were expressed at readily detectable levels in resting B cells, whereas Puma, Bik, and Bad were only weakly detected. The expression of Bim and Noxa did not vary after 24 hours in culture, with or without mitogenic stimulation. Bid was undetectable after 24 hours in nonactivated B cells and was only weakly detected in activated B cells. The disappearance of Bid after 24 hours is most likely because of its caspase-mediated cleavage. Accordingly, this could be prevented by the addition of the broad-spectrum caspase-inhibitor QVD-OPH (data not shown). Bad and Bik expression levels were increased in nonactivated cells (mostly apoptotic) but were not significantly augmented in response to stimulation with SAC (Bik) or even slightly decreased (Bad). The pattern of Puma expression differed substantially from the other BH3-only proteins. Puma was not detected in freshly isolated resting B cells or in nonactivated cells after 24 hours in culture. In contrast, expression of Puma was strongly up-regulated after 24 hours of SAC-mediated activation (Figure 1A). Regarding the antiapoptotic proteins, expression of Bcl-2 and Bcl-xL remained unchanged whereas the levels of Mcl-1 and A1/Bfl1 were diminished in nonactivated (and mostly dying) B cells but were augmented in response to mitogenic stimulation.
Expression of Puma was up-regulated in activated human B cells. (A) Human purified tonsillar B lymphocytes (2 × 106 cells/mL) were left untreated or stimulated with mitogenic doses of the S aureus Cowan 1 strain (pansorbin: SAC; 1/10 000) for 24 hours. Expression levels of various Bcl-2 family proteins and tubulin (loading control) were analyzed by immunoblotting. The percentages of apoptotic cells were determined by flow cytometric analysis. Data represent mean (± SEM; B). Human purified tonsillar B lymphocytes (2 × 106 cells/mL) were stimulated with SAC (1/10 000) in the presence or absence of the broad-spectrum caspase inhibitor QVD-OPH (10nM) to prevent apoptosis. Expression levels of Puma and tubulin (loading control) were evaluated by immunoblotting. The percentages of apoptotic cells were determined by flow cytometric analysis. Data represent mean (± SEM). (C) Human purified tonsillar B lymphocytes (2 × 106 cells/mL) were stimulated for 24 hours in the presence or absence of SAC (1/10 000), or anti–human μ antibodies (10 μg/mL) or anti–human CD40 antibodies (5 μg/mL) either by themselves or in combination. Expression levels of Puma and tubulin (loading control) were analyzed by immunoblotting. DNA synthesis was quantified by measuring incorporation of 3H-thymidine during the last 16 hours of culture. Results are representative of 4 independent experiments.
Expression of Puma was up-regulated in activated human B cells. (A) Human purified tonsillar B lymphocytes (2 × 106 cells/mL) were left untreated or stimulated with mitogenic doses of the S aureus Cowan 1 strain (pansorbin: SAC; 1/10 000) for 24 hours. Expression levels of various Bcl-2 family proteins and tubulin (loading control) were analyzed by immunoblotting. The percentages of apoptotic cells were determined by flow cytometric analysis. Data represent mean (± SEM; B). Human purified tonsillar B lymphocytes (2 × 106 cells/mL) were stimulated with SAC (1/10 000) in the presence or absence of the broad-spectrum caspase inhibitor QVD-OPH (10nM) to prevent apoptosis. Expression levels of Puma and tubulin (loading control) were evaluated by immunoblotting. The percentages of apoptotic cells were determined by flow cytometric analysis. Data represent mean (± SEM). (C) Human purified tonsillar B lymphocytes (2 × 106 cells/mL) were stimulated for 24 hours in the presence or absence of SAC (1/10 000), or anti–human μ antibodies (10 μg/mL) or anti–human CD40 antibodies (5 μg/mL) either by themselves or in combination. Expression levels of Puma and tubulin (loading control) were analyzed by immunoblotting. DNA synthesis was quantified by measuring incorporation of 3H-thymidine during the last 16 hours of culture. Results are representative of 4 independent experiments.
We next activated B cells for 24 hours with mitogenic doses of SAC in the presence or absence of the caspase inhibitor QVD-OPH to establish whether the increased Puma levels were associated with the activation or apoptotic status of B lymphocytes (Figure 1B). Caspase inhibition strongly attenuated apoptosis of nonactivated B cells, but did not modify the levels of Puma in SAC-activated or nonstimulated B cells. Up-regulation of Puma was not restricted to SAC-mediated activation; it was also observed in the presence of more physiologic stimuli, such as those transmitted through the BCR and CD40. Indeed, mitogenic stimulation with a combination of anti-μ and anti-CD40 antibodies promoted B-cell proliferation (verified by H3-thymidine uptake), and also strongly up-regulated expression of Puma. Treatments with either anti-μ or anti-CD40 antibody alone, which are not potently mitogenic by themselves, did not significantly increase the levels of Puma (Figure 1C).
Collectively, these data demonstrate that mitogenic stimulation of human B lymphocytes in vitro causes a specific increase in the levels of the proapoptotic BH3-only protein Puma.
Expression of Puma was associated with germinal center–like cells
We next investigated whether similar changes in the levels of Puma occur during the activation of B lymphocytes in vivo using in situ staining for Puma expression in histologic sections of human tonsils. As previously reported,28 germinal center B cells were mostly Bcl-2 negative (Figure 2Ai). In contrast, staining with an anti-Puma antibody was found to produce a concentrated signal inside the germinal centers (Figure 2Aii), where it was colocalized with staining for the pro-survival Bcl-2 family member Mcl-1 (Figure 2Aiii), which has recently been reported to be essential for the survival of germinal center B cells.29 Localization of Bcl-xL was observed inside and outside the germinal center structures (Figure 2Aiv). These data show that the up-regulation of Puma is associated with B lymphocyte activation and that germinal center B cells express relatively high levels of Puma. In addition, in SAC-activated B cells, Puma clearly associated with Mcl-1 as demonstrated by immunoprecipitation followed by Western blotting, whereas no such association was observed with Bcl-xl (Figure 2B) or Bcl-2 (data not shown). Because Burkitt lymphoma has been shown to be derived from germinal center centroblasts,30 we next assessed the levels of Puma expression in 3 different Burkitts lymphoma derived cell lines BJAB, BL41, and Ramos, and compared them to the levels found in resting normal (nontransformed) B cells. Indeed, the levels of Puma were significantly higher in the Burkitt lymphoma derived cell lines compared with the control resting B cells (Figure 2C). The hallmark of Burkitt lymphoma is the chromosomal translocation of the c-myc oncogene into the Ig loci, resulting in deregulated c-Myc overexpression. It has recently been shown that c-Myc overexpression is responsible for the “centroblast-like” phenotype of the Burkitt lymphoma cells and that treatment of these cells with a specific c-Myc inhibitor, 10058-F4 inhibits expression of proteins associated with the germinal center phenotype, such as Bcl-6.31 We therefore explored whether the modification of this “germinal center phenotype” was also associated with a change in the regulation of Puma expression. Indeed, we observed a parallel decrease in the levels of Bcl-6 and Puma proteins from BL41 cells in response to treatment with various doses of 10058-F4, whereas the levels of Bcl-2 or GAPDH were not altered (Figure 2D). To rule out that the decrease of Puma expression caused by 10058-F4 treatment could be because of caspase-3 dependent degradation of Puma,23 we treated 2 different Burkitt lymphoma cells lines, BL41 (Figure 2E) and Ramos (Figure 2F), with this Myc inhibitor in presence or absence of the caspase inhibitor QVD-OPH. Again, levels of Bcl-6 and Puma decreased significantly in response to treatment with the c-Myc inhibitor alone and this reduction was not affected by the addition of QVD-OPH. As c-Myc has been reported to bind to the Puma's promoter32 we examined whether inhibition of c-Myc had an impact on the Puma mRNA levels. No changes in Puma mRNA levels were observed in SAC-activated B cells (Figure 2G), or in BL41 (data not shown) when they were exposed to the Myc inhibitor 10058-F4. This indicates that Puma expression must be regulated by a more complex mechanism in this context. Collectively, these data demonstrate that Puma expression is associated with the germinal center activated B-cell phenotype, both in normal as well as cancerous human B cells.
Expression of Puma in normal and malignant B cells was associated with a germinal center phenotype. (A) Expression of Bcl-2 (i), Puma (ii), Mcl-1 (iii), and Bcl-xL (iv) was assessed in histologic sections of human lymph nodes by in situ immunostaining (×100 magnification). As a control, the same tonsil sample was stained with normal rabbit serum (at a dilution 1/8000) using the same protocol that was used for detection of PUMA (data not shown). (B) Tonsilar B cells (2 × 106 cells/mL) were activated in culture with SAC (1/10 000) for 24 hours. Cells were lysed in CHAPS buffer and cell lysates (50 μg protein) were immuno-precipitated with antibodies against Puma or control Ig. Immune complexes as well as input from Puma immunoprecipitates were analyzed by immunoblotting with antibodies against Mcl-1, Bcl-xL, or Puma. The asterisk represents a nonspecific cross-reactive band. (C) Expression of Puma and tubulin (loading control) was analyzed by immunoblotting in 3 Burkitt lymphoma–derived cell lines (BJAB, BL41, and Ramos) and compared with the expression levels in purified human tonsillar B lymphocytes. (D) BL41 cells were cultured for 24 hours in presence of various doses of the c-Myc inhibitor 10058-F4. Expression levels of Puma, Bcl-2, Bcl-6, and GAPDH (loading control) were analyzed by immunoblotting. (E-F) BL41 cells (E) or Ramos cells (F) were treated in culture for 24 hours with the c-Myc inhibitor 10058-F4 (20μM) in the presence or absence of the broad spectrum caspase inhibitor QVD-OPH (10nM). Expression levels of Puma, Bcl-2, Bcl-6, and GAPDH (loading control) were examined by immunoblotting. (G) Tonsilar B cells (2 × 106 cells/mL) were activated with SAC (1/10 000) in the absence or presence of the c-Myc inhibitor 10058-F4 (20μM) for 24 hours: mRNA levels for Puma and GAPDH were analyzed for various cycles of PCR. Results are representative of 3 independent experiments.
Expression of Puma in normal and malignant B cells was associated with a germinal center phenotype. (A) Expression of Bcl-2 (i), Puma (ii), Mcl-1 (iii), and Bcl-xL (iv) was assessed in histologic sections of human lymph nodes by in situ immunostaining (×100 magnification). As a control, the same tonsil sample was stained with normal rabbit serum (at a dilution 1/8000) using the same protocol that was used for detection of PUMA (data not shown). (B) Tonsilar B cells (2 × 106 cells/mL) were activated in culture with SAC (1/10 000) for 24 hours. Cells were lysed in CHAPS buffer and cell lysates (50 μg protein) were immuno-precipitated with antibodies against Puma or control Ig. Immune complexes as well as input from Puma immunoprecipitates were analyzed by immunoblotting with antibodies against Mcl-1, Bcl-xL, or Puma. The asterisk represents a nonspecific cross-reactive band. (C) Expression of Puma and tubulin (loading control) was analyzed by immunoblotting in 3 Burkitt lymphoma–derived cell lines (BJAB, BL41, and Ramos) and compared with the expression levels in purified human tonsillar B lymphocytes. (D) BL41 cells were cultured for 24 hours in presence of various doses of the c-Myc inhibitor 10058-F4. Expression levels of Puma, Bcl-2, Bcl-6, and GAPDH (loading control) were analyzed by immunoblotting. (E-F) BL41 cells (E) or Ramos cells (F) were treated in culture for 24 hours with the c-Myc inhibitor 10058-F4 (20μM) in the presence or absence of the broad spectrum caspase inhibitor QVD-OPH (10nM). Expression levels of Puma, Bcl-2, Bcl-6, and GAPDH (loading control) were examined by immunoblotting. (G) Tonsilar B cells (2 × 106 cells/mL) were activated with SAC (1/10 000) in the absence or presence of the c-Myc inhibitor 10058-F4 (20μM) for 24 hours: mRNA levels for Puma and GAPDH were analyzed for various cycles of PCR. Results are representative of 3 independent experiments.
Puma was not required for B-cell activation
To delineate a role for Puma in B-cell homeostasis, the effects of its loss on B-cell activation were examined by analyzing the responses of splenic B lymphocytes from Puma-deficient mice (Puma−/−) to various mitogens. Murine splenic B cells exhibited a strong up-regulation of Puma levels after the combined stimulation with anti-μ and anti-CD40 antibodies, as previously observed in human B cells (Figure 3A). Increased levels of Puma were also observed after stimulation with lipopolysaccharide (LPS). B-cell activation was monitored by changes in cell size (forward light scatter; FSC) and granularity (side scatter; SSC). Activated B cells were found in fraction R1, while apoptotic cells (shrunken cells) appeared in fraction R2 (Figure 3Bi and ii). The R1 fraction of enlarged cells corresponds to activated B cells, because > 93% of the cells were CD19+ and CD69+ (Figure 3Biii). The impact of Puma deficiency on B-cell activation were examined by comparing the responses to LPS or anti-μ/anti-CD40 stimulation between wild-type and Puma−/− B lymphocytes (Figure 3C). No differences were noticed in the ability of these mitogenic stimuli to promote B-cell activation between B cells from wild-type and Puma−/− mice, as evaluated by H3-thymidine incorporation and FACS based enumeration of activated B cells.
Puma was not required for B-cell activation. (A) Wild-type splenic B cells (2 × 106 cells/mL) were cultured for 48 hours in the presence or absence of LPS (10 μg/mL), or Fab'2 anti–mouse μ antibody fragments (10 μg/mL) plus anti–mouse CD40 antibodies (5 μg/mL). Expression levels of Puma and tubulin (loading control) were assessed by immunoblotting. (B) Splenic B cells (2 × 106 cells/mL) from wild-type mice were either left untreated or stimulated with Fab'2 anti–mouse μ antibody fragments (10 μg/mL) plus anti–mouse CD40 antibodies (5 μg/mL) for 48 hours. Cells were then labeled with FITC–anti-CD69 and PE–anti-CD19 antibodies and analyzed by flow cytometry. Enlarged activated B cells (R1) with increased forward scatter (FSC) were quantified by flow cytometric analysis and CD19+/CD69+ cells were quantified within the R1 fraction. (C) Splenic B cells (2 × 106 cells/mL) from wild-type (Wt) or Puma-deficient (Puma−/−) mice were either left untreated or stimulated with LPS (10 μg/mL) or the combination of Fab'2 anti–mouse μ antibody fragments (10 μg/mL) plus anti–mouse CD40 antibodies (5 μg/mL) for 48 hours. The percentages of activated B cells were determined by flow cytometric analysis and B-cell proliferation was quantified by measuring 3H-thymidine incorporation into cellular DNA during the last 16 hours of culture. Data represent mean (± SEM). (D) Frozen spleen sections from wild-type and Puma−/− mice that had been immunized 14 days earlier with 100 μg NP coupled to KLH, were stained with antibodies to B220 to identify follicles (red) and GL7 for germinal centers (green; ×20 magnification). Results are representative of 3 independent experiments.
Puma was not required for B-cell activation. (A) Wild-type splenic B cells (2 × 106 cells/mL) were cultured for 48 hours in the presence or absence of LPS (10 μg/mL), or Fab'2 anti–mouse μ antibody fragments (10 μg/mL) plus anti–mouse CD40 antibodies (5 μg/mL). Expression levels of Puma and tubulin (loading control) were assessed by immunoblotting. (B) Splenic B cells (2 × 106 cells/mL) from wild-type mice were either left untreated or stimulated with Fab'2 anti–mouse μ antibody fragments (10 μg/mL) plus anti–mouse CD40 antibodies (5 μg/mL) for 48 hours. Cells were then labeled with FITC–anti-CD69 and PE–anti-CD19 antibodies and analyzed by flow cytometry. Enlarged activated B cells (R1) with increased forward scatter (FSC) were quantified by flow cytometric analysis and CD19+/CD69+ cells were quantified within the R1 fraction. (C) Splenic B cells (2 × 106 cells/mL) from wild-type (Wt) or Puma-deficient (Puma−/−) mice were either left untreated or stimulated with LPS (10 μg/mL) or the combination of Fab'2 anti–mouse μ antibody fragments (10 μg/mL) plus anti–mouse CD40 antibodies (5 μg/mL) for 48 hours. The percentages of activated B cells were determined by flow cytometric analysis and B-cell proliferation was quantified by measuring 3H-thymidine incorporation into cellular DNA during the last 16 hours of culture. Data represent mean (± SEM). (D) Frozen spleen sections from wild-type and Puma−/− mice that had been immunized 14 days earlier with 100 μg NP coupled to KLH, were stained with antibodies to B220 to identify follicles (red) and GL7 for germinal centers (green; ×20 magnification). Results are representative of 3 independent experiments.
We next investigated whether Puma-deficiency interfered with the formation of germinal centers. Puma-deficient and control wild-type mice were immunized with NP-KLH and 14 days later germinal centers identified by staining of spleen sections with antibodies specific to GL7 (green) and B220 (red). As shown in Figure 3D, similar morphology of germinal center were present in mice of both genotypes. These data show that loss of Puma does not perturb in vitro B-cell activation and germinal center formation in vivo.
Puma deficiency prolonged survival of activated B cells in vitro
We next assessed whether loss of Puma affected the survival of activated B cells in vitro (Figure 4). Wild-type and Puma−/− splenic B lymphocytes were stimulated with a mitogenic combination of anti-μ plus anti-CD40 antibodies (Figure 4A) or with LPS (Figure 4B), and the percentages of viable (PI-) activated B cells were measured at different time points. As shown in Figure 4A, the percentages of wild-type B cells activated with anti-μ plus anti-CD40 antibodies reached a maximum at day 2, and then decreased until day 8. The numbers of Puma-deficient activated B cells also reached a maximum at day 2, but in contrast to the wild-type B cells, their viability was maintained at a high level considerably longer. Indeed, 19% of mitogenically activated Puma−/− B cells were still viable on day 6 of culture compared with only 3% for the wild-type B cells. A similar survival advantage was observed when B cells were activated by LPS (Figures 4B). Both Puma and Mcl-1 were up-regulated in wild-type B cells in response to mitogenic stimulation. Although the kinetics differed slightly according to the mitogenic stimulus used, the pattern of up-regulation of Puma and Mcl-1 were similar and in accordance with the data presented in Figures 1 and 2 for human B cells. Moreover, enumeration of apoptotic cells 6 days after mitogenic activation showed that whereas all wild-type B cells were apoptotic, apoptosis in Puma-deficient B cells activated by anti-μ plus anti-CD40 antibodies or LPS was significantly reduced (72.6% and 82.4% respectively) for anti-μ plus CD40 and LPS treatment (Figure 4C). Labeling of cells with the dye CFSE showed that these long-term surviving mitogen activated Puma−/− B cells were cycling at the same rate as activated wild-type B cells (Figure 4D). These data demonstrate that Puma is a key factor in promoting apoptosis of mitogenically stimulated B cells in culture and thereby indicate that Puma may play a critical role in regulating the developmentally programmed apoptosis of activated B cells in vivo.
Puma-deficient B cells were more resistant than wild-type B cells to apoptosis on in vitro activation. (A-B) Wild-type and Puma-deficient (Puma−/−) splenic B cells (2 × 106 cells/mL) were stimulated for 8 days in culture by the combination of Fab'2 anti–mouse μ antibody fragments (10 μg/mL) plus anti–mouse CD40 antibodies (5 μg/mL; A) or LPS (10 μg/mL; B). Activated B cells were quantified by flow cytometry (see Figure 3C) from day 2 to day 8. Data represent the mean (± SEM) of n = 6 mice for each genotype. (*P < .05, **P < .01). Expression levels of Puma, Mcl-1, and GAPDH (loading control) proteins were determined by immunoblotting. (C) Wild-type (Wt) and Puma-deficient (Puma−/−) splenic B cells (2 × 106 cells/mL) were stimulated for 6 days by the combination of Fab'2 anti–mouse μ antibody fragments (10 μg/mL) plus anti–mouse CD40 antibodies (5 μg/mL) or LPS (10 μg/mL). The percentages of apoptotic cells were assessed by flow cytometry. Data represent mean (± SEM) of n = 5 mice per genotype. (**P < .01). (D) Wild-type (Wt) and Puma-deficient (Puma−/−) B cells were labeled with CFSE and then stimulated in culture with Fab'2 anti–mouse μ antibody fragments (10 μg/mL) plus anti–mouse CD40 antibodies (5 μg/mL). B-cell proliferation (reflected by dilution of CFSE fluorescence intensity) was assessed by FACS analysis at days 0 and 4 of activation. Results shown are representative of 3 independent experiments.
Puma-deficient B cells were more resistant than wild-type B cells to apoptosis on in vitro activation. (A-B) Wild-type and Puma-deficient (Puma−/−) splenic B cells (2 × 106 cells/mL) were stimulated for 8 days in culture by the combination of Fab'2 anti–mouse μ antibody fragments (10 μg/mL) plus anti–mouse CD40 antibodies (5 μg/mL; A) or LPS (10 μg/mL; B). Activated B cells were quantified by flow cytometry (see Figure 3C) from day 2 to day 8. Data represent the mean (± SEM) of n = 6 mice for each genotype. (*P < .05, **P < .01). Expression levels of Puma, Mcl-1, and GAPDH (loading control) proteins were determined by immunoblotting. (C) Wild-type (Wt) and Puma-deficient (Puma−/−) splenic B cells (2 × 106 cells/mL) were stimulated for 6 days by the combination of Fab'2 anti–mouse μ antibody fragments (10 μg/mL) plus anti–mouse CD40 antibodies (5 μg/mL) or LPS (10 μg/mL). The percentages of apoptotic cells were assessed by flow cytometry. Data represent mean (± SEM) of n = 5 mice per genotype. (**P < .01). (D) Wild-type (Wt) and Puma-deficient (Puma−/−) B cells were labeled with CFSE and then stimulated in culture with Fab'2 anti–mouse μ antibody fragments (10 μg/mL) plus anti–mouse CD40 antibodies (5 μg/mL). B-cell proliferation (reflected by dilution of CFSE fluorescence intensity) was assessed by FACS analysis at days 0 and 4 of activation. Results shown are representative of 3 independent experiments.
After immunization, Puma−/− mice accumulated more antigen-specific B cells than wild-type mice
To assess the role of Puma in B-cell activation and survival in vivo, we monitored the primary immune response after NP-KLH injection in wild-type and Puma−/− mice. The number of antigen-specific B cells (NP+IgG1+B220+) was first measured by immunostaining and FACS analysis at 14, 28, and 42 days after immunization. Analysis of the spleens from Puma−/− mice showed that in comparison to immunized wild-type mice their numbers of antigen-specific B cells were increased by ∼ 3-fold at day 28 and by ∼ 2-fold at day 42 (Figure 5A). No difference between wild-type and Puma−/− mice was found when comparing the numbers of antigen-specific B cells in the blood (Figure 5B). These results demonstrate that Puma plays a critical role in the normal developmentally programmed death of antigen-specific B cells during the shutdown of a T cell–dependent humoral immune response.
Immunized Puma-deficient mice exhibited more antigen-specific CD38high memory B cells compared with wild-type mice. Wild-type (Wt) and Puma-deficient (Puma−/−) mice were injected intra-peritoneally with 100 μg NP coupled to KLH. After 14, 28, or 42 days, leukocytes were collected from the spleen, blood or bone marrow, then subjected to enumeration of AFCs by ELISPOT or stained with the indicated antibodies and analyzed by flow cytometry. (A-B) Viable IgG1+B220+ (but IgM−IgD−Gr1−Mac1−) B cells that can bind the immunizing hapten NP coupled to the fluorescent protein, allophycocyanin (APC), from spleen (A) and blood (B) were identified by flow cytometry. The total numbers of antigen-specific NP+IgG1+B220+ B cells in the spleen and their percentages in blood are presented in A and B, respectively. Data represent mean (± SD) of n = 3 at d14, n = 7-9 at d28 and at d42, n = 3 mice per genotype. (**P < .01, *** P < .005). (C-D) Frequencies of NP+IgG1+B220+ AFCs in the spleens (C, number) and bone marrow (D, percentage) of wild-type or Puma−/− mice. The total column represents all AFCs (high plus low affinity for NP; ie, binding to NP20) and the high-affinity (antibodies binding to NP2) AFCs are represented by the black portion of the column. Affinity maturation is calculated as the ratio of NP2/NP20 cells and this is indicated at the top of each column. Data represent mean (± SD) of n = 3 at d14, n = 6 at d28, and at d42, n = 3 mice per genotype (P > .05). (E-F) NP+IgG1+B220+ B cells with a memory phenotype (CD38high) were quantified by flow cytometry. The total numbers of memory B cells in the spleens (E) and the percentages of memory B cells in the blood (F) are indicated. Data represent mean (± SD) of n = 3 at d14, n = 4-9 at d28, and at d42, n = 3 mice per genotype. (**P < .01, *** P < .005).
Immunized Puma-deficient mice exhibited more antigen-specific CD38high memory B cells compared with wild-type mice. Wild-type (Wt) and Puma-deficient (Puma−/−) mice were injected intra-peritoneally with 100 μg NP coupled to KLH. After 14, 28, or 42 days, leukocytes were collected from the spleen, blood or bone marrow, then subjected to enumeration of AFCs by ELISPOT or stained with the indicated antibodies and analyzed by flow cytometry. (A-B) Viable IgG1+B220+ (but IgM−IgD−Gr1−Mac1−) B cells that can bind the immunizing hapten NP coupled to the fluorescent protein, allophycocyanin (APC), from spleen (A) and blood (B) were identified by flow cytometry. The total numbers of antigen-specific NP+IgG1+B220+ B cells in the spleen and their percentages in blood are presented in A and B, respectively. Data represent mean (± SD) of n = 3 at d14, n = 7-9 at d28 and at d42, n = 3 mice per genotype. (**P < .01, *** P < .005). (C-D) Frequencies of NP+IgG1+B220+ AFCs in the spleens (C, number) and bone marrow (D, percentage) of wild-type or Puma−/− mice. The total column represents all AFCs (high plus low affinity for NP; ie, binding to NP20) and the high-affinity (antibodies binding to NP2) AFCs are represented by the black portion of the column. Affinity maturation is calculated as the ratio of NP2/NP20 cells and this is indicated at the top of each column. Data represent mean (± SD) of n = 3 at d14, n = 6 at d28, and at d42, n = 3 mice per genotype (P > .05). (E-F) NP+IgG1+B220+ B cells with a memory phenotype (CD38high) were quantified by flow cytometry. The total numbers of memory B cells in the spleens (E) and the percentages of memory B cells in the blood (F) are indicated. Data represent mean (± SD) of n = 3 at d14, n = 4-9 at d28, and at d42, n = 3 mice per genotype. (**P < .01, *** P < .005).
Loss of Puma provoked accumulation of memory B cells but not AFCs
On immunization, Bcl-2 transgenic and Bim-deficient mice accumulated abnormally increased numbers of antibody-forming B cells (AFCs) and memory B cells.6,7,13 Because Puma proved to be a critical regulator of the survival of activated B cells in culture, we measured the numbers of these 2 cell populations in immunized Puma−/− mice.
ELISPOT assays were performed to measure the frequency and number of NP+ IgG1+ AFCS in the spleen and bone marrow of immunized Puma−/− and wild-type mice. The numbers of NP-specific AFCs in the spleens (Figure 5C) and the percentages of NP-specific AFCs in the bone marrow (Figure 5D) at 14, 28 and 42 days after immunization were not significantly different between the 2 groups of mice. This indicates that Puma deficiency does not affect the AFC population. We also observed no difference in the ratio of high-affinity to total (high plus low affinity) anti-NP antibody producing AFC between Puma−/− and wild-type mice. This ratio was assessed by measuring the differential binding to low haptenated (NP2) or high haptenated (NP20) carrier protein of the anti-NP antibody produced by the IgG1+ AFC cells.33,34
The proportions and total numbers of memory B cells, identified by their levels of CD38 expression,3,27 were next determined. Compared with wild-type animals, the numbers of memory B cells (CD38high) in the spleens of Puma−/− mice were ∼ 4-fold higher at day 28 and day 42 after immunization (Figure 5E). No difference in the numbers of NP-specific memory B cells was detected in the blood between the wild-type and Puma−/− mice (Figure 5F). Moreover analysis of the numbers of splenic NP+IgG1+ antigen-specific B cells (Figure 6A), splenic CD38high memory B cells (Figure 6B), and the frequency of bone marrow resident AFCs (Figure 6C) 5 days after a secondary injection of NP-KLH showed all 3 cells populations remained higher in the Puma−/− mice compared with the wild-type controls. Booster immunization elicited a clear increase in the frequency of NP-specific AFCs in the bone marrow of Puma−/− mice, demonstrating that Puma-deficient memory B cells are functional, without significantly changing the number of memory B cells in either group (Figure 6C).
Puma-deficient mice contained more antigen-specific CD38high memory B cells and AFCs than wild-type mice after secondary immunization. Wild-type and Puma−/− mice were injected intraperitoneally with 100 μg NP coupled to KLH at day 0 and day 42. Five days after the second injection (day 47), leukocytes were collected from the spleen or bone marrow and subjected to ELISPOT for enumeration of AFCs or stained with the indicated antibodies and then analyzed by flow cytometry to determine the numbers of memory B cells. Numbers of splenic NP+IgG1+B220+ B cells (A) and NP+IgG1+B220+ CD38high memory B cells (B) of wild-type or Puma−/− mice. (C) Frequencies of anti-NP Ig secreting AFCs in the bone marrow of wild-type or Puma−/− mice. The total column represents all AFCs (high plus low affinity for NP; ie, binding to NP20) and the high-affinity (antibodies binding to NP2) AFCs are represented by the black portion of the column. Data represent mean (± SD) of n = 3 at d42 and at d47, n = 7 mice per genotype. (**P < .01, *** P < .005).
Puma-deficient mice contained more antigen-specific CD38high memory B cells and AFCs than wild-type mice after secondary immunization. Wild-type and Puma−/− mice were injected intraperitoneally with 100 μg NP coupled to KLH at day 0 and day 42. Five days after the second injection (day 47), leukocytes were collected from the spleen or bone marrow and subjected to ELISPOT for enumeration of AFCs or stained with the indicated antibodies and then analyzed by flow cytometry to determine the numbers of memory B cells. Numbers of splenic NP+IgG1+B220+ B cells (A) and NP+IgG1+B220+ CD38high memory B cells (B) of wild-type or Puma−/− mice. (C) Frequencies of anti-NP Ig secreting AFCs in the bone marrow of wild-type or Puma−/− mice. The total column represents all AFCs (high plus low affinity for NP; ie, binding to NP20) and the high-affinity (antibodies binding to NP2) AFCs are represented by the black portion of the column. Data represent mean (± SD) of n = 3 at d42 and at d47, n = 7 mice per genotype. (**P < .01, *** P < .005).
As Bcl-2 overexpression6,7 and Bim deficiency13 have been shown to affect the process of affinity maturation by allowing the abnormal survival of low-affinity antibody producing activated B cells and AFCs, the impact of Puma deficiency on this process was also investigated. The NP response of B cells has been reported to involve mainly the VH186.2 gene.33,35 We therefore amplified and sequenced the rearranged VH186.2-constant region γ1 cDNA from single FACS-sorted splenic B220+NP+IgG1+ cells with a memory phenotype (CD38high) from both wild-type and Puma−/− mice. Results of the sequence analyses are summarized in Table 1. In contrast to the Bcl-2 transgenic6,7 and Bim−/− mice,13 no difference in the frequencies of IgV gene mutations were apparent between the Puma−/− and wild-type mice. There were no significant differences in the percentages of sequences with zero mutation between Puma−/− B cells (7.1%) and wild-type B cells (10.5%). There were also no differences in the average of the total numbers of mutations per VH186.2 gene (4.9 vs 6.6 for Puma−/− and wild-type B cells, respectively). Finally, the well-described affinity-enhancing mutation (W33L) in VH186.2 position 33 was present at similar frequencies in Puma−/− (42.9%) and control B cells (36.8%).
Summary of VH186.2 sequences from NP-specific IgG1 B cells
| . | Wild-type . | Puma−/− . |
|---|---|---|
| Single cell PCR, no. | 24 | 53 |
| Positive PCR, no. | 17 | 28 |
| Positive VH186.2, no. | 14 | 19 |
| Range mutations per VH186.2, no. | 0-8 | 0-12 |
| Sequences with zero mutation, % | 7.1 | 10.5 |
| Average total mutations per VH186.2, no. (SD) | 4.9 ± 2.3 | 6.6 ± 3.4 |
| W33L substitution, n/N (%) | 6/14 (42.9) | 7/19 (36.8) |
| CDR1+CDR2, R/S (ratio) | 37/5 (7.4) | 51/17 (3.0) |
| FW1-FW3, R/S (ratio) | 16/10 (1.6) | 33/20 (1.7) |
| . | Wild-type . | Puma−/− . |
|---|---|---|
| Single cell PCR, no. | 24 | 53 |
| Positive PCR, no. | 17 | 28 |
| Positive VH186.2, no. | 14 | 19 |
| Range mutations per VH186.2, no. | 0-8 | 0-12 |
| Sequences with zero mutation, % | 7.1 | 10.5 |
| Average total mutations per VH186.2, no. (SD) | 4.9 ± 2.3 | 6.6 ± 3.4 |
| W33L substitution, n/N (%) | 6/14 (42.9) | 7/19 (36.8) |
| CDR1+CDR2, R/S (ratio) | 37/5 (7.4) | 51/17 (3.0) |
| FW1-FW3, R/S (ratio) | 16/10 (1.6) | 33/20 (1.7) |
Details of the nucleotide sequences of IgVH186.2 genes are summarized, comparing wild-type with Puma−/− B cells.
R/S indicates ratio of replacement to silent mutations in all sequences; CDR, complementary-determining region; and FW, framework region.
Collectively, these data demonstrated that Puma is critical for the regulation of CD38high memory B-cell survival but does not impact on IgV gene somatic hyper-mutation and affinity maturation of the humoral immune response.
Discussion
Humoral immunity is a complex process involving both positive and negative selection of antigen-stimulated B cells, leading to the generation of antibody-forming cells (AFCs) involved in the primary response and memory B cells responsible for immunologic memory. Regulation of B-cell survival is a critical aspect of this process and it has been shown that some proteins of the Bcl-2 family play an important role in controlling apoptosis of B cells during humoral immune responses.7-9,13,29,36 In the present study, we show that the BH3-only protein Puma is a major regulator of the survival of antigen-specific memory B cells and therefore impacts on immunologic memory. Our data show that Puma expression is strongly increased in both human and murine B cells in response to in vitro stimulation with mitogens, such as SAC or LPS, or with more physiologic signals, such as ligation of the BCR and CD40. These findings indicate that up-regulation of Puma is more related to the activation status of B cells per se rather than the stimulus used. Moreover, we show that Puma up-regulation in vitro is correlated with in vivo expression of Puma in activated B cells within germinal centers and also associated with the germinal center–like phenotype of human Burkitt lymphoma. The mechanisms that cause Puma up-regulation in mitogen stimulated B cells are presently unclear and is independent of p53 because mitogen stimulated p53−/− B cells express similar levels of Puma as wild-type B cells (data not shown). Although Puma is rapidly up-regulated on mitogenic activation of B cells, our results do not support a role for Puma in the early phase of B-cell activation. Firstly, no differences in the percentages or total numbers of live activated B cells were observed between Puma−/− and wild-type B cells stimulated in vitro for 24-48 hours with LPS or the combination of anti-μ plus anti-CD40 antibodies. Secondly, the formation of germinal centers was not impaired in immunized Puma−/− mice. Thirdly, 14 days after immunization, Puma-deficient mice had a similar number of antigen-specific B cells compared with wild-type mice and this was not associated with changes in the ratio of high/low affinity AFC or number of memory B cells, in contrast to what was observed in Bcl-2 transgenic6,7,26 or Bim-deficient mice.13
The mechanisms that attenuate the proapoptotic effects of Puma during the early phase of B-cell activation in vitro and within germinal centers in vivo are not understood. Puma induces apoptosis by binding and antagonizing all of the 5 pro-survival Bcl-2 family members or, possibly more controversially, by directly activating the proapoptotic multi-BH domain protein Bax.37-42 The proapoptotic effect of Puma might therefore be counteracted by one or several antiapoptotic proteins of the Bcl-2 family. Mcl-1 is an outstanding candidate for this role because it has recently been shown to be critical for the formation and of the germinal center reaction and establishment of B-cell memory.29 Several of our results support this hypothesis. We observed high levels of Mcl-1 within the germinal center and a considerable up-regulation of Mcl-1 in B cells during mitogenic activation in vitro. Moreover, Puma and Mcl-1 are coexpressed and a portion of these 2 proteins are actually associated with each other in SAC-activated B cells and in Burkitt lymphoma cells (data not shown). Similar to Mcl-1, A1/Bfl-1 was also found to be up-regulated in B cells during in vitro activation, which extends a previous report showing that CD40 ligation elicited increased transcription of A1/Bfl-1 in B cells.43,44 A1/Bfl-1 may therefore also serve to neutralize the proapoptotic function of Puma during early stages of B-cell activation. However, further experiments using additional tools and approaches will be necessary to clarify the role of these proteins in this process.
BH3-only proteins are important regulators of the death of antigen-activated B cells.45 Studies performed on mice deficient for both Bim and Puma have shown that these 2 potently proapoptotic BH3-only proteins have overlapping functions in T- and B- cell homeostasis.18 Compared with Bim−/− or Puma−/− (single knock-out) B cells, Bim−/−Puma−/− (double knock-out) B cells are more resistant to a range of apoptotic stimuli, including cytokine deprivation. Interestingly, comparison of nonimmunized Bim−/−Puma−/−, Bim−/−, Puma−/−, and Bcl-2 transgenic mice showed that only the bim−/−puma−/− mice had a similar elevation in immunoglobulin levels to Bcl-2 transgenic animals, indicating that Puma and Bim exert overlapping roles in controlling the humoral immune response. Indeed, our results support a role for Puma in the normal developmentally programmed death of antigen-stimulated B cells. This was demonstrated both by in vitro and in vivo observations. Mitogenically activated Puma−/− B cells had prolonged in vitro survival compared with wild-type B cells. Furthermore, Puma−/− mice immunized with NP-KLH antigen display an enhanced accumulation of antigen specific B cells at days 28 and 42 compared with wild-type mice. This increase of antigen specific B cells after primary immunization was not associated with an increase of NP-specific AFCs but rather with an increase of CD38high memory B cells. This increased memory was subsequently manifested in the increased number of antigen NP-specific AFC in Puma−/− mice boosted with a secondary injection of NP-KLH. The increase in antigen-specific IgG memory B cells appears to be mostly restricted to the spleen, consistent with the recent work of Aiba and colleagues showing that NP-specific CD38highIgG1+ memory B cells are preferentially localized adjacent to contracted germinal centers in the spleen but not present in significant numbers in the recirculating pool.46 However, analysis of immunized Puma−/− mice clearly showed that Puma must exert a different and specific role in the control of B-cell activation compared with that of Bim. Whereas Bim is responsible for the elimination of low affinity Ig-producing activated B cells and AFCs in germinal centers, loss of Puma did not interfere with the antigen-mediated selection of high-affinity Ig-producing memory B cells and AFCs. Instead, Puma is specifically involved in the control of the long-term survival of memory B lymphocytes. Interestingly, a role for Puma in the survival of antigen-specific CD8 T cells has recently been observed in mice infected with the HSV-1 virus,47 indicating that Puma is involved in controlling the survival of both activated B and T cells.
In conclusion, using both in vitro and in vivo approaches with human as well as murine models, our data show that Puma plays a major role in the control of the T-mediated B-cell response. Our present study demonstrates that Puma is a major regulator of memory B-cell survival and therefore a key molecule in controlling the immune response process.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to K. Vella, G. Siciliano, A. Naughton, K. Pioch, N. Iannarella, J. Allen, and G. Duc for expert animal care; B. Helbert and C. Young for genotyping; Dr F. Battye, V. Milovac, C. Tarlinton, and J. Garbe for cell sorting; and N. Bidere, D. Arnoult, and A. Villunger for very stimulating discussions and technical help.
This work was supported by fellowships and grants from Inserm, Ligue Nationale contre le Cancer (Equipe labellisée), Agence Nationale de la Recherche (ANR), Cancéropole IdF (projet ERABL), the National Health and Medical Research Council (Australia; programs 257502 and 356202), the Leukemia & Lymphoma Society (New York; SCOR grant 7015), and the National Cancer Institute (National Institutes of Health grants CA80188 and CA43540). C.C. and S.F. hold fellowships from Fondation pour la Recherche Médicale, Association pour le Recherche sur le Cancer (ARC), and Deutsche Forschungsgemeinschaft.
National Institutes of Health
Authorship
Contribution: C.C. and S.F. planned and performed most of the experiments, wrote the manuscript, and share first authorship. M.T.A., P.H., and C.A. helped with several experiments; P.B. M.R. and G.L. contributed essential reagents and ideas and helped with experiments; and A.S., D.M.T., and A.V. conceived the experimental plan, helped plan experiments, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for S.F. is Universitätsklinikum, Ulm, Germany
Correspondence: Dr Aimé Vazquez, Inserm U.1014, Hôpital Paul Brousse, 14 Avenue Paul Vaillant-Couturier, F-94807 Villejuif, France; e-mail: aime.vazquez@inserm.fr.
References
Author notes
C.C. and S.F. share first authorship.
A.S., D.M.T., and A.V. contributed equally to this article.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal