Abstract
Targeting antigens to dendritic cell (DC)–specific receptors, such as DC-SIGN, induces potent T cell-mediated immune responses. DC-SIGN is a transmembrane C-type lectin receptor with a long extracellular neck region and a carbohydrate recognition domain (CRD). Thus far, only antibodies binding the CRD have been used to target antigens to DC-SIGN. We evaluated the endocytic pathway triggered by antineck antibodies as well as their intracellular routing and ability to induce CD8+ T-cell activation. In contrast to anti-CRD antibodies, antineck antibodies induced a clathrin-independent mode of DC-SIGN internalization, as demonstrated by the lack of colocalization with clathrin and the observation that silencing clathrin did not affect antibody internalization in human DCs. Interestingly, we observed that anti-neck and anti-CRD antibodies were differentially routed within DCs. Whereas anti-CRD antibodies were mainly routed to late endosomal compartments, anti-neck antibodies remained associated with early endosomal compartments positive for EEA-1 and MHC class I for up to 2 hours after internalization. Finally, cross-presentation of protein antigen conjugated to antineck antibodies was approximately 1000-fold more effective than nonconjugated antigen. Our studies demonstrate that anti-neck antibodies trigger a distinct mode of DC-SIGN internalization that shows potential for targeted vaccination strategies.
Introduction
Dendritic cells (DCs) play a key role in initiating adaptive immune responses by capturing antigens and presenting them to T cells. The discovery of receptors that are mainly expressed by DCs, such as several members of the C-type lectin receptor (CLR) family, allows for vaccination strategies that target antigens directly to DCs in vivo.1 Targeted delivery of antigens through CLRs stimulates antigen presentation and results in immunity when DC maturation stimuli are coadministered.2 Although most of the vaccines currently on the market mainly induce humoral responses, many DC-targeted vaccines also induce strong cytotoxic T cell (CTL) responses.1 This is attributed to the ability of certain DC subsets to present exogenous antigens on MHC class I, a process called cross-presentation.
CLRs bind carbohydrate structures in a Ca2+-dependent manner via their carbohydrate recognition domain (CRD). The CRD binds specific mannose, galactose, or fucose structures present on self or non–self-proteins.3 Human DC-SIGN represents a member of the CLR family that has been explored for DC vaccination strategies. The extracellular part of DC-SIGN is composed of a C-terminal CRD and a neck region consisting of 7 complete, and 1 incomplete, 23-residue tandem repeat regions. DC-SIGN has been demonstrated to recognize many pathogens, including viruses, bacteria, fungi, and parasites.4 After binding, these pathogens are internalized and pathogen-derived antigens are presented via MHC class I and II molecules to CD8+ and CD4+ T cells, respectively.5,6 By analogy, vaccination strategies targeting antigens to the CRD of DC-SIGN resulted in antigen presentation via MHC classes I and II.7-10 In vivo, targeted delivery of antigens to the CRD of DC-SIGN in Rag2−/− γC−/− mice reconstituted with human immune cells also induced antigen-specific T-cell responses.11
Thus far, both antibodies and sugar ligands have been exploited to target antigens to the CRD of DC-SIGN. However, what precisely triggers receptor internalization is still unknown. The observation that DC-SIGN is effectively internalized by single-chain antibodies suggests that internalization is not dependent on receptor cross-linking, as is essential when targeting Fc receptors.11-13 We previously demonstrated that ligand binding to the CRD of DC-SIGN results in clathrin-mediated internalization of the receptor and routing to late endosomal, LAMP-1, and MHC class II positive, compartments.8,9,14,15 The observation of Sierra-Filardi et al, showing that antibodies recognizing the neck domain of DC-SIGN display an enhanced ability to trigger receptor internalization compared with CRD-binding antibodies,16 prompted us to initiate a detailed study on antigen uptake and processing when targeting different epitopes of DC-SIGN. We hypothesized that intracellular trafficking of CLRs may be affected depending on receptor triggering, as has been demonstrated for Dectin-1.17 This in turn could affect antigen processing and presentation.
We used microscopy and immunologic approaches to determine whether targeting distinct receptor epitopes affects DC-SIGN internalization, intracellular trafficking, and antigen presentation. Interestingly, we observed that internalization of antibodiesbinding to the neck region was independent of clathrin and resulted in prolonged localization of the receptor in early endosomal compartments. Moreover, these antibodies showed potential for DC vaccination strategies because antigens targeted via the neck domain were efficiently cross-presented to CD8+ T cells.
Methods
Reagents and antibodies
Antibodies used included the following: anti-CRD antibody AZN-D1,18 antineck antibody H200, and anti-CRD antibody 1B1019 from Santa Cruz Biotechnology; anti-neck antibody DCN46,20 anti-clathrin heavy chain antibody, and anti–EEA-1 antibody from BD Biosciences PharMingen; anti–MHC-I antibody W6/32 and anti–MHC-II antibody Q5/13, both ascites; anticlathrin light chain antibody CON.1 from DIANOVA; rabbit anti-LAMP1 antibody from Sigma-Aldrich; anti-CD63 antibody from PeliCluster Sanquin; and Alexa-conjugated secondary antibodies, Lysotracker-Red, Alexa-conjugated ovalbumin (OVA), Alexa monoclonal antibody labeling kit, and LipofectaminTM 2000 from Invitrogen.
Mice
OT-I mice21 on the C57BL/6 background were bred at the Central Animal Laboratory. Transgenic mice carrying the human DC-SIGN cDNA under the murine CD11c promoter were described previously and were bred at the Twincore or the Helmholtz Center for Infection Research.22 Drinking water and standard laboratory food pellets were provided ad libitum. The experiments were performed under specific pathogen-free conditions and in accordance with institutional, state, and federal guidelines.
Cells
CHO cell lines stably expressing DC-SIGN wild-type, DC-SIGN-Δ-Repeat, or DC-SIGN-Δ-CRD were established by LipofectaminTM 2000 transfection. Human immature DCs were generated from peripheral blood monocytes of healthy donors as reported elsewhere.23 Mouse bone marrow cells were isolated from femurs and tibias of mice and cultured in RPMI 1640 with Glutamax (Invitrogen), 10% heat-inactivated FCS (Hyclone, Perbio), 100 μg/mL penicillin, 100 μg/mL streptomycin, and 50μM 2-mercaptoethanol (all from PAA Laboratories) supplemented with GM-CSF supernatant.24 Bone marrow–derived DCs (BMDCs) were routinely checked for purity and maturation markers by flow cytometry. OT-I mice were killed, and lymph node and spleen suspensions were made. The OT-I cells were sorted using Miltenyi Biotec CD8+ magnetic cell sorting kit for antigen presentation assays. Phenotypic characterization of OT-I cells was performed using a FACSCalibur (BD Biosciences).
DNA constructs
Antibody internalization assay
Either immature DCs or cell lines stably expressing DC-SIGN wild-type, Δ-Repeat, or Δ-CRD mutants were incubated with anti-CRD or anti-neck antibodies (at saturating concentrations) in serum-free medium for 20 minutes on ice. Isotype controls were included in all of the experiments to correct for nonspecific binding. After washing the unbound antibodies in ice-cold medium, half of the cells were further incubated for 15 minutes on ice to prevent internalization, whereas the other half was shifted to 37°C for 15 minutes to induce internalization. Subsequently, cells were incubated on ice to stop internalization, washed with ice-cold PBS containing 3% BSA, and incubated with Alexa488-conjugated goat-anti–mouse antibodies for 20 minutes on ice to stain the antibodies still bound to the cell surface. Unbound antibodies were washed away and cells were fixed in 1% paraformaldehyde. The mean fluorescence (MF) of the samples was measured by flow cytometry using the FACSCalibur (BD Biosciences), and the percentage of internalized antibodies was calculated as: (MF 4°C − MF 37°C)/MF 4°C × 100.
RNA interference
Immature DCs were transfected with 100nM siRNA with transfection reagent DF4 (Dharmacon RNA Technologies), according to the manufacturer's protocol. The siRNAs used were Clathrin OTP SMARTpool (L-004001-01) and ON-TARGET plus siCONTROL nontargeting pool (D-001810-10-05) as control (Dharmacon RNA Technologies). This protocol resulted in nearly 80% transfection efficiency as determined by flow cytometry of cells transfected with siGLO RISC-free siRNA (D-001600-01-05). Silencing of clathrin was confirmed by Western blot (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Confocal laser scanning microscopy
Double labeling experiments with antineck and anti-CRD antibodies were performed by allowing immature DCs to attach to fibronectin-coated coverslips for 60 minutes at 37°C in serum-free medium. Cells were fixed with 2% paraformaldehyde, washed, and incubated with 3% BSA, 10mM glycine, and 2% human serum to prevent nonspecific binding of primary antibodies. Subsequently, cells were incubated with antineck and anti-CRD antibodies for 30 minutes at room temperature. Subsequently, cells were washed and incubated with isotype-specific Alexa-conjugated secondary antibodies.
For the internalization experiments, immature DCs were allowed to adhere to fibronectin-coated coverslips for 60 minutes at 37°C in serum-free medium. Cells were transferred to 4°C and incubated with serum-free medium containing 3% BSA, 10mM glycine, and 2% human serum to prevent nonspecific binding of primary antibodies. Subsequently, cells were incubated with anti–DC-SIGN antibodies and/or OVA–Alexa-647 for 20 minutes at 4°C and washed. Internalization was induced by adding prewarmed serum-free medium and shifting the cells to 37°C for various periods of time. After paraformaldehyde fixation and saponin permeabilization, cells were stained with anti-CD63, anti-LAMP1, anti–EEA-1, anti–MHC-I, anti–MHC-II, or anti-clathrin antibodies and isotype-specific Alexa-conjugated secondary antibodies. Isotype controls were included in all of the experiments. For time-lapse imaging experiments, immature DCs were allowed to adhere to fibronectin-coated glass-bottom WillCo Dishes for 60 minutes at 37°C in serum-free medium without phenol red. Subsequently, cells were labeled with Lysotracker Red, washed, and incubated with AlexaFluor-488–conjugated anti-neck or anti-CRD antibodies. Cells were analyzed directly after addition of the antibodies for 90 minutes at 37°C. Samples were analyzed using a Zeiss LSM 510 confocal microscope equipped with a type S heated stage CO2 controller and a Plan Apochromat 63 × 1.4 oil immersion differential interference contrast lens (Carl Zeiss). Images were processed with NIH ImageJ Version 1.44a software (rsb.info.nih.gov/ij). The Pearson correlation coefficient was calculated using the JACoP plugin.27
Generation of antibody/OVA conjugates
Endotoxin-free OVA (Profos Ag) was conjugated to the antineck DCN46 or an isotype control antibody. Therefore, the cross-linking agent sulfosuccinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate (sSMCC; Pierce Chemical) was conjugated to the antibodies according to the manufacturer's protocol. Protected sulfhydryl groups were introduced to OVA using N-succinimidyl-S-acetylthiopropionate (Pierce Chemical) and were reduced with hydroxylamine hydrochloride (Pierce Chemical) using the manufacturer's protocol. Sulfhydryl-modified OVA was then added to the sSMCC-modified antibodies at a molar ratio of 5:1 and incubated overnight at 4°C. The antibody/OVA conjugates were purified by protein A column equilibrated in PBS to remove free OVA protein. Subsequently, the conjugates were concentrated by using a 100-kDa MWCO Amicon Centriplus centrifugal filter (Millipore). Protein content of the concentrated samples was determined by Coomassie Plus assay kit (Pierce Chemical), and the conjugates were characterized by SDS-PAGE. OVA content of the conjugates was determined by sandwich ELISA as described before.28
T-cell proliferation assay
DC-SIGN transgenic BMDCs were used for cross-presentation studies at day 7 of culture, by which > 65% of the cells expressed the DC-specific marker CD11c. In antigen binding experiments, BMDCs were incubated at 10 000 cells per well in 96-well round-bottom plates in the absence or presence of 20 μg/mL antineck/OVA or isotype-OVA for 1 hour at 4°C. In antigen uptake experiments, 5000 BMDCs were incubated in the absence or presence of isotype/OVA or antineck/OVA conjugates or nonconjugated OVA at the indicated concentrations for 3 hours at 37°C. Next, cells of both the binding and uptake experiments were washed, BMDC maturation was induced by adding 2 μg/mL lipopolysaccharide (LPS; Sigma-Aldrich), and cells were cocultured with 50 000 OT-I or OT-II T cells. Subsequently, [3H]-thymidine (1 μCi/well; MP Biomedicals) was added for 16 hours to detect T-cell proliferation. Cells were harvested onto filters, and [3H]-thymidine incorporation was assessed using a β-counter. IFN-γ levels were determined in culture supernatants 2 days after the start of the coculture by standard sandwich ELISA using antimouse IFN-γ capturing (clone R4-6A2; BD Biosciences) and detection (clone XMG 1.2; BioLegend) antibodies.
Results
Binding and internalization characteristics of various DC-SIGN antibodies
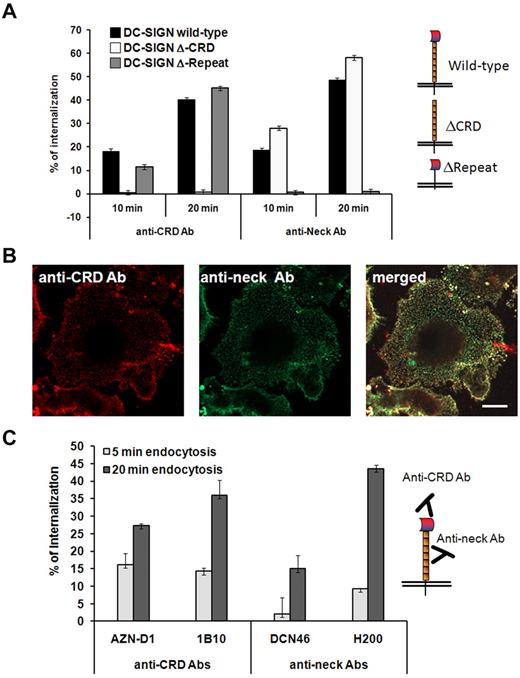
We first characterized binding and internalization of various DC-SIGN antibodies recognizing distinct receptor epitopes. CHO cells expressing the wild-type DC-SIGN, or mutants lacking the CRD or neck domain were incubated with the well-characterized neck-binding antibodies DCN46 and H200,16,19,20 or with the anti-CRD antibodies AZN-D1 and 1B10 to confirm their specificity. The anti-CRD antibody AZN-D1 induced internalization of both wild-type receptor and the mutant lacking the neck domain, whereas the antineck antibody H200 induced internalization of both the wild-type receptor and the mutant lacking the CRD (Figure 1A). Because it is known that DC-SIGN forms multimers and can be present in microdomain clusters, this might directly affect antibody recognition.29 Therefore, we determined whether antineck and anti-CRD antibodies recognize the same DC-SIGN molecules on the cell surface. Figure 1B shows a similar DC-SIGN staining pattern irrespective of whether DCs were labeled with antineck or anti-CRD antibodies. Next, we determined the efficiency by which various anti-CRD and antineck domain antibodies induce receptor internalization in human DCs. Approximately 25% to 45% of the DC-SIGN molecules disappeared from the cell surface within 20 minutes after addition of the anti-CRD antibodies 1B10 and AZN-D1 or the antineck antibody H200, whereas this was 15% in case of the antineck antibody DCN46 (Figure 1C). This indicates that not all antibodies recognizing the antineck domain are more effective inducers of DC-SIGN internalization, as has been suggested before.16
Binding of antibodies to DC-SIGN neck region triggers endocytosis. (A) CHO cells stably transfected with wild-type DC-SIGN or DC-SIGN lacking the neck (ΔRepeat) or lacking the CRD (ΔCRD) were incubated with anti-CRD (AZN-D1) or anti-neck (H200) antibody at 4°C, washed, and incubated for 10 or 20 minutes at 37°C to induce endocytosis. Cells were analyzed by flow cytometry, and the percentage of internalization was calculated. (B) Steady-state confocal microscopy image of immature DCs labeled with antineck (H200, green) and anti-CRD (AZN-D1, red) antibodies. Both antibodies label the same DC-SIGN molecules on one representative cell of several images from 2 experiments. Scale bar represents 5 μm. (C) Anti-neck and anti-CRD antibody internalization by immature DCs after 5 or 20 minutes at 37°C. Experiments were performed in triplicate, and 1 representative experiment of 3 is shown. Data represent mean ± SD.
Binding of antibodies to DC-SIGN neck region triggers endocytosis. (A) CHO cells stably transfected with wild-type DC-SIGN or DC-SIGN lacking the neck (ΔRepeat) or lacking the CRD (ΔCRD) were incubated with anti-CRD (AZN-D1) or anti-neck (H200) antibody at 4°C, washed, and incubated for 10 or 20 minutes at 37°C to induce endocytosis. Cells were analyzed by flow cytometry, and the percentage of internalization was calculated. (B) Steady-state confocal microscopy image of immature DCs labeled with antineck (H200, green) and anti-CRD (AZN-D1, red) antibodies. Both antibodies label the same DC-SIGN molecules on one representative cell of several images from 2 experiments. Scale bar represents 5 μm. (C) Anti-neck and anti-CRD antibody internalization by immature DCs after 5 or 20 minutes at 37°C. Experiments were performed in triplicate, and 1 representative experiment of 3 is shown. Data represent mean ± SD.
Targeting DC-SIGN via the neck region results in clathrin-independent internalization
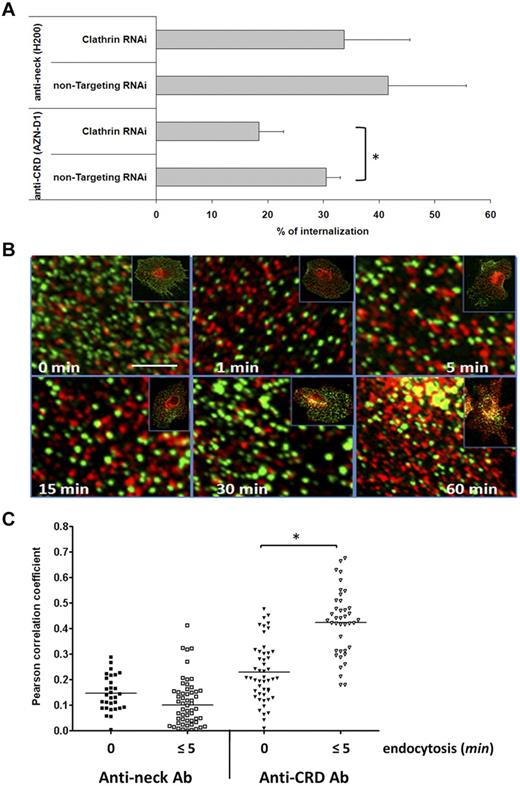
Our previous findings showed that pathogen uptake via DC-SIGN is clathrin-mediated.15 The anti-CRD antibody AZN-D1 also triggers clathrin-dependent internalization of DC-SIGN, which may be related to the fact that this antibody blocks binding of HIV-1 to DC-SIGN, suggesting that HIV-1 and AZN-D1 bind DC-SIGN in the same region.15,18 Here, we directly compared the effect of silencing of clathrin expression in human DCs by RNAi on the internalization of CRD- and neck-binding antibodies. Western blot analysis showed clathrin expression was efficiently, although not completely, blocked by RNAi treatment (supplemental Figure 1). Interestingly, no significant effect on DC-SIGN internalization was obtained with the neck-binding antibody H200 when silencing clathrin expression in human DCs by RNAi, whereas substantial reduced internalization was observed with the anti-CRD antibody AZN-D1 (Figure 2A). To confirm clathrin-independent internalization when triggering the neck domain, the intracellular localization of DC-SIGN and clathrin was assessed over time by confocal microscopy. Although previous studies revealed extensive colocalization of the anti-CRD antibody AZN-D1 and clathrin during the first 15 minutes of endocytosis,15 the anti-neck antibody H200 did not colocalize with clathrin (Figure 2B). This is further substantiated by the observation that the Pearson correlation coefficient determining the degree of H200 and clathrin colocalization did not change over time, whereas it showed a significant increase during the first 5 minutes of endocytosis in case of AZN-D1 (Figure 2C).
Endocytosis via DC-SIGN neck domain is clathrin-independent. (A) Clathrin or control siRNA knockdown immature DCs were incubated with anti-CRD (AZN-D1) or anti-neck (H200) antibody, and endocytosis was induced. Cells were analyzed by flow cytometry, and the percentage of internalization was determined. Experiments were performed in triplicate. Data represent mean values of 3 independent experiments ± SD. *P < .02. For H200: P > 0.5 (unpaired t test). (B) Immature DCs were incubated with antineck antibody (green) at 4°C, washed, and either kept on ice (steady state, 0 minutes) or shifted to 37°C for the indicated time points to trigger endocytosis. After fixation and permeabilization, clathrin was labeled (red), and the samples were analyzed by confocal microscopy. The pictures are enlarged areas taken from the cells shown in the small insets. Scale bar represents 2 μm. (C) Pearson colocalization coefficient plot of anti-CRD or anti-neck antibodies and clathrin of multiple cells in steady state (0 minutes) and after triggering endocytosis (≤ 5 minutes). One representative experiment of 3 is shown. *P < .01 (t test).
Endocytosis via DC-SIGN neck domain is clathrin-independent. (A) Clathrin or control siRNA knockdown immature DCs were incubated with anti-CRD (AZN-D1) or anti-neck (H200) antibody, and endocytosis was induced. Cells were analyzed by flow cytometry, and the percentage of internalization was determined. Experiments were performed in triplicate. Data represent mean values of 3 independent experiments ± SD. *P < .02. For H200: P > 0.5 (unpaired t test). (B) Immature DCs were incubated with antineck antibody (green) at 4°C, washed, and either kept on ice (steady state, 0 minutes) or shifted to 37°C for the indicated time points to trigger endocytosis. After fixation and permeabilization, clathrin was labeled (red), and the samples were analyzed by confocal microscopy. The pictures are enlarged areas taken from the cells shown in the small insets. Scale bar represents 2 μm. (C) Pearson colocalization coefficient plot of anti-CRD or anti-neck antibodies and clathrin of multiple cells in steady state (0 minutes) and after triggering endocytosis (≤ 5 minutes). One representative experiment of 3 is shown. *P < .01 (t test).
Targeting DC-SIGN via the neck region reduces trafficking to late endosomal compartments
Next, we assessed the possibility that clathrin-dependent and -independent DC-SIGN internalization mechanisms supply distinct endosomal compartments. Anti-CRD antibodies and the CRD-binding HIV-1 protein gp120 are known to be routed to acidic lysosomal compartments within 60 to 90 minutes.9,14 Here, we performed live cell imaging experiments to follow internalization of fluorescently labeled anti-neck and anti-CRD antibodies in human DCs. In contrast to the anti-CRD antibody AZN-D1, the antineck antibody H200 showed little colocalization with acidic organelles within the first 60 minutes after endocytosis (Figure 3A). Moreover, analysis of DCs internalizing the anti-neck antibodies H200 and DCN46 by confocal microscopy revealed that, even after 2 hours, only a minority of the antibodies colocalized with the lysosomal marker CD63 (Figure 3B). Together, these data suggest that DC-SIGN antibodies targeting the neck and CRD domain end up in distinct endosomal compartments.
Endocytosis via DC-SIGN neck domain leads to decreased routing to the lysosomal compartments. (A) Immature DCs were incubated with Alexa488-conjugated anti-CRD or anti-neck antibodies (green) at 4°C, washed, labeled with Lysotracker to stain the lysosomes (red), and shifted at 37°C to trigger endocytosis. Cells were analyzed by time-lapse confocal microscopy. Snapshots were taken at the indicated time points. Images represent one focal plane in the middle of the cell body. Representative cells from multiple experiments are shown. Scale bar represents 10 μm. (B) Immature DCs were incubated with antineck antibody H200 or DCN46 (green) at 4°C, washed, and shifted to 37°C for 2 hours. After fixation and permeabilization, the CD63+ lysosomal compartment (red) was labeled, and the samples were analyzed by confocal microscopy. Note that, at the 2-hour time point, only intracellular molecules are visualized because extracellular membrane-bound antibodies were removed by acid-strip treatment of cells before fixation. Scale bars represent 10 μm.
Endocytosis via DC-SIGN neck domain leads to decreased routing to the lysosomal compartments. (A) Immature DCs were incubated with Alexa488-conjugated anti-CRD or anti-neck antibodies (green) at 4°C, washed, labeled with Lysotracker to stain the lysosomes (red), and shifted at 37°C to trigger endocytosis. Cells were analyzed by time-lapse confocal microscopy. Snapshots were taken at the indicated time points. Images represent one focal plane in the middle of the cell body. Representative cells from multiple experiments are shown. Scale bar represents 10 μm. (B) Immature DCs were incubated with antineck antibody H200 or DCN46 (green) at 4°C, washed, and shifted to 37°C for 2 hours. After fixation and permeabilization, the CD63+ lysosomal compartment (red) was labeled, and the samples were analyzed by confocal microscopy. Note that, at the 2-hour time point, only intracellular molecules are visualized because extracellular membrane-bound antibodies were removed by acid-strip treatment of cells before fixation. Scale bars represent 10 μm.
Targeting DC-SIGN via the neck region prolongs retention in early endosomes
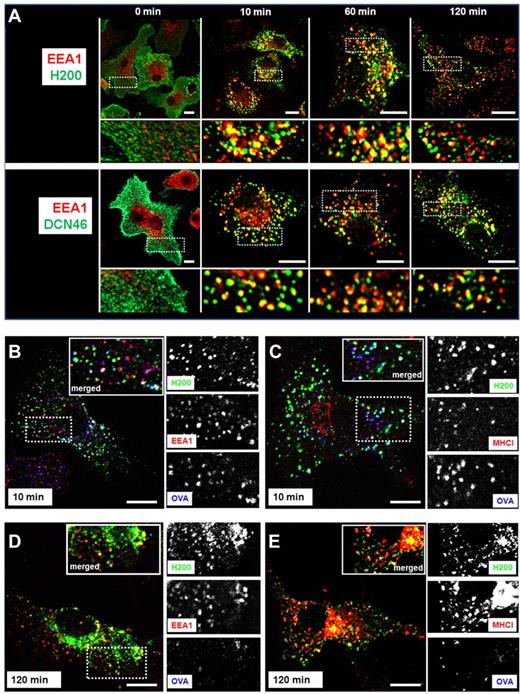
We sought to further characterize the nature of the endosomal compartments supplied by anti-neck antibodies. Interestingly, the mannose receptor (MR) represents another CLR that was shown to deliver antigenic cargo to nonlysosomal compartments. The model antigen OVA shuttles to an early endosomal EEA-1+ and MHC-I+ compartment when it enters the cell via the MR, with subsequent antigen presentation via MHC class I.30 Our results show that, similar to what was found for the MR, the 2 anti-neck antibodies DCN46 and H200 extensively colocalized with the early endosomal marker EEA-1 as late as 2 hours after internalization (Figure 4A). Because detainment of antigen in early endosomal compartments is associated with increased cross-presentation via MHC class I,30,31 we analyzed whether the anti-neck H200 antibody would colocalize with OVA and MHC class I molecules. DCs fed with fluorescently labeled OVA and H200 antibody showed that both proteins colocalized with EEA-1+ and MHC class I+ compartments as early as 10 minutes after internalization (Figure 4B-C). Part of the internalized H200 colocalized with OVA and EEA-1+ and MHC class I+ compartments for up to 2 hours (Figure 4D-E). Note that all of the antibody routing experiments were performed in the presence of human serum to exclude involvement of Fc receptors in skewing the intracellular routing of the various DC-SIGN antibodies. Experiments performed in the presence of 10% human Fc receptor blocking reagent also showed neck-binding antibodies colocalizing with EEA-1+ for up to 5 hours after internalization (supplemental Figure 2). These data reveal that antibodies triggering the DC-SIGN neck domain reside in early endosomal compartments for prolonged periods of time.
Triggering DC-SIGN neck domain leads to a prolonged localization of the antigen in early endosomes and MHC class I compartments. (A) Immature DCs were incubated with anti-neck antibodies H200 or DCN46 (green) at 4°C, washed, and shifted at 37°C for the indicated time points. Cells (except for t = 0 samples) were acid-strip treated to remove extracellular membrane-bound antibodies, fixed, permeabilized, stained with anti-EEA-1 antibody (red), and analyzed by confocal microscopy. Representative images from at least 3 different experiments are shown. Scale bars represent 10 μm. Immature DCs were incubated with antineck antibody H200 (green) and OVA-Alexa647 (blue) at 4°C, and then treated as in panel A. After acid-strip, fixation, and permeabilization, the samples were stained with anti-EEA-1 (B,D) or anti-MHC-I (C,E) antibodies (red) and analyzed by confocal microscopy. Representative images from 2 independent experiments are shown. Scale bars represent 10 μm.
Triggering DC-SIGN neck domain leads to a prolonged localization of the antigen in early endosomes and MHC class I compartments. (A) Immature DCs were incubated with anti-neck antibodies H200 or DCN46 (green) at 4°C, washed, and shifted at 37°C for the indicated time points. Cells (except for t = 0 samples) were acid-strip treated to remove extracellular membrane-bound antibodies, fixed, permeabilized, stained with anti-EEA-1 antibody (red), and analyzed by confocal microscopy. Representative images from at least 3 different experiments are shown. Scale bars represent 10 μm. Immature DCs were incubated with antineck antibody H200 (green) and OVA-Alexa647 (blue) at 4°C, and then treated as in panel A. After acid-strip, fixation, and permeabilization, the samples were stained with anti-EEA-1 (B,D) or anti-MHC-I (C,E) antibodies (red) and analyzed by confocal microscopy. Representative images from 2 independent experiments are shown. Scale bars represent 10 μm.
Antigens targeted to DC-SIGN neck region are cross-presented
The confocal microscopy data suggest that antineck domain antibodies may constitute excellent tools to target antigens into cellular compartments that facilitate cross-presentation. Because proper T-cell stimulation requires stimulation with mature DCs, we first confirmed that DC maturation using a TLR stimulus would not affect the routing of the anti-neck antibody (supplemental Figure 3). Next, we determined whether antigens targeted to the DC-SIGN neck domain are presented to antigen-specific naive CD8+ and CD4+ T cells via MHC classes I and II, respectively. For this purpose, OVA was conjugated as the antigen to the anti-neck antibody DCN46 (anti-neck/OVA) and, as a control, to an isotype-specific antibody (isotype/OVA). Analysis of the conjugates revealed that, on average, 3 molecules of OVA were attached to a single antibody (data not shown). We opted for DCN46 as the neck-targeting antibody because it does not affect DC maturation or the capacity of immature and mature DCs to induce primary and secondary immune responses.20 In contrast, H200 has been reported to induce DC-SIGN signaling and reduce the ability of DCs to mature and induce T-cell responses.19 BMDCs from mice carrying the human DC-SIGN transgene under the promoter of CD11c were used as antigen-presenting cells.22 These cells were used previously to determine cross-presentation of glycan-modified antigens that bind the CRD of DC-SIGN.10 Expression of human DC-SIGN was detected on 62% of the CD11c+ cells in BMDCs derived from transgenic mice (supplemental Figure 4A). Similar to the human DCs, triggering the neck domain of human DC-SIGN in mouse BMDCs carrying the transgene resulted in delayed routing of antibodies to lysosomal compartments (supplemental Figure 4B). We performed experiments wherein antibody/OVA conjugates were allowed to bind to the BMDCs, excess antigen was removed, and BMDCs were allowed to internalize, mature, and present antigen to CD4+ (OT-II) and CD8+ (OT-I) T cells. The results show that specific binding of the anti-neck/OVA conjugate to DC-SIGN was sufficient to induce proliferation of both OT-II and OT-I T cells (Figure 5A). Next, BMDCs were allowed to take up antigen at various concentrations for a prolonged period of time before induction of DC maturation and coculturing with the OT-I (Figure 5B) and OT-II cells (Figure 5C). In these experiments, isotype/OVA conjugates did not induce T-cell proliferation, showing that Fc receptors and the MR hardly contributed to cross-presentation of antibody/OVA conjugates. By contrast, anti-neck/OVA conjugates induced strong OT-I (Figure 5B) and OT-II (Figure 5C) proliferation at > 1000 times lower antigen concentrations compared with nonconjugated OVA. Moreover, proliferation of both OT-I and OT-II cells correlated with the production of IFN-γ, showing that the T cells were properly activated (Figure 5B-C). In conclusion, both MHC class I and II-restricted antigenic epitopes are efficiently presented to T cells on targeted delivery to the neck domain of DC-SIGN.
Antigens targeted to the DC-SIGN neck domain on DCs are presented to CD4+ and CD8+ T cells. The specificity and efficacy by which antibody/OVA conjugates induce antigen presentation via MHC I and II were determined using BMDCs from human DC-SIGN transgenic mice. (A) To specifically deliver antigen to the neck domain of DC-SIGN, anti-neck/OVA and its control isotype/OVA were incubated with transgenic BMDCs at 4°C. Next, LPS was added to the medium to induce BMDC maturation. OT-I or OT-II T cells were added and cells were co-cultured for 3 days before determining T-cell proliferation. Two independent experiments were performed showing similar results. Data represent mean values of 1 experiment performed in triplicate ± SEM. *P < .05 (t test). **P < .001 (t test). To evaluate the efficacy of neck-targeted antigens, BMDCs were incubated with various concentrations of anti-neck/OVA, isotype/OVA, or nonconjugated OVA for 3 hours. Next, antigens were removed and LPS was added to the medium to induce BMDC maturation, and (B) OT-I or (C) OT-II T cells were added. T-cell proliferation and IFN-γ levels were determined. Two experiments were performed with similar results. Data represent mean ± SEM for 1 experiment performed in duplicate.
Antigens targeted to the DC-SIGN neck domain on DCs are presented to CD4+ and CD8+ T cells. The specificity and efficacy by which antibody/OVA conjugates induce antigen presentation via MHC I and II were determined using BMDCs from human DC-SIGN transgenic mice. (A) To specifically deliver antigen to the neck domain of DC-SIGN, anti-neck/OVA and its control isotype/OVA were incubated with transgenic BMDCs at 4°C. Next, LPS was added to the medium to induce BMDC maturation. OT-I or OT-II T cells were added and cells were co-cultured for 3 days before determining T-cell proliferation. Two independent experiments were performed showing similar results. Data represent mean values of 1 experiment performed in triplicate ± SEM. *P < .05 (t test). **P < .001 (t test). To evaluate the efficacy of neck-targeted antigens, BMDCs were incubated with various concentrations of anti-neck/OVA, isotype/OVA, or nonconjugated OVA for 3 hours. Next, antigens were removed and LPS was added to the medium to induce BMDC maturation, and (B) OT-I or (C) OT-II T cells were added. T-cell proliferation and IFN-γ levels were determined. Two experiments were performed with similar results. Data represent mean ± SEM for 1 experiment performed in duplicate.
Discussion
The present study demonstrates that the intracellular routing of DC-SIGN depends on the epitope targeted to trigger internalization. Antibodies directed against the neck domain result in clathrin-independent internalization and prolonged residence in early endosomal, MHC I+ compartments, whereas antibodies binding to the CRD induce clathrin-mediated internalization and preferential routing to lysosomal compartments. This suggests that the neck domain of DC-SIGN represents a promising target for DC-specific vaccination strategies aiming to induce CTL responses.
The molecular mechanisms involved in the process of cross-presentation remain poorly understood. Initially, cross-presentation was thought to require antigens to escape from endosomal compartments into the cytoplasm, proteasomal processing, relocation to the endoplasmic reticulum, and subsequent loading onto MHC I molecules. Recent evidence demonstrates the importance of early endosomes in cross-presentation, to carry peptide transporters and facilitate loading of peptides onto MHC I without the need of antigen relocalization to the endoplasmic reticulum.30,32,33 Antigens retained in early endosomal, MHC I+ compartments are presented on MHC classes I and II, whereas routing to lysosomal compartments selectively inhibits class I presentation.31,34 In line with these studies, we found that antibody-antigen conjugates internalized into early endosomes via the neck domain of DC-SIGN result in effective MHC class I and II-mediated antigen presentation. Notwithstanding, it has been shown before by our group as well as others that antigens targeted to the CRD of DC-SIGN can also be cross-presented, despite the fact that they are routed to lysosomal compartments.8,10 Possibly, targeting the CRD-domain routes part of the antigen toward a recently defined antigen storage compartment distinct from early endosomal and MHC class II loading compartments. This storage compartment stains positive for the lysosomal marker LAMP1, negative for MHC classes I and II, and provides a continuous supply of antigens for MHC class I presentation.35
Regrettably, it was impossible to make a fair comparison between antigen presentation efficiency after differential routing of DC-SIGN antibodies. We performed experiments to compare antigen presentation induced by anti-neck/OVA to similar conjugates generated by coupling the anti-CRD antibody AZN-D1 to OVA. The data show that both class I and II epitopes were more efficiently presented when targeted by the antineck antibody, both in the presence and absence of a DC maturation stimulus, and at relatively low antigen concentrations (supplemental Figure 5). However, differences in the binding affinity between the 2 distinct antibody/OVA conjugates (supplemental Figure 6) and the efficacy by which these 2 antibodies induce receptor internalization (Figure 1C) will inevitably have affected the amount of antigen that entered the cells. The observed differences in antigen presentation will therefore not merely reflect differences in antigen routing. Nevertheless, the data identify the anti-neck antibody DCN46 to target DCs more efficiently for antigen presentation than the anti-CRD AZN-D1 antibody that was used so far.8,9,11
Our findings suggest that targeting antigens to distinct receptor epitopes, and thereby distinct endosomal compartments with different protease activity, probably affect the processing of antigens and thereby result in presentation of different epitopes. This is exemplified by a recent study assessing induction of T-cell responses against the tumor-associated antigen NY-ESO-1 after internalization via distinct pathways. Antigen-presenting cells that were treated with NY-ESO-1 targeted to the CLRs DEC-205 and MR presented a peptide repertoire that was distinct from cells treated with soluble protein.36 Consequently, targeting antigens to DC-SIGN via both neck and CRD domains may provide a means to broaden the peptide repertoire that is presented to T cells.
Apart from mediating pathogen binding and internalization, DC-SIGN is known to activate intracellular signal pathways on pathogen recognition. The receptor is associated with a signalosome complex consisting of the scaffold proteins LSP1, KSR1, and CNK and the kinase Raf-1. Whereas pathogens displaying fucose ligands actively dissociate the KSR1–CNK–Raf-1 complex from the DC-SIGN signalosome resulting in suppression of inflammatory responses, mannose-expressing pathogens activate Raf-1 via the signalosome and induce expression of proinflammatory cytokines, such as IL-12 and IL-6.37 Besides sugar ligands, certain antibodies have been described to induce DC-SIGN signaling. Strikingly, these include both CRD and neck domain binding antibodies, which trigger distinct signaling pathways. The anti-CRD antibody MR-1 signals via ERK1/2 and enhances TNF-α and LPS-induced IL-10 production by DCs.38 The anti-neck antibody H200 mimics HIV-1–induced DC-SIGN signaling via the small GTPase protein RhoA.19 These studies show that antibodies, similar to sugar ligands, can be harnessed to induce transcriptional programs in antigen-presenting cells that skew immune responses toward a specific immunologic outcome, which is useful for targeted vaccination strategies.
In addition to exogenous ligands, the CRD domain of DC-SIGN recognizes various endogenous glycoproteins with known functions in the immune system, including ICAM-2 and ICAM-3, Mac-1, and sialylated immunoglobulins.39,40 Consequently, CRD-blocking antibodies not only hamper pathogen recognition by DCs but probably affect DC function in multiple ways. Notably, T cells expressing sugar residues recognized by DC-SIGN show reduced proliferation in an alloreactive setting in the presence of CRD-blocking antibodies.41 Neck-binding antibodies are less likely to interfere with DC-SIGN function as the CRD is still available to bind sugar residues, which favors their use in targeted vaccination strategies. Although many DC-SIGN molecules are removed from the cell surface after antibody binding, generally half of the > 100 000 receptor molecules remains available for ligand binding on the cell surface for a prolonged period of time.7-9
There is one other study that describes prolonged retention of a DC-SIGN ligand in an early endosomal compartment. DC-SIGN binds hepatitis C virus via its envelope glycoproteins E1 and E2. Immature DCs internalize hepatitis C virus-like particles and route them to an EEA-1+ compartment where they reside for up to 24 hours.42 Because other DC-SIGN ligands binding to the same binding site as E1 and E2 are delivered to lysosomal compartments, the authors suggested that the differential routing of HCV is dependent on the ligand and not on the binding site. In contrast, our experiments now show that antibodies are differentially routed depending on whether they bind to the CRD or neck domain. Possibly, distinct conformational changes are induced in individual DC-SIGN molecules, tetramers, or DC-SIGN clusters depending on both the nature of the ligand and the binding site, which result in different modes of internalization. Anti-neck antibodies may provide useful tools defining the molecular mechanisms responsible for skewing intracellular routing after pathogen-receptor interaction.
A detailed understanding of how binding affects the mode of receptor internalization, signaling, routing, and antigen presentation will allow rational design of antibody-based vaccines. Our current findings show that the choice of antibody not merely determines targeting efficiency but also dictates receptor routing, and thereby antigen handling by the cell. This calls for a reevaluation of current strategies targeting DC-SIGN and reveals the neck domain as a promising target to reach the appropriate intracellular compartment to induce cellular immune responses, allowing activation of CD8+ T cells and provision of T-cell help without completely blocking receptor function.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Microscopic Imaging Center of the Nijmegen Centre for Molecular Life Sciences for use of their facilities and Ingrid Zeelenberg, Inge Reinieren-Beeren, and Jack Fransen for technical assistance.
This work was supported by The Netherlands Organization for Scientific Research (Veni 916.66.028 and Meervoud 836.09.002, A.C.; TOP 9120.6030; and Spinoza Prize), Immunanomap (MRTN-CT-2006-035946) funded by the European Commission (C.G.F.), and the Sonderforschungsbereich (SFB900; T.S.).
Authorship
Contribution: P.J.T., W.G., L.B., L.J.C., and B.J. performed experiments; A.C., P.J.T., and C.G.F. designed and interpreted experiments; T.S. provided essential materials; P.J.T. and A.C. wrote the manuscript; and A.C. directed the project.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alessandra Cambi, Department of Tumor Immunology, Nijmegen Centre for Molecular Life Sciences, Radboud University Nijmegen Medical Centre, Nijmegen, PO Box 9101, 6500 HB Nijmegen, The Netherlands; e-mail: A.Cambi@ncmls.ru.nl.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal