Abstract
Trousseau syndrome is classically defined as migratory, heparin-sensitive but warfarin-resistant microthrombi in patients with occult, mucinous adenocarcinomas. Injecting carcinoma mucins into mice generates platelet-rich microthrombi dependent on P- and L-selectin but not thrombin. Heparin prevents mucin binding to P- and L-selectin and mucin-induced microthrombi. This model of Trousseau syndrome explains resistance to warfarin, which inhibits fluid-phase coagulation but not selectins. Here we found that carcinoma mucins do not generate microthrombi in mice lacking P-selectin glycoprotein ligand-1 (PSGL-1), the leukocyte ligand for P- and L-selectin. Furthermore, mucins did not activate platelets in blood from PSGL-1–deficient mice. Mucins induced microthrombi in radiation chimeras lacking endothelial P-selectin but not in chimeras lacking platelet P-selectin. Mucins caused leukocytes to release cathepsin G, but only if platelets were present. Mucins failed to generate microthrombi in cathepsin G-deficient mice. Mucins did not activate platelets in blood from mice lacking cathepsin G or protease-activated receptor-4 (PAR4), indicating that cathepsin G activates platelets through PAR4. Using knockout mice and blocking antibodies, we found that mucin-triggered cathepsin G release requires L-selectin and PSGL-1 on neutrophils, P-selectin on platelets, and Src family kinases in both cell types. Thus, carcinoma mucins promote thrombosis through adhesion-dependent, bidirectional signaling in neutrophils and platelets.
Introduction
Trousseau syndrome is classically defined as a thrombotic event preceding or appearing concomitantly with a visceral malignancy.1,2 The hallmarks are venous and arterial microthrombi with secondary microangiopathic hemolytic anemia. Notwithstanding its classic definition, Trousseau syndrome is sometimes used to describe any thrombotic complication associated with cancer.2 Early studies of Trousseau syndrome emphasized the activation of fluid-phase coagulation. A cysteine protease in carcinoma extracts was reported to directly activate factor X.3 Many tumors express tissue factor,4 and tissue factor-bearing microparticles derived from platelets5 or tumor cells6,7 have been observed. However, heparin prevents recurrent thrombosis much more effectively than vitamin K antagonists such as warfarin.1,8-13 Although heparin might block tissue factor-triggered coagulation more effectively than warfarin, its clinical superiority implies that other mechanisms also contribute to Trousseau syndrome. The association of Trousseau syndrome with mucinous adenocarcinomas suggests a pathogenic role for tumor-secreted mucins.1 Carcinoma cells express higher levels of mucins,14 and elevated levels of these mucins and their fragments circulate in patients.15,16 Mucins bearing highly sialylated O-glycans selectively resist clearance and circulate for long periods.17
During inflammation or injury, interactions of selectins with their glycosylated ligands initiate adhesion of leukocytes to activated platelets and/or endothelial cells.18 L-selectin is expressed on leukocytes. Thrombin and other agonists cause platelets and endothelial cells to mobilize P-selectin from secretory granules to the plasma membrane.19 The dominant leukocyte ligand for P- and L-selectin is P-selectin glycoprotein ligand-1 (PSGL-1), a transmembrane homodimer.18 Carcinoma mucins display sialylated, fucosylated, and sulfated O-glycans that also bind to P- and L-selectin.20 When injected intravenously into mice, pyrogen-free preparations of carcinoma mucins form platelet-rich, fibrin-containing microthrombi in lungs and other organs.21 Mucins do not form microthrombi in mice lacking P- or L-selectin.21 Pretreating mice with heparin significantly reduces mucin-induced microthrombi, whereas pretreating mice with hirudin, a thrombin inhibitor, diminishes fibrin within the platelet aggregates but does not alter their size or frequency. Thus, mucins do not require thrombin generation to trigger platelet-rich thrombi.21 These results suggest a fluid-phase coagulation-independent mechanism for Trousseau syndrome and provide a rationale for its frequent association with mucin-rich adenocarcinomas. Notably, some of the diverse glycosaminoglycans of heparin bind to and block the adhesive functions of P- and L-selectin.22 This might explain why heparin is much more effective than warfarin in preventing thrombosis in Trousseau syndrome.
The mechanisms by which carcinoma mucins activate platelets and generate microthrombi are not well understood. Mucins activate platelets in whole blood even in the presence of hirudin to block thrombin generation.21 Platelet activation requires L-selectin, suggesting a key role for leukocytes.21 Engaging either L-selectin or PSGL-1 can trigger signaling cascades in neutrophils, including the activation of Src family kinases (SFKs).23-25 These signals might cause neutrophils to release mediators that activate platelets. In testing this hypothesis, we found that carcinoma mucins required both PSGL-1 and platelet (but not endothelial) P-selectin to form platelet-rich microthrombi in vivo. We identified the neutrophil proteinase cathepsin G as a key mediator of mucin-induced platelet activation in vitro and in vivo. Cathepsin G used protease-activated receptor-4 (PAR4) to stimulate platelets. Mucin-triggered secretion of cathepsin G required L-selectin and PSGL-1 in neutrophils, P-selectin in platelets, and SFKs in both cell types. Thus, carcinoma mucins initiate thrombosis through adhesion-dependent, reciprocal activation of neutrophils and platelets.
Methods
Reagents
Rat monoclonal antibodies (mAbs) to murine CD41 (cloneMWReg 30) and L-selectin (clone Mel-14), R-phycoerythrin (PE)–labeled rat mAbs to murine CD41 mAb (clone MWReg 30) and CD45 (clone 30-F11), and FITC-labeled rat mAbs to murine P-selectin (clone RB40.34), CD45.1 (clone A20), and CD45.2 (clone 104) were from BD Biosciences. Rat anti-murine PSGL-1 mAbs 4RB12 and 4RA1026 were gifts from Dietmar Vestweber (Max Planck Institute, Muenster, Germany). Cy3-labeled donkey anti–rat IgG was from Jackson ImmunoResearch Laboratories. HRP-conjugated goat anti–mouse IgG, phosphatase inhibitor cocktail, and 4%-20% gradient polyacrylamide gels were from Thermo Scientific. Murine P-selectin-IgM chimera was described previously.27 Tissue culture-grade PBS and goat anti–human IgM antibody were from Invitrogen. Hirudin (Refludan) was from Aventis Pharmaceuticals. Tissue-Tek OCT compound (OCT) was from Sakura Finetek. Vectashield mounting medium was from Vector Laboratories. Cathepsin G and its substrate, Suc-AAPF-pNA, were from Biomol International. The PAR4 receptor agonist peptide AYPGKF was described previously.28
Mice
All mice were bred at least 10 generations into the C57BL/6J (B6, CD45.2) background. PSGL-1–deficient mice (PSGL-1−/−) and PAR4-deficient mice (PAR4−/−) were generated as described.27,29 P-selectin–deficient (P-sel−/−) B6, wild-type (WT) B6, and SJL-Ptprca Pep3b/BoyJ-C57BL/6J (SJL, CD45.1) mice were purchased from The Jackson Laboratory. Mice deficient in cathepsin G30 and neutrophil elastase31 (CG−/−/NE−/−) were gifts from Timothy Ley (Washington University). CG−/−/NE−/− mice were bred with C57BL/6J mice to generate cathepsin G-deficient mice (CG−/−) and neutrophil elastase–deficient mice (NE−/−). We confirmed normal blood counts and normal prothrombin times and activated partial thromboplastin times in mice lacking either or both proteinases. Mice lacking Hck, Fgr, and Lyn (Hck−/−/Fgr−/−/Lyn−/−)32 were gifts from Clifford Lowell (University of California at San Francisco). All experiments were performed in compliance with protocols approved by the Institutional Animal Care and Use Committee of the Oklahoma Medical Research Foundation.
Cells
Murine bone marrow leukocytes, isolated as described,33 were suspended at 2 × 107 cells/mL in HBSS with calcium and magnesium (Mediatech). Whole blood was collected by heart puncture into tubes containing hirudin (50 μg/mL final concentration) and centrifuged at 100g for 10 minutes. The platelet-rich plasma was centrifuged at 800g for 10 minutes. The resulting platelet pellet was washed twice with 0.12M sodium chloride, 0.0129M trisodium citrate, 0.03M D-glucose, pH 6.5 and resuspended at 109 platelets/mL in HBSS with calcium and magnesium.
Purification of carcinoma mucin fragments
Mucin fragments from human colonic adenocarcinoma LS180 cells were isolated under stringent conditions to remove coagulants, pyrogens, and other contaminants as described.21
Mucin-induced thrombus formation in vivo
Mucin-induced thrombus formation in vivo was performed as described.21 Briefly, tissue culture–grade saline (0.1 mL) or purified mucins (100 μg) dissolved in 0.1 mL saline were injected into the lateral tail vein of anesthetized mice. After 5 minutes the mice were killed. Lung morphology was preserved by intratracheal perfusion of OCT. The thoracic cavity was opened and lung tissues were collected. Cryosections of lung tissues (5 μm) were fixed with acetone, blocked in 10% rabbit serum, incubated with rat anti–mouse CD41 mAb and Cy3-conjugated donkey anti–rat IgG (H&L) at room temperature, and mounted with Vectashield mounting medium. Twenty random fields per tissue section were digitally captured with a 20 × Plan-Fluor dry objective lens (numerical aperture 0.45) on a Nikon Eclipse TE2000-E microscope connected to a Photometrics Coolsnap-ES camera. Immunofluorescently labeled (CD41/Cy3) platelet-positive pixels were acquired with Metamorph 7.5 software and quantified with Adobe Photoshop.
Bone marrow transplantation
To differentiate whether P-selectin on platelets or endothelial cells is required for mucin-induced thrombosis, chimeric mice were generated by bone marrow transplantation as described.34 Briefly, either WT SJL (CD45.1) or P-sel−/− B6 (CD45.2) bone marrow cells (1 × 106 cells/recipient) were injected intravenously into lethally irradiated (9.5 Gy) P-sel−/− B6 or WT SJL recipients (10 weeks of age), respectively, to generate endothelial cell-specific P-sel−/− or platelet-specific P-sel−/− chimeras. After 12 weeks, peripheral leukocytes were examined by flow cytometry using antibodies to CD45.1 or CD45.2 to determine donor hematopoietic cell engraftment.
Mucin-induced platelet activation in vitro
Platelet activation in whole blood was measured as described.21 Briefly, blood was collected from mice by heart puncture into tubes containing hirudin (50 μg/mL final concentration). In some experiments, EDTA (20mM final concentration) was added. Ten microliters of control buffer or buffer containing mucins (100 μg/mL final concentration) was added to 100 μL blood in siliconized cuvettes, and the mixture was stirred for 5 minutes at 37°C. Reacted blood (5 μL) was diluted into 20 μL HEPES-buffered saline containing PE-conjugated rat anti–mouse CD41 mAb (5 μg/mL final) and FITC-conjugated rat anti–mouse P-selectin (5 μg/mL final). The mixture was incubated without stirring for 15 minutes at room temperature and then diluted and immediately analyzed by flow cytometry. The percentage of P-selectin (FITC)–positive cells was determined in the population of platelets gated by PE fluorescence. P-selectin expression in control buffer-treated samples was subtracted as background.
Cathepsin G enzymatic assay
Control buffer (20 μL) or 20 μL buffer containing 0.2 × 108 washed platelets was added to 100 μL buffer containing 2 × 106 leukocytes. After stirring for 5 minutes at 37°C, control buffer (20 μL) or 20 μL buffer containing mucin (100 μg/mL final) was added. After again stirring for 5 minutes at 37°C, the reaction was stopped by pelleting the cells at 1000g at 4°C. Supernatants were placed in wells of a 96-well microtiter plate. The cathepsin G substrate suc-AAPF-pNA (510μM final) was added to each well. After 2 hours at 37°C, the reaction was terminated by adding phenylmethylsulfonyl fluoride (3.5mM final) and incubating on ice for 30 minutes. Absorbance at 405 nm was then immediately determined with a VersaMax microplate reader (Molecular Devices). The concentration of released cathepsin G in each supernatant was calculated from a standard curve prepared with increasing concentrations of purified cathepsin G. Cathepsin G enzymatic activity in control buffer-treated samples was subtracted as background.
Statistics
Data are expressed as mean ± SEM. Comparisons used the Student t test (unpaired and 2-tailed). A P value < .05 was considered to be significant.
Results
Mucins require PSGL-1 to generate platelet-rich microthrombi in vivo and to activate platelets in whole blood
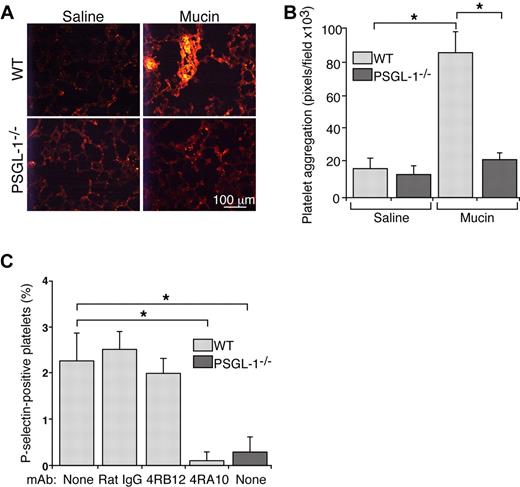
When injected intravenously into mice, pyrogen-free preparations of carcinoma mucins form platelet-rich microthrombi in lungs and other organs.21 Mucins do not form microthrombi in mice lacking P- or L-selectin.21 Both selectins bind to carcinoma mucins,20 but they also bind to PSGL-1 on leukocytes.18 To determine whether mucins require PSGL-1 to generate microthrombi, we injected carcinoma mucins or control saline intravenously into WT or PSGL-1−/− mice. Mucins rapidly generated small intravascular platelet-rich microthrombi, detected with mAb to the platelet marker CD41, in lungs of WT mice (Figure 1A-B), confirming previous results.21 In contrast, mucin injection yielded only rare, small intravascular platelet-rich microthrombi in lungs of PSGL-1−/− mice, which were indistinguishable from those in WT or PSGL-1−/− mice injected with control saline. These data demonstrate that mucins require PSGL-1 to generate platelet-rich microthrombi in vivo.
Mucins require PSGL-1 to generate platelet-rich microthrombi in vivo and to activate platelets in whole blood. (A) Representative images of lung sections from WT or PSGL-1−/− mice prepared 5 minutes after intravenous injection of mucins or saline. The sections were labeled with antibody to the platelet marker, CD41. (B) Quantification of CD41-positive pixels in lung sections. The data represent the mean ± SEM, n = 4-7 mice for each group. *P < .001. (C) Hirudin-anticoagulated whole blood from WT or PSGL-1−/− mice was incubated with mucins in the presence or absence of control rat IgG, nonblocking anti–PSGL-1 mAb 4RB12 or blocking anti–PSGL-1 mAb 4RA10. The percentage of P-selectin–positive platelets was measured by flow cytometry. P-selectin expression in control buffer-treated samples was subtracted as background. The data represent the mean ± SEM from 5 experiments. *P < .01.
Mucins require PSGL-1 to generate platelet-rich microthrombi in vivo and to activate platelets in whole blood. (A) Representative images of lung sections from WT or PSGL-1−/− mice prepared 5 minutes after intravenous injection of mucins or saline. The sections were labeled with antibody to the platelet marker, CD41. (B) Quantification of CD41-positive pixels in lung sections. The data represent the mean ± SEM, n = 4-7 mice for each group. *P < .001. (C) Hirudin-anticoagulated whole blood from WT or PSGL-1−/− mice was incubated with mucins in the presence or absence of control rat IgG, nonblocking anti–PSGL-1 mAb 4RB12 or blocking anti–PSGL-1 mAb 4RA10. The percentage of P-selectin–positive platelets was measured by flow cytometry. P-selectin expression in control buffer-treated samples was subtracted as background. The data represent the mean ± SEM from 5 experiments. *P < .01.
Carcinoma mucins do not activate platelets in buffer or in platelet-rich plasma,20 and they require L-selectin–expressing leukocytes to activate platelets in whole blood.21 We incubated hirudin-anticoagulated blood from WT or PSGL-1−/− mice with mucins or control buffer and then used flow cytometry to quantify platelet activation by expression of P-selectin on platelets. P-selectin expression in control buffer-treated samples was subtracted as background. Mucins induced a low but significant increase in P-selectin expression on platelets from WT mice (Figure 1C). This increased expression was prevented by adding blocking anti–PSGL-1 mAb 4RA10 but not nonblocking anti–PSGL-1 mAb 4RB12 or control IgG. Furthermore, mucins did not induce P-selectin expression on platelets from PSGL-1−/− mice (Figure 1C). These results demonstrate that mucins require PSGL-1 to activate platelets in whole blood.
Mucins require P-selectin on platelets but not on endothelial cells to generate microthrombi in vivo
In whole blood, mucins activate platelets and therefore increase surface expression of P-selectin.21 In vivo, P-selectin is expressed on activated endothelial cells as well as platelets.18 We transplanted WT or P-sel−/− bone marrow into lethally irradiated WT or P-sel−/− recipient mice. After 12 weeks, > 95% of circulating leukocytes in recipient mice expressed the CD45.1 or CD45.2 marker from donor mice. Injected mucins generated equivalent platelet microthrombi in WT mice and in mice lacking P-selectin in endothelial cells. By contrast, mucins did not generate microthrombi in mice lacking P-selectin in platelets (Figure 2A-B). Thus, mucins require P-selectin on platelets but not on endothelial cells to generate microthrombi in our model.
Mucins require P-selectin on platelets but not on endothelial cells to generate microthrombi in vivo. Transplantation of bone marrow into lethally irradiated mice was used to generate mice lacking P-selectin in platelets or in endothelial cells. (A) Representative images of lung sections prepared 5 minutes after intravenous injection of mucins or saline. (B) Quantification of CD41-positive pixels in lung sections. The data represent the mean ± SEM, n = 5-6 for each group. *P < .001.
Mucins require P-selectin on platelets but not on endothelial cells to generate microthrombi in vivo. Transplantation of bone marrow into lethally irradiated mice was used to generate mice lacking P-selectin in platelets or in endothelial cells. (A) Representative images of lung sections prepared 5 minutes after intravenous injection of mucins or saline. (B) Quantification of CD41-positive pixels in lung sections. The data represent the mean ± SEM, n = 5-6 for each group. *P < .001.
Mucins require the neutrophil proteinase, cathepsin G, to generate platelet-rich microthrombi in vivo, and require cathepsin G and PAR4 to activate platelets in whole blood
Leukocytes are required for mucin-induced platelet activation,21 suggesting that they might release a platelet agonist(s). One potent agonist is the neutrophil proteinase, cathepsin G.35-38 Another candidate is neutrophil elastase, which alone does not activate platelets but potentiates the effects of low doses of cathepsin G.39 To determine whether mucins use either of these proteinases to generate microthrombi in vivo, we injected mucins into WT mice or into mice lacking cathepsin G (CG−/−), neutrophil elastase (NE−/−) or both proteinases (CG−/−/NE−/−). Mice lacking either or both proteinases are healthy, have normal blood counts, and exhibit normal neutrophil recruitment and function in response to some but not all inflammatory challenges.30,31,40,41 Mucins rapidly generated platelet-rich microthrombi in WT or NE−/− mice but not in CG−/−/NE−/− or CG−/− mice (Figure 3A-B). Furthermore, mucins activated platelets in hirudin-treated blood from WT mice but not from CG−/−/NE−/− or CG−/− mice (Figure 3C-D). Mucin-induced platelet activation in blood from NE−/− mice was modestly decreased (Figure 3D), suggesting that elastase augments the actions of cathepsin G. These data demonstrate that mucins require cathepsin G to generate microthrombi in vivo and to activate platelets in vitro.
Mucins require the neutrophil proteinase, cathepsin G, to generate platelet-rich microthrombi in vivo, and require cathepsin G and PAR4 to activate platelets in whole blood. (A) Representative images of lung sections from mice of the indicated genotype prepared 5 minutes after intravenous injection of mucins or saline. The sections were labeled with antibody to the platelet marker, CD41. (B) Quantification of CD41-positive pixels in lung sections. The data represent the mean ± SEM, n = 7 for each group. *P < .001. (C,D) Hirudin-anticoagulated whole blood from mice of the indicated genotype was incubated with mucins. The percentage of P-selectin–positive platelets was measured by flow cytometry. P-selectin expression in control buffer-treated samples was subtracted as background. The data represent the mean ± SEM from 5 experiments. (E) Hirudin-anticoagulated whole blood from mice of the indicated genotype was incubated with mucins or the PAR4 receptor agonist AYPGKF peptide at the indicated concentration. The percentage of P-selectin–positive platelets was measured by flow cytometry. P-selectin expression in control buffer-treated samples was subtracted as background. The data represent the mean ± SEM from 5 experiments. *P < .01; **P < .05.
Mucins require the neutrophil proteinase, cathepsin G, to generate platelet-rich microthrombi in vivo, and require cathepsin G and PAR4 to activate platelets in whole blood. (A) Representative images of lung sections from mice of the indicated genotype prepared 5 minutes after intravenous injection of mucins or saline. The sections were labeled with antibody to the platelet marker, CD41. (B) Quantification of CD41-positive pixels in lung sections. The data represent the mean ± SEM, n = 7 for each group. *P < .001. (C,D) Hirudin-anticoagulated whole blood from mice of the indicated genotype was incubated with mucins. The percentage of P-selectin–positive platelets was measured by flow cytometry. P-selectin expression in control buffer-treated samples was subtracted as background. The data represent the mean ± SEM from 5 experiments. (E) Hirudin-anticoagulated whole blood from mice of the indicated genotype was incubated with mucins or the PAR4 receptor agonist AYPGKF peptide at the indicated concentration. The percentage of P-selectin–positive platelets was measured by flow cytometry. P-selectin expression in control buffer-treated samples was subtracted as background. The data represent the mean ± SEM from 5 experiments. *P < .01; **P < .05.
Cathepsin G activates human platelets through PAR4.42 Mucins triggered platelet activation in hirudin-anticoagulated blood from WT or PAR4 heterozygous mice (PAR4+/−) but not from PAR4-deficient (PAR4−/−) mice (Figure 3E). These data indicate that mucin-induced release of cathepsin G activated murine platelets through PAR4. As a positive control, we used the PAR4 receptor agonist peptide AYPGFK.28 At a low concentration (50μM), the peptide activated a small percentage of platelets in hirudin-treated blood from control mice but not from PAR4−/− mice (Figure 3E). At a high concentration (500μM), the peptide caused platelets to form aggregates that were not detected by flow cytometry. Adding EDTA prevented aggregation, revealing that the high peptide concentration stimulated > 90% of WT platelets but did not stimulate PAR−/− platelets (data not shown).
Mucins require L-selectin, PSGL-1, and platelet P-selectin to stimulate secretion of cathepsin G from neutrophils
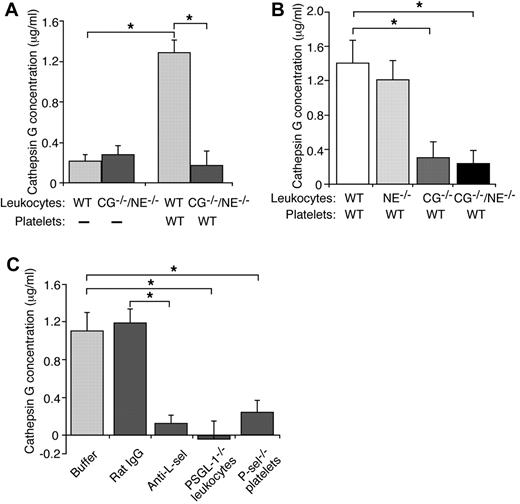
Carcinoma mucins have binding sites for L-selectin,20 and L-selectin is required for mucins to induce platelet-rich microthrombi in vivo and platelet activation in vitro.21 We asked whether mucin binding to L-selectin induces leukocytes to secrete cathepsin G. We incubated leukocytes with control buffer or mucins, pelleted the cells, and measured the concentration of secreted cathepsin G by an enzymatic assay. Activity in control buffer-treated samples was subtracted as background. Mucin treatment resulted in similarly low, presumably nonspecific enzymatic activity in supernatants from WT or CG−/−/NE−/− leukocytes (Figure 4A). Thus, mucin binding to L-selectin is not sufficient to signal secretion of cathepsin G. We next incubated mucins with a mixture of washed platelets and leukocytes. In the presence of WT platelets, mucins significantly increased secretion of cathepsin G from WT or NE−/− leukocytes but not from CG−/− or CG−/−/NE−/− leukocytes (Figure 4A-B). These data demonstrate that mucins require platelets to stimulate secretion of cathepsin G from neutrophils.
Mucins require L-selectin, PSGL-1, and platelet P-selectin to stimulate secretion of cathepsin G from neutrophils. (A-B) Leukocytes from mice of the indicated genotype were incubated with mucins in the presence or absence of washed platelets from WT mice. After pelleting the cells, the concentration of cathepsin G was measured using an enzymatic assay. Activity in control buffer-treated samples was subtracted as background. (C) Leukocytes and platelets of the indicated genotype were incubated with mucin in the presence or absence of control rat IgG or anti–L-selectin mAb Mel-14. After pelleting the cells, the concentration of cathepsin G was measured using an enzymatic assay. Activity in control buffer-treated samples was subtracted as background. The data represent the mean ± SEM from 3 experiments. *P < .01.
Mucins require L-selectin, PSGL-1, and platelet P-selectin to stimulate secretion of cathepsin G from neutrophils. (A-B) Leukocytes from mice of the indicated genotype were incubated with mucins in the presence or absence of washed platelets from WT mice. After pelleting the cells, the concentration of cathepsin G was measured using an enzymatic assay. Activity in control buffer-treated samples was subtracted as background. (C) Leukocytes and platelets of the indicated genotype were incubated with mucin in the presence or absence of control rat IgG or anti–L-selectin mAb Mel-14. After pelleting the cells, the concentration of cathepsin G was measured using an enzymatic assay. Activity in control buffer-treated samples was subtracted as background. The data represent the mean ± SEM from 3 experiments. *P < .01.
Mucins require PSGL-1, L-selectin, and P-selectin to activate platelets in vitro. A mAb to L-selectin, but not control rat IgG, blocked mucin-induced secretion of cathepsin G in a mixture of WT platelets and WT leukocytes (Figure 4C). Mucins did not stimulate secretion of cathepsin G in a mixture of WT platelets and PSGL-1−/− leukocytes or in a mixture of P-sel−/− platelets and WT leukocytes (Figure 4C). These data demonstrate that mucins require L-selectin, PSGL-1, and platelet P-selectin to induce secretion of cathepsin G from neutrophils.
Mucins require SFKs in both leukocytes and platelets to stimulate secretion of cathepsin G
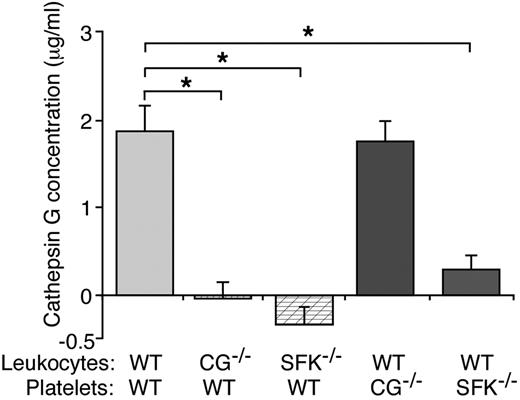
In some situations, engaging L-selectin or PSGL-1 activates SFKs that propagate downstream signaling events in leukocytes.23-25 We asked whether mucin binding to L-selectin and/or P-selectin binding to PSGL-1 might activate SFKs to signal release of cathepsin G. For this purpose we used Hck−/−/Fgr−/−/Lyn−/− mice, which lack all 3 SFKs expressed in neutrophils32 and 2 (Fgr and Lyn) of several SFKs (Fgr, Lyn, Lck, Fyn, Yes, and Src) expressed in platelets.43 In the presence of WT platelets, mucins stimulated release of cathepsin G from WT leukocytes but not from CG−/− or Hck−/−/Fgr−/−/Lyn−/− leukocytes (Figure 5). Mucins also stimulated release of cathepsin G from WT leukocytes mixed with CG−/− platelets. Remarkably, they did not induce cathepsin G release from WT leukocytes mixed with Hck−/−/Fgr−/−/Lyn−/− platelets (Figure 5). Platelets express Fgr and Lyn but not Hck.43 These data demonstrate that mucins require at least one of the SFKs in neutrophils and at least Fgr or Lyn in platelets to stimulate secretion of cathepsin G.
Mucins require SFKs in both leukocytes and platelets to stimulate secretion of cathepsin G. Leukocytes and platelets of the indicated genotype were incubated with mucins. After pelleting the cells, the concentration of cathepsin G was measured using an enzymatic assay. Activity in control buffer-treated samples was subtracted as background. The data represent the mean ± SEM from 5 experiments. *P < .01.
Mucins require SFKs in both leukocytes and platelets to stimulate secretion of cathepsin G. Leukocytes and platelets of the indicated genotype were incubated with mucins. After pelleting the cells, the concentration of cathepsin G was measured using an enzymatic assay. Activity in control buffer-treated samples was subtracted as background. The data represent the mean ± SEM from 5 experiments. *P < .01.
Discussion
Injecting carcinoma mucins intravenously into mice generates platelet-rich microthrombi like those in patients with classically defined Trousseau syndrome. The microthrombi form independently of fluid-phase blood coagulation but require P- and L-selectin,21 which bind to carcinoma mucins.20 Here we have demonstrated that mucins form microthrombi through a signal amplification loop in platelets and neutrophils. Signaling requires selectin/PSGL-1–mediated interactions between platelets and neutrophils, SFKs in both cell types, cathepsin G released from neutrophils, and PAR4 on platelets.
Mucins required PSGL-1 to generate platelet-rich microthrombi in vivo and to activate platelets in hirudin-treated (thrombin-inactivated) whole blood. Therefore, mucin binding to L-selectin on leukocytes and P-selectin on platelets is not sufficient to trigger thrombosis. PSGL-1 must also bind to P-selectin to bring leukocytes in close contact with platelets. Binding of PSGL-1 to L-selectin may bring leukocytes in contact with other leukocytes. In our model, mucin-induced formation of microthrombi required P-selectin from platelets but not from endothelial cells, suggesting an important role for platelet P-selectin in the pathogenesis of Trousseau syndrome. In some patients, P-selectin on activated endothelial cells may augment thrombosis by concentrating platelet-neutrophil aggregates along vascular surfaces.
Mucins induced platelet-rich microthrombi in mice lacking elastase but not in mice lacking cathepsin G. Therefore, cathepsin G is the critical neutrophil enzyme for mucin-generated microthrombi in vivo. Mucins required release of cathepsin G from neutrophils to activate platelets in vitro. Mucin-induced platelet activation was slightly diminished in blood from elastase-deficient mice. Cathepsin G is a well-documented and potent platelet agonist.35-38 Elastase is a much weaker agonist. However, it potentiates the stimulatory effects of low concentrations of cathepsin G in vitro39 and might contribute to mucin-triggered thrombosis in vivo. Close contacts between neutrophils and platelets are presumably required to provide a protected environment for cathepsin G activity36 by preventing rapid dilution and/or inactivation of the enzyme by plasma antiproteinases.37 Binding of platelet P-selectin to neutrophil PSGL-1 mediates these close contacts,44 providing a rationale for the roles of both molecules during mucin-induced thrombosis.
How do mucins stimulate neutrophils to secrete cathepsin G? The enzyme is stored in azurophilic granules, which are more difficult to mobilize than other secretory granules in neutrophils.45 Incubating only mucins with leukocytes did not discharge cathepsin G, indicating that signals propagated through L-selectin are not sufficient. In agreement, antibody crosslinking of L-selectin releases secretory vesicles and secondary and tertiary granules, but not azurophilic granules.46 Mucins required L-selectin and PSGL-1 on neutrophils and P-selectin on platelets to induce cathepsin G release. They also required at least one of the SFKs in neutrophils: Hck, Fgr, and Lyn. Engaging L-selectin or PSGL-1 activates these SFKs, which propagate downstream signals.23-25 Cooperative binding of mucins to L-selectin and of P-selectin to PSGL-1 might suffice to trigger secretion of cathepsin G. However, mucins also required Fgr and/or Lyn in platelets to stimulate release of cathepsin G from neutrophils. This SFK-dependent stimulation of platelets might mobilize mediators that facilitate secretion of cathepsin G from azurophilic granules. Stimulated platelets release many chemokines, including CXCL1 and CXCL4 that activate neutrophils through G protein-coupled receptors.47
How do mucins cause activation of SFKs in platelets? Binding of mucins or PSGL-1 to P-selectin might first activate SFKs. Signaling through P-selectin has received less attention than signaling through other adhesion receptors. However, anti–P-selectin antibodies cause transient Ca2+ fluxes in histamine-stimulated human endothelial cells,48 and interactions of neutrophil PSGL-1 with P-selectin enhance secretion of metalloproteinase 2 from platelets.49 Binding of cathepsin G might further activate SFKs. Cathepsin G activates human platelets through PAR4,42 consistent with our finding that mucin-induced activation of murine platelets required both cathepsin G and PAR4. PARs propagate multiple signals through heterotrimeric G proteins, including activation of SFKs as late events.50
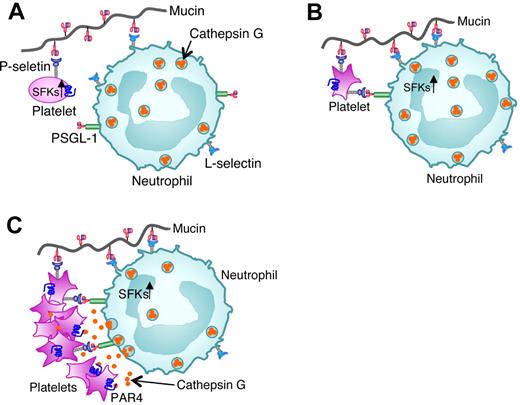
Taken together, our data suggest a mechanistic framework for mucin-triggered thrombosis (Figure 6). Binding of mucins to L-selectin on neutrophils and to low levels of P-selectin on resting platelets brings these cells into closer proximity. Binding of mucins and neutrophil PSGL-1 to P-selectin then activates SFKs in platelets. These kinases propagate sufficient signals to release agonists for neutrophils and/or to mobilize more P-selectin to the platelet surface. More P-selectin binding to PSGL-1 and mucin binding to L-selectin activate SFKs in neutrophils. These SFK-dependent signals cooperate with signaling from platelet-derived agonists to trigger cathepsin G release into the protected space between adherent neutrophils and platelets. Binding of cathepsin G to PAR4 further activates platelets to mobilize more P-selectin and release more soluble agonists. Meanwhile, integrins may stabilize platelet-neutrophil conjugates through inside-out and outside-in signaling.51 Our model emphasizes mucin-induced bidirectional signaling in platelets and neutrophils in a feedback amplification loop that requires direct adhesive contacts. Although highly reproducible, mucin-induced activation of murine platelets was limited in vitro. Mucins also partially activated human platelets in hirudin-anticoagulated whole blood collected by venipuncture, but only in the first few milliliters (M.G.W. and A.V., unpublished observations, April 2002). This suggests that ex vivo priming of platelets immediately after venipuncture sensitized them to mucins. Similar priming of murine platelets may have occurred during cardiac puncture. Although mucins only partially activated platelets in vitro, they dramatically increased platelet-rich microthrombi in vivo. The microcirculation may provide a favorable environment for mucins to bridge, activate, and trap circulating platelets and neutrophils. Furthermore, patients with cancer may release chemokines or other mediators that prime circulating platelets and neutrophils for further activation by mucins.52,53
Model for mucin-induced platelet-neutrophil interactions in Trousseau syndrome. (A) Binding of mucins to L-selectin on neutrophils and to low levels of P-selectin on resting platelets first brings these cells into closer proximity. (B) Binding of mucins and neutrophil PSGL-1 to P-selectin then activates SFKs in platelets. (C) P-selectin binding to PSGL-1 and mucin binding to L-selectin next activate SFKs in neutrophils. SFK-dependent signals in platelets and neutrophils cooperate with signaling from platelet-derived agonists to trigger cathepsin G release into the protected space between adherent neutrophils and platelets. Binding of cathepsin G to PAR4 further activates platelets to mobilize more P-selectin and cause platelet aggregation.
Model for mucin-induced platelet-neutrophil interactions in Trousseau syndrome. (A) Binding of mucins to L-selectin on neutrophils and to low levels of P-selectin on resting platelets first brings these cells into closer proximity. (B) Binding of mucins and neutrophil PSGL-1 to P-selectin then activates SFKs in platelets. (C) P-selectin binding to PSGL-1 and mucin binding to L-selectin next activate SFKs in neutrophils. SFK-dependent signals in platelets and neutrophils cooperate with signaling from platelet-derived agonists to trigger cathepsin G release into the protected space between adherent neutrophils and platelets. Binding of cathepsin G to PAR4 further activates platelets to mobilize more P-selectin and cause platelet aggregation.
The mechanism to trigger thrombosis that we describe does not require thrombin or fluid-phase blood coagulation and applies most to patients with mucinous adenocarcinomas and migratory thrombi in classically defined Trousseau syndrome. Thrombosis in cancer patients is likely to have multiple causes, including excessive blood coagulation.2 Cancer patients frequently have inflammatory challenges that could stimulate endothelial cells to mobilize P-selectin. Therefore, P-selectin on endothelial cells as well as platelets might trigger thrombosis in some patients with mucinous adenocarcinomas. During inflammation, leukocyte-derived microparticles bearing tissue factor are recruited to damaged vascular surfaces through interactions of PSGL-1 with P-selectin, triggering fibrin deposition.54 Some cancer cells shed microparticles that express tissue factor6 and also PSGL-1.7 These microparticles, like those from leukocytes, can promote thrombosis by interacting with P-selectin at sites of inflammation.7 Furthermore, neutrophil-derived nucleosomes and serine proteinases can cooperate to trigger platelet activation and fibrin formation.40,55 Our studies provide insights into mucin-dependent but thrombin-independent thrombosis in patients with Trousseau syndrome. The usual clinical superiority of heparin (which blocks mucin binding to P- and L-selectin) over warfarin suggests that the mechanism for thrombosis that we describe is often dominant. Therefore, our findings may lead to new therapies for this serious complication in cancer patients.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Michael McDaniel and Lacromioara Ivanciu for advice on preparation and staining of tissue sections; and Dietmar Vestweber, Timothy Ley, and Clifford Lowell for valuable reagents.
This work was supported by National Institutes of Health grants HL34363, HL85607, HL65185, HL44907, RR18758, and CA38701. T.D. was supported by American Heart Association (WSA) postdoctoral fellowship 09POST2230289.
National Institutes of Health
Authorship
Contribution: B.S., M.G.W., L.Y., and J.M.M. performed research; T.D. and S.R.C. contributed key reagents; R.P.M. designed research; B.S., M.G.W., T.D., S.R.C., L.X., A.V., and R.P.M. analyzed data; and B.S. and R.P.M. wrote the manuscript, with input from all authors.
Conflict-of-interest disclosure: R.P.M. has equity in Selexys, a company that is developing inhibitors of selectins and selectin ligands. The remaining authors declare no competing financial interests.
The current affiliation for M.G.W. is College of Medicine, University of Illinois at Urbana-Champaign, Urbana, IL.
Correspondence: Rodger P. McEver, MD, Cardiovascular Biology Research Program, Oklahoma Medical Research Foundation, 825 NE 13th St, Oklahoma City, OK 73104; e-mail: rodger-mcever@omrf.org.