Abstract
CD8+ cytotoxic T lymphocytes play a major role in defense against intracellular pathogens, and their functions are specified by antigen recognition and innate cytokines. IL-12 and IFN-α/β are potent “signal 3” cytokines that are involved in both effector and memory cell development. Although the majority of effector cells are eliminated as inflammation resolves, some survive within the pool of memory cells and retain immediate effector function. In this study, we demonstrate that IL-12 instructs a unique program of effector cell differentiation that is distinct from IFN-α/β. Moreover, effector memory (TEM) cells within peripheral blood display many common attributes of cells differentiated in vitro in response to IL-12, including proinflammatory cytokine secretion and lytic activity. A pattern of IL-12–induced genes was identified that demarcate TEM from central memory cells, and the ontologies of these genes correlated precisely with their effector functions. Further, we uncovered a unique program of gene expression that was acutely regulated by IL-12 and reflected in stable gene expression patterns within TEM, but not T central memory cells in vivo. Thus, this study directly links a selective set of IL-12–induced genes to the programming of effector functions within the stable population of human CD8+ TEM cells in vivo.
Introduction
The pool of circulating CD8+ cytotoxic T lymphocytes (CTLs) is remarkably heterogeneous, consisting of antigen inexperienced naive cells as well as multiple populations of effector and memory subsets.1 Various models have been proposed to explain the genesis of memory subsets, and a significant body of evidence suggests that long-lived memory cells are derived from early effector cells that survive contraction as antigen loads wane.2 If so, then the diverse spectrum of memory cells that fall into either effector memory (TEM) or central memory (TCM) categories would also be predicted to be derived from a common pool of primary effectors.3 Regardless of their origins, TEM and TCM differ substantially in their functional capabilities, and the extrinsic signals that regulate their development are complex and multifactorial. Among these factors, innate cytokines are key signals that regulate effector cell differentiation,4 with IL-12 and type I interferon (IFN-α/β) playing important roles in both effector and memory cell development.5
In humans, CD8+ subsets have been distinguished based on their differential expression of various markers, including CD45RA, CD27, CD28, CCR7, CD62L, CD127, and CXCR3.6-9 Based solely on the expression of CCR7 and CD45RA, early studies defined 4 main subsets: naive (CD45RA+/CCR7+), TCM (CD45RA−/CCR7+), TEM (CD45RA−/CCR7−), and effector memory-RA (TEMRA, CD45RA+/CCR7−).10 Aside from their differential expression of CD45RA and other cell surface markers, TEM uniformly exhibit rapid effector functions in response to antigen activation. Further, TEM do not generally require CD28-mediated costimulation or innate cytokines to secrete proinflammatory cytokines or to lyse target cells.11,12 Thus, if TEM cells are direct descendants of effector CTLs, then the maintenance of effector functions within TEM would suggest early programming during the priming phase of infection.
In mice, both IL-12 and IFN-α/β exert somewhat overlapping and redundant effects by driving effector cell development.5,13-17 Although IL-12 seems to be more potent at promoting IFN-γ secretion, both IL-12 and IFN-α/β are equally effective at regulating lytic activity in murine CD8+ T cells. In contrast, IL-12 and IFN-α/β appear to play very distinct roles in priming human CD8+ T-cell responses. Recently, Ramos et al demonstrated a preferential role for IL-12 over IFN-α/β in driving cytokine expression and lytic activity in human CTLs.18 Conversely, IFN-α/β enhanced the development of a subpopulation of cells that displayed phenotypic and functional characteristics of TCM. Programming of effectors by IL-12 was accompanied by progression of cells through cell division and was altered by the strength of TCR engagement. IL-12 programmed effector cell development, which was regulated by induction of the IL-12 receptor at each division as cells proliferated in response to TCR activation. In contrast, IFN-α/β tended to slow the progression of cell division in some cells, and this effect correlated with induction of the IFN-α/β receptor-2 subunit on cells that acquired a TCM phenotype.18 These observations suggest that the functional heterogeneity observed within the pool of memory CD8+ T cells in vivo may result from the differential responsiveness of memory precursors to IL-12 and IFN-α/β during priming.19
In this study, we wished to determine whether the initial signals delivered by IL-12 and IFN-α/β regulated either distinct or overlapping programs of gene expression, and whether these patterns were altered during proliferation. Further, we sought to assess the differential gene expression patterns of memory subpopulations from peripheral blood. This approach allowed for the identification of a subset of genes that were both acutely regulated by IL-12 during priming and also stably expressed in TEM in vivo. As a result, we have identified a defined program of IL-12–mediated gene expression with the potential to regulate effector cell development and maintenance of the TEM phenotype.
Methods
Human subjects
Informed consent was obtained in accordance with the Declaration of Helsinki, and peripheral blood was collected by venipuncture from healthy adult donors as approved by the Internal Review Board at the University of Texas Southwestern Medical Center.
Isolation of and culture of human CD8+ T cells
CD8+CD45RA+ cells were isolated by negative selection with BD IMag kit (Human Naive CD8+ T Cell Enrichment Set-DM), and purity was routinely > 90%. Purified CD8+CD45RA+ cells were labeled with CFSE and cultured at 1×106 cells/mL in 96-well tissue culture treated plates coated with 1.5 μg/mL of anti-CD3+anti-CD28 antibodies in complete IMDM supplemented with 10% FBS. All cells received rhIL-2 (200 U/mL) and anti–human IFN-γ (5 μg/mL), whereas the variable cytokine polarization conditions included neutralized (anti–human IL-12 [5 μg/mL]), IL-12 (recombinant-human IL-12 [10 ng/mL]), IFN-α (anti–human IL-12 [5 μg/mL], recombinant-human IFN-α(A) [1000 U/mL]), and IL-12 + IFN-α (recombinant-human IL-12 [10 ng/mL], recombinant-human IFN-α(A) [1000 U/mL]).
Cytotoxicity assays
CD8+ cells were sorted by FACS based on chemokine receptors and were subjected to a redirected lysis assay as previously described.18 Briefly, anti-CD3–coated THP-1 target cells were labeled by culturing in the presence of 150 μCi Na2[51Cr]O4 in complete growth media for 1.5 hours. Target cells were washed and incubated with CTLs at various E:T ratios for 4 hours at 37°C. Specific cytotoxicity was measured by scintillation counting of 51Cr released in the media.
RNA isolation and microarray analysis
RNA was isolated according to the manufacturer's recommendations using Arcturus PicoPure RNA Isolation Kit. RNA was submitted to the University of Texas Southwestern Medical Center Genomics and Microarray Core Facility where the integrity and quality of the RNA were tested using Bioanalyzer Chip. cDNA synthesis and hybridization onto Illumina SingleColor HumanWG-6_V3_0_R1 platform was according to the manufacturer's instructions. The microarray results were submitted to GEO and assigned the accession number GSE27337.
Raw image files from the scanned chip were first analyzed using GenomeStudio Data Analysis Software Version 1.8.0 to obtain signal intensity values, and sample probe profiles were generated without normalization. Data were then exported to GeneSpring GX 11 Version 4.0 software (Agilent Technologies) for further statistical analysis and visualization. Using GeneSpring, a gene-level expression file was obtained with quantile normalization of all samples. Raw data were normalized, and a 1-way ANOVA test with unequal variance was performed to identify genes that were differentially expressed among the donor samples and under any of the culture conditions. A fold change of > 1.7 was then applied to identify differentially expressed genes. Gene ontology analysis on selected gene sets as well as pathway search was performed using the web-based tool: “Database for Annotation, Visualization, and Integrated Discovery” (DAVID).20,21
Results
IL-12 selectively programs the development of CCR7loCXCR3hi effector CTLs
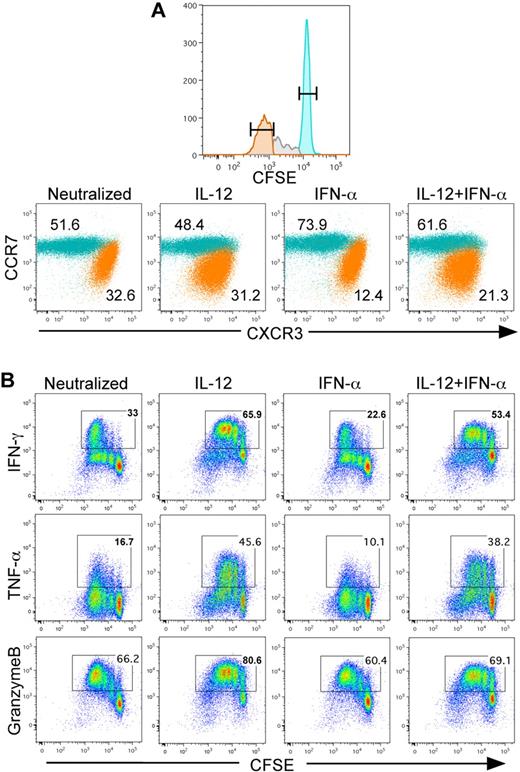
Recently, Ramos et al demonstrated that the innate cytokines IL-12 and IFN-α/β differentially regulated the development of effector and memory functions in human CD8+ T cells.18 By tracking cell division in response to TCR activation, the IL12Rβ2 subunit was increased by IL-12 activation at each cell division, thereby increasing IL-12 responsiveness. This observation suggests that effector functions regulated by IL-12 would be increased at each cell division. To address this, CD8+CD45RA+ T cells were purified from peripheral blood of healthy adult human donors, labeled with CFSE, and then activated with plate-bound anti-CD3/anti-CD28 for 3 days under various defined cytokine conditions. Based on prior titration experiments,18 the cytokine concentrations used here were chosen to maximize the relative frequency of both divided and undivided populations that differentially responded to IL-12 and IFN-α. As expected, we identified populations of cells that differentially expressed CCR7 and CXCR3 regardless of whether the cells were activated with either IL-12 or IFN-α (Figure 1A). Further, cells that were retained in the undivided population were found to be predominantly CCR7hi and CXCR3lo. In contrast, cells that rapidly divided lost expression of CCR7 and were CXCR3hi, which is consistent with previously reported effector CTL markers. Further, regardless of cytokine activation, the dividing cells displayed higher expression of CXCR3 than the undivided population, which remained predominantly CCR7hi.
IL-12 programs CCR7loCXCR3hi effctor CD8+ T cells in vitro. (A) CD8+CD45RA+ T cells were labeled with CFSE and stimulated with plate-bound anti-CD3+anti-CD28 for 3 days in the presence of indicated cytokine conditions. On day 3, the cells were split 1:10 with 100 U/mL of rhIL-2. On day 4, cells were stained for CCR7 and CXCR3, and data are gated on the undivided CFSEhi (teal) and divided CFSElo (orange) populations. The percentage of each population is indicated within the dot plots. (B) Cytokine polarized CD8+ T cells (same as in panel A) were restimulated with 80 ng/mL of phorbol myristate acetate and 1μM of ionomycin in the presence of monensin for 4 hours. Cells were then fixed and stained for intracellular IFN-γ, TNF-α, and granzyme B.
IL-12 programs CCR7loCXCR3hi effctor CD8+ T cells in vitro. (A) CD8+CD45RA+ T cells were labeled with CFSE and stimulated with plate-bound anti-CD3+anti-CD28 for 3 days in the presence of indicated cytokine conditions. On day 3, the cells were split 1:10 with 100 U/mL of rhIL-2. On day 4, cells were stained for CCR7 and CXCR3, and data are gated on the undivided CFSEhi (teal) and divided CFSElo (orange) populations. The percentage of each population is indicated within the dot plots. (B) Cytokine polarized CD8+ T cells (same as in panel A) were restimulated with 80 ng/mL of phorbol myristate acetate and 1μM of ionomycin in the presence of monensin for 4 hours. Cells were then fixed and stained for intracellular IFN-γ, TNF-α, and granzyme B.
CTLs cultured in vitro were also examined for effector molecule expression. As the cells divided, daughter cells progressively expressed increased levels of IFN-γ and TNF-α that was significantly enhanced by IL-12, but not IFN-α (Figure 1B). Granzyme B was expressed uniformly in the most divided population of cells, and IL-12 enhanced granzyme B during earlier divisions (Figure 1B). Although CXCR3 expression increased in divided cells regardless of innate cytokine exposure, IL-12, but not IFN-α, significantly increased IFN-γ, TNF-α, and granzyme B expression within cells as a function of proliferation. Collectively, these data indicate that enhanced sensitivity to IL-12, as previously reported, directly correlates with acquisition of effector functions and suggests an instructive program of effector cell differentiation by IL-12.
CCR7loCXCR3hi CD8+ T cells in human peripheral blood are functional effectors
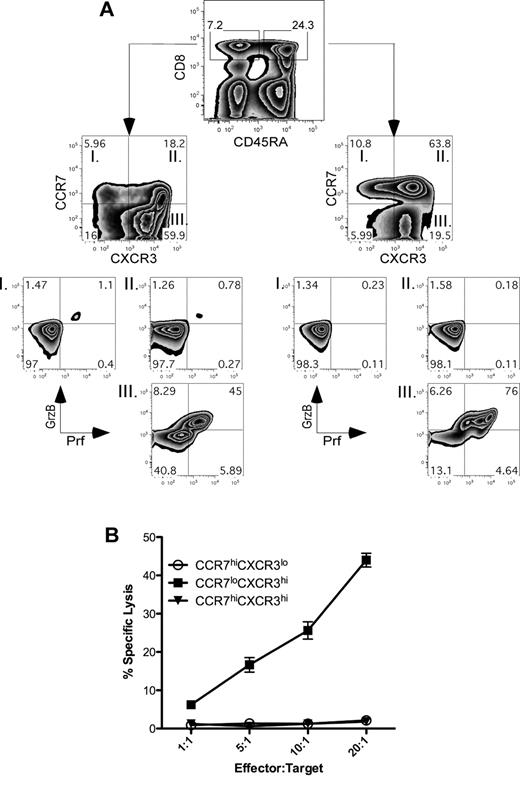
The differential expression of various markers, including CD45RA, CCR7, and CXCR3, on human CD8+ cell populations reflects the functional behaviors of these cells in vivo.8,9 For example, CXCR3 plays an important role in migration of the effector CTLs to sites of infection22,23 and thus has been associated as a marker for distinguishing the effector subsets. The induction of the CXCR3hiCCR7lo cells in our in vitro assays was verified as a parallel functional subset within the CD8+ T-cell pool by staining PBMCs for the surface markers CD8, CD45RA, CCR7, and CXCR3 (Figure 2A). Both CD45RA+ and CD45RA− CD8+ cells contained variegated populations of cells that express CCR7 and CXCR3 (Figure 2A top panel). A proportion of the CD8+ T cells with low expression of CCR7 coexpressed the lytic effector molecules perforin and granzyme B regardless of their expression of CD45RA (Figure 2A bottom panels quadrant III). Using these chemokine receptors as cell surface markers, we purified the following CD8+ T-cell subsets directly from human peripheral blood by FACS sorting: CCR7hiCXCR3lo, CCR7hiCXCR3hi, and CCR7loCXCR3hi. Because all of these populations exist within both the CD45RA+ and CD45RA− population, CD45 was not included as a marker for sorting in this experiment. Immediately after purification, these subsets were then assessed for their ability to kill target cells in a redirected lysis assay. Expression of perforin and granzyme B within the CCR7loCXCR3hi population (Figure 2A) correlated precisely with their ability to mediate killing activity in a redirected lysis assay (Figure 2B). These data demonstrate that lytic activity within the CCR7loCXCR3hi population is a stable property of these cells in homeostasis.
CCR7loCXCR3hi CD8+ T cells display an effector phenotype. (A) PBMCs from healthy human blood were stained for surface receptors CD8, CD45RA, CXCR3, CCR7, and intracellular perforin and granzyme B. Four individual donors were used in replicate experiments, and representative plots from one experiment are shown. (B) Healthy human PBMCs were stained with antibodies specific for CD8, CCR7, and CXCR3 and sorted into 3 separate CD8+ populations as indicated in the figure. Redirected lysis assay was carried out at the indicated E:T ratios using anti–human anti-CD3–coated THP-1 target cells. The result was repeated with cells isolated from 2 healthy donor samples with similar results.
CCR7loCXCR3hi CD8+ T cells display an effector phenotype. (A) PBMCs from healthy human blood were stained for surface receptors CD8, CD45RA, CXCR3, CCR7, and intracellular perforin and granzyme B. Four individual donors were used in replicate experiments, and representative plots from one experiment are shown. (B) Healthy human PBMCs were stained with antibodies specific for CD8, CCR7, and CXCR3 and sorted into 3 separate CD8+ populations as indicated in the figure. Redirected lysis assay was carried out at the indicated E:T ratios using anti–human anti-CD3–coated THP-1 target cells. The result was repeated with cells isolated from 2 healthy donor samples with similar results.
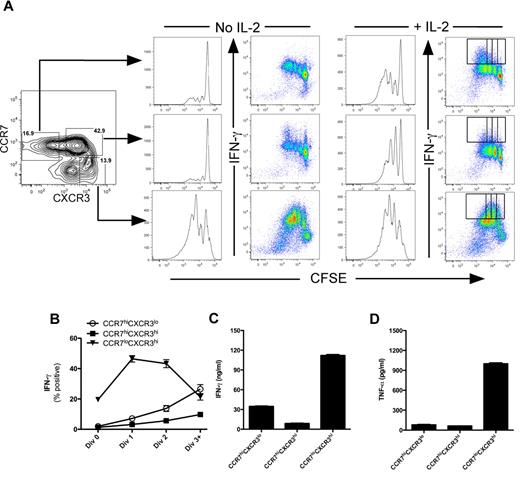
We further assessed the function of these purified cell populations in response to TCR activation by determining whether CCR7loCXCR3hi cells were capable of proliferation and proinflammatory cytokine expression. Cells were purified from peripheral blood as described in Figure 3 and assessed for proliferation and effector function as the cells divided in response to TCR activation. In the absence of exogenous IL-2, only the CCR7loCXCR3hi cells were capable of rapid proliferation and acquisition of IFN-γ expression (Figure 3A bottom panels). However, addition of IL-2 was sufficient to promote cell division within both the CCR7hiCXCR3lo and CCR7hiCXCR3hi populations (Figure 3A right panel). Regardless of their proliferative capacity, IFN-γ was expressed predominantly by cells within the CCR7loCXCR3hi population (Figure 3B). In response to anti-CD3 stimulation, IFN-γ and TNF-α secretion correlated well with the selective cytokine expression pattern within the CCR7loCXCR3hi population (Figure 3C-D). Taken together, these data demonstrate that the circulating TEM (CCR7loCXCR3hi) population within human peripheral blood possesses immediate effector capabilities (Figures 2–3), and their functions and phenotype are paralleled by cells that differentiated in response to IL-12 in vitro (Figure 1).18
CCR7loCXCR3hi effector memory CD8+ T cells secrete effector cytokines. CCR7hiCXCR3lo, CCR7hiCXCR3hi, and CCR7loCXCR3hi CD8+ T cells were sorted from healthy human PBMCs and labeled with CFSE. The cells were stimulated with plate-bound anti-CD3 for 3 days in the absence or presence of 200 U/mL of rhIL-2. The cells were restimulated with phorbol myristate acetate and ionomycin in the presence of brefeldin A for 4 hours on day 3 after stimulation. Proliferation was measured by CFSE dilution, and IFN-γ was measured by intracellular staining. (B) The percentage of cells positive for IFN-γ expression as a function of cell division are plotted as mean ± SEM for the cells receiving exogenous IL-2 as indicated by the gates in panel A (far right dot plots). IFN-γ (C) and TNF-α (D) were measured in the supernatants by ELISA.
CCR7loCXCR3hi effector memory CD8+ T cells secrete effector cytokines. CCR7hiCXCR3lo, CCR7hiCXCR3hi, and CCR7loCXCR3hi CD8+ T cells were sorted from healthy human PBMCs and labeled with CFSE. The cells were stimulated with plate-bound anti-CD3 for 3 days in the absence or presence of 200 U/mL of rhIL-2. The cells were restimulated with phorbol myristate acetate and ionomycin in the presence of brefeldin A for 4 hours on day 3 after stimulation. Proliferation was measured by CFSE dilution, and IFN-γ was measured by intracellular staining. (B) The percentage of cells positive for IFN-γ expression as a function of cell division are plotted as mean ± SEM for the cells receiving exogenous IL-2 as indicated by the gates in panel A (far right dot plots). IFN-γ (C) and TNF-α (D) were measured in the supernatants by ELISA.
IL-12 selectively regulates an effector CTL gene expression signature
CTL effector cell differentiation is dynamic, being regulated by multiple signals that are integrated as the cells divide in response to antigen stimulation. If stable populations of TEM cells retain some of the original transcriptional programs that regulated their development, then comparing their gene expression signatures to cells acutely activated by IL-12 in vitro could reveal these pathways. As a first approach, we examined changes in gene expression profiles as cells differentiated in response to IL-12, IFN-α, or IL-12 + IFN-α. CD8+CD45RA+ T cells were isolated from peripheral blood of 5 healthy human subjects, labeled with CFSE, and stimulated in vitro under defined cytokine conditions for 3.5 days. CFSEhi (undivided) and CFSElo (divided) were purified by cell sorting as described in Figure 1 followed by RNA isolation and microarray analysis.
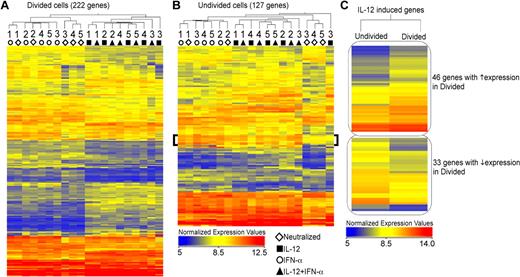
We first performed an unsupervised 2-way hierarchical cluster analysis of genes that were significantly altered by 1.7-fold compared with the neutralized control, and the normalized intensity values of these genes are displayed in the heat-maps (Figure 4). As shown in Figure 4A (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), cells that divided in the presence of IL-12 displayed a unique gene expression signature that was distinct from that of IFN-α, and this pattern was conserved among the 5 donors. Further, this signature of IL-12–regulated genes was observed in both the absence and presence of IFN-α. Although the differences in gene expression profiles were less pronounced within the undivided population, we identified a cluster of genes that were distinctly regulated by IFN-α (Figure 4B bracket). The differential regulation of IL-12–induced genes was further pronounced comparing fold-change values of sets of genes regulated by IL-12 within the undivided versus divided populations (Figure 4C; supplemental Table 2). For each gene that was regulated by IL-12, the magnitude of induction was greater in the divided population compared with the undivided cells. Thus, the differential induction of IL-12–regulated genes within the actively dividing population correlated precisely with increased sensitivity to IL-12 and acquisition of effector function observed in Figure 1 and in previous studies.18
IL-12 and IFN-α regulate expression of unique gene sets in human CD8+ T cells. (A-B) Negatively isolated CD8+CD45RA+ T cells were polarized in vitro for 3 days under the defined conditions as indicated: neutralized (◊), IL-12 (■), IFN-α (○), and IL-12 + IFN-α (▴) and CFSElo (shown in orange in Figure 1A) and CFSEhi (shown in teal in Figure 1A) cells were sorted for RNA extraction and microarray analysis. A statistically significant gene list (P ≤ .01) was obtained using normalized expression values of the 5 donors, and ANOVA Unequal Variance (Welch) was used as the statistical test. Cytokine-stimulated samples were compared with the neutralized condition to obtain a list of genes with at least 1.7-fold difference. Hierarchical clustering of both conditions and entities (genes) is represented in the heat-maps of normalized expression values, both within divided (A) and undivided (B) populations. Individual donors are indicated as numbers under the dendrogram. The bracket in panel B indicates a cluster of IFN-α–stimulated genes. (C) Genes regulated by IL-12 (compared with neutralized) were hierarchically clustered based on their expression pattern within the divided and undivided populations.
IL-12 and IFN-α regulate expression of unique gene sets in human CD8+ T cells. (A-B) Negatively isolated CD8+CD45RA+ T cells were polarized in vitro for 3 days under the defined conditions as indicated: neutralized (◊), IL-12 (■), IFN-α (○), and IL-12 + IFN-α (▴) and CFSElo (shown in orange in Figure 1A) and CFSEhi (shown in teal in Figure 1A) cells were sorted for RNA extraction and microarray analysis. A statistically significant gene list (P ≤ .01) was obtained using normalized expression values of the 5 donors, and ANOVA Unequal Variance (Welch) was used as the statistical test. Cytokine-stimulated samples were compared with the neutralized condition to obtain a list of genes with at least 1.7-fold difference. Hierarchical clustering of both conditions and entities (genes) is represented in the heat-maps of normalized expression values, both within divided (A) and undivided (B) populations. Individual donors are indicated as numbers under the dendrogram. The bracket in panel B indicates a cluster of IFN-α–stimulated genes. (C) Genes regulated by IL-12 (compared with neutralized) were hierarchically clustered based on their expression pattern within the divided and undivided populations.
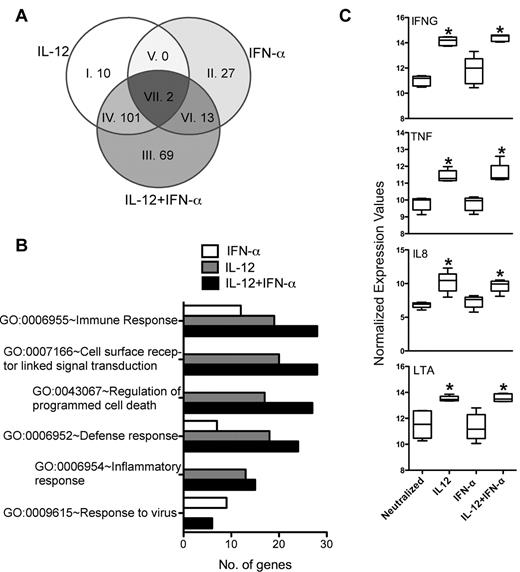
In mice, both IL-12 and IFN-α can promote effector CTL development, albeit IL-12 being more potent in this activity than IFN-α.16 Agarwal et al have further demonstrated that these cytokine signaling pathways regulate an overlapping spectrum of effector response genes, such as IFN-γ and granzyme B, suggesting some level of redundancy between these signals in mice.24 Although IL-12 was more potent, we also found that both IL-12 and IFN-α induced IFN-γ secretion in murine CD8+ OT-I cells, and the effect of IFN-α was abolished in IFN-α/β receptor-deficient cells (supplemental Figure 1). In contrast, we found very little effect of IFN-α in regulating any aspect of human CTL effector function above the level displayed by cells activated under neutralizing conditions (Figure 1),18 suggesting that IL-12 and IFN-α play nonredundant roles in regulating CTL effector functions. To distinguish genes that were either regulated distinctly or commonly by IL-12 and IFN-α, we compared the gene lists by Venn analysis. The differences in activation by IL-12 and IFN-α were reflected by the unique spectrum of genes that were differentially regulated within the dividing population. Of the 222 genes that were assessed in Figure 4A, we found that only 2 genes (CXCl10 and FASL) were commonly regulated by both IL-12 and IFN-α (Figure 5A Venn segment VII, and in supplemental Table 1). In contrast, 101 genes were regulated exclusively by IL-12 regardless of the presence of IFN-α (Figure 5A Venn segment IV). These genes were further analyzed with the “DAVID” bioinformatics resource by assessing biological meaning of this gene list based on gene ontologies.20,21,25 The major ontology terms that explained the gene pool included the following ontology definitions: immune response, cell surface receptor-linked signal transduction, regulation of programmed cell death, defense response, inflammatory response, and response to virus (Figure 5B). These ontology terms included genes that were selectively induced by IL-12 in the dividing population, such as IFNG, TNF, IL8, and LTA (lymphotoxin-α; Figure 5C). Conversely, genes regulated exclusively by IFN-α were included only within the immune response, defense response, and response to virus categories, which included the canonical interferon-sensitive genes IRF7, OASL, MX1, and ISG15 (supplemental Table 1). The robust induction of these genes within the IFN-α–treated population underscores their selective responsiveness to IFN-α in this assay. Thus, the patterns of gene expression and the functional annotation of those genes highlight the unique pathways that are distinctly regulated by IL-12 and IFN-α in human CD8+ T cells. Overall, we found that IL-12, but not IFN-α, selectively programmed effector functions within actively dividing CD8+ T cells, and these functions corresponded to the known biologic activities of the genes that were selectively regulated by IL-12.
IL-12, but not IFN-α, regulates effector gene expression in human CD8+ T cells. (A) Differentially regulated genes by IL-12, IFN-α, and IL-12 + IFN-α were analyzed by Venn diagram to identify any genes commonly regulated by the different cytokine conditions. Individual segments are numbered corresponding to the gene list in supplemental Table 1. (B) Genes up-regulated by at least 1.7-fold were used to determine the Gene Ontology (GO) of the biological processes. The number of genes within a specific GO term is used as an indication of the magnitude of their functional correlation. (C) Normalized expression values of selected genes representing effector molecules are plotted as box-whisker bars (whiskers represent range).
IL-12, but not IFN-α, regulates effector gene expression in human CD8+ T cells. (A) Differentially regulated genes by IL-12, IFN-α, and IL-12 + IFN-α were analyzed by Venn diagram to identify any genes commonly regulated by the different cytokine conditions. Individual segments are numbered corresponding to the gene list in supplemental Table 1. (B) Genes up-regulated by at least 1.7-fold were used to determine the Gene Ontology (GO) of the biological processes. The number of genes within a specific GO term is used as an indication of the magnitude of their functional correlation. (C) Normalized expression values of selected genes representing effector molecules are plotted as box-whisker bars (whiskers represent range).
Gene expression profiles of effector and naive/central memory CD8+ T cells in peripheral blood
As demonstrated in Figures 2 and 3, circulating CD8+ T cells within the CCR7loCXCR3hi subpopulation were capable of robust effector response on TCR stimulation. Based on our functional analysis, we predicted that these TEM cells would stably express a subset of genes whose functions would be predictive of the effector phenotype of this population. We addressed this by comparing the gene expression profiles of CCR7hiCXCR3lo and CCR7loCXCR3hi cells purified directly from peripheral blood of 4 healthy adults by cell sorting. The CCR7hiCXCR3lo potentially contain mixed populations of both naive and central memory cells yet are functionally similar given their relatively low levels of cytokine secretion shown in Figure 3C and D. We generated a list of significantly regulated genes with differential expression of > 1.7-fold by performing a pair-wise t test (P < .01) between the 2 cell subsets from each of the 4 donor samples. A 2-way unsupervised hierarchical clustering of these genes revealed a clear segregation of gene expression patterns that differed between the CCR7hiCXCR3lo and CCR7loCXCR3hi populations, and the normalized expression values of these genes are depicted in the heat-map in Figure 6A and listed in supplemental Table 3. Small differences in the absolute expression values for each gene were detected among the 4 donors, yet these differences did not significantly impact the hierarchy of the individual clusters of genes differentially expressed by the 2 subpopulations (Figure 6A).
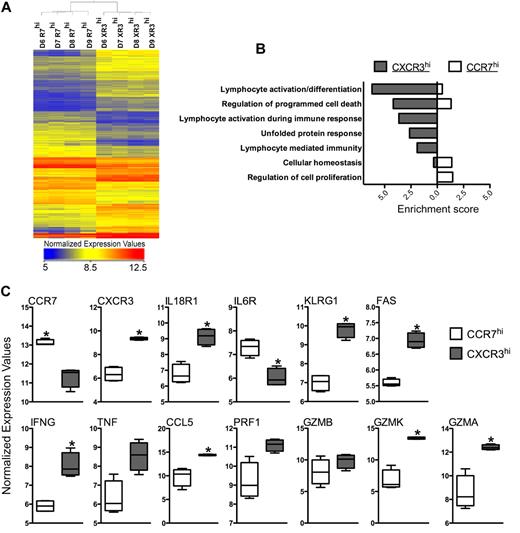
CCR7loCXCR3hi sorted from healthy human peripheral blood show effector/effector memory gene expression pattern. (A) The heat-map represents the normalized expression values of CD8+CCR7hiCXCR3lo (R7hi) and CD8+CCR7loCXCR3hi (XR3hi) cells isolated from 4 individual healthy human donors (D6-D9) by FACS sorting. A statistically significant (P ≤ .01) gene list was obtained from the 2 subsets using a pair-wise t test. A list of differentially regulated genes (≥ 1.7-fold) in 4 donors was generated, and hierarchical clustering of both cell types and entities was performed. (B) Genes with higher expression values in CXCR3hi or CCR7hi cells (compared with each other) were used to determine Functional Annotation Clustering of Biological Processes using DAVID. Enrichment score of the cluster is used as an indication for how strongly groups of differentially expressed genes are involved within each functional cluster. (C) Normalized expression values of selected genes are plotted as box-whisker bars (whiskers represent range).
CCR7loCXCR3hi sorted from healthy human peripheral blood show effector/effector memory gene expression pattern. (A) The heat-map represents the normalized expression values of CD8+CCR7hiCXCR3lo (R7hi) and CD8+CCR7loCXCR3hi (XR3hi) cells isolated from 4 individual healthy human donors (D6-D9) by FACS sorting. A statistically significant (P ≤ .01) gene list was obtained from the 2 subsets using a pair-wise t test. A list of differentially regulated genes (≥ 1.7-fold) in 4 donors was generated, and hierarchical clustering of both cell types and entities was performed. (B) Genes with higher expression values in CXCR3hi or CCR7hi cells (compared with each other) were used to determine Functional Annotation Clustering of Biological Processes using DAVID. Enrichment score of the cluster is used as an indication for how strongly groups of differentially expressed genes are involved within each functional cluster. (C) Normalized expression values of selected genes are plotted as box-whisker bars (whiskers represent range).
To further assess the potential biological processes of these genes, functional annotation clustering was performed within DAVID, which grouped related ontologies and ranked their overall importance by an enrichment score (Figure 6B). A high enrichment score (≥ 1.3) is indicative of a more important role played by a specific biological process within the gene set of interest. Specific ontologies describing the gene sets that were more highly expressed in CXCR3hi cells than in the CCR7hi population were clustered in groups representing lymphocyte activation and differentiation, programmed cell death, unfolded protein response, and effector response. In contrast, gene sets that were elevated in CCR7hi cells compared with CXCR3hi cells clustered uniquely in groups describing regulation of cellular metabolic processes and RNA processing. As predicted by their effector phenotype and reflected by the ontology analysis, we found that many of the genes known to mediate effector functions were more highly expressed in the CXCR3hi population, including IFN-γ, CCL5, and FasL (Figure 6C). In addition, granzymes A and K were elevated in the CXCR3hi cells, but, interestingly, perforin and granzyme B were not significantly different between the 2 populations (Figure 6C). Effector cell surface receptors, such as IL-18R1, KLRG1, and IL-2Rβ, were also significantly enriched within the CXCR3hi subset. Thus, the genes that have significantly higher expression in TEM CXCR3hi cells were found to be part of functional clusters relating to immune response.
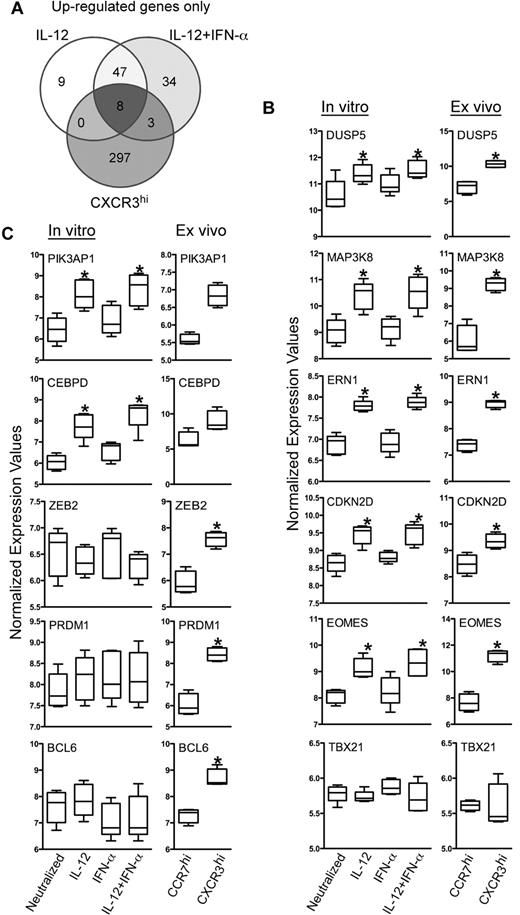
IL-12 drove the differentiation of acute effector cells that exhibited many of the functional attributes of circulating TEM cells in vivo. However, it was not clear what components of this acute signaling pathway were stably expressed during the memory cell transition. Given their common functional activities, we predicted that we would find similarities in gene expression patterns between cells activated by IL-12 in vitro and the CXCR3hi CD8+ TEM cells sorted from peripheral blood. Conversely, the differences in gene expression between these 2 cell types may reveal subsets of genes transiently expressed during acute stimulation, which may lead to secondary gene expression pathways in TEM cells persistent under homeostatic conditions in vivo. This analysis focused on the IL-12–induced genes that were selectively regulated in the dividing population described in Figure 4A. IL-12– and IL-12 + IFN-α–induced genes from dividing cells were compared with the set of genes that were more highly expressed in CXCR3hi cells purified directly from peripheral blood (supplemental Table 4). Of the 308 genes elevated within the CXCR3hi population in vivo, Venn analysis identified 8 of these genes that were regulated acutely by IL-12 in vitro, most notably IFN-γ and Eomes (Figure 7A Venn center segment; supplemental Table 4). However, the majority of the IL-12–induced genes expressed in acutely activated dividing cells were not differentially expressed in the CXCR3hi cells, indicating transience in the majority of the IL-12–regulated pathways or differences in activation status.
Effector memory CD8+ T cells isolated ex vivo and IL-12 programmed effector CD8+ T cells generated in vitro share a set of commonly regulated genes. Differentially expressed genes from the ex vivo samples were reassessed to identify genes expressed > 1.7-fold higher in all donors within the CXCR3hi compared with the CCR7hi cells. These genes were then assessed for commonality with the induced genes identified in cells activated with IL-12 and IL-12 + IFN-α culture conditions. (A) Genes up-regulated by either IL-12 or IL-12 + IFN-α and also increased in CD8+CCR7loCXCR3hi cells are shown in the Venn diagram. (B-C) Normalized expression values of selected genes are plotted as box-whisker bars (whiskers represent range).
Effector memory CD8+ T cells isolated ex vivo and IL-12 programmed effector CD8+ T cells generated in vitro share a set of commonly regulated genes. Differentially expressed genes from the ex vivo samples were reassessed to identify genes expressed > 1.7-fold higher in all donors within the CXCR3hi compared with the CCR7hi cells. These genes were then assessed for commonality with the induced genes identified in cells activated with IL-12 and IL-12 + IFN-α culture conditions. (A) Genes up-regulated by either IL-12 or IL-12 + IFN-α and also increased in CD8+CCR7loCXCR3hi cells are shown in the Venn diagram. (B-C) Normalized expression values of selected genes are plotted as box-whisker bars (whiskers represent range).
This Venn analysis enabled the identification of specific genes that represented 3 possible forms of differential regulation: (1) induced by IL-12 and elevated in CXCR3hi cells, (2) induced by IL-12 but not differentially expressed in CXCR3hi cells, and (3) no acute regulation by IL-12 but elevated in CXCR3hi cells. As would be expected based on the differences in the activation state of the cells, only a small subset of genes were induced by IL-12 and remained elevated in TEM compared with their TCM counterparts (Figure 7A center segment). By focusing on molecular regulators, such as signaling intermediates and transcription factors, we found that DUSP5, MAP3K8, ERN1, CDKN2D, and EOMES were acutely induced by IL-12 and selectively elevated within the CXCR3hi population (Figure 7A Venn center segment, B). Interestingly, the T-box family member TBX21 (T-bet), which is closely related to EOMES and involved in Th1 development, was neither regulated by IL-12 nor differentially expressed in TEM versus TCM cell in vivo (Figure 7B bottom panel). We confirmed this pattern of expression of EOMES and TBX21 in cells isolated from an independent donor by quantitative PCR (supplemental Figure 2). In contrast, PIK3AP1 and CEBPD were acutely induced by IL-12, and although marginally elevated in CXCR3hi cells, their normalized expression values were not significantly different from CCR7hi cells (Figure 7C). Finally, the transcriptional regulators ZEB2, PRDM1 (Blimp-1), and BCL6 were not significantly regulated by either IL-12 or IFN-α in vitro, yet they were significantly elevated in the CXCR3hi cells, suggesting an alternate mode of regulation (Figure 7C). Thus, this combinatorial analysis revealed specific transcriptional pathways regulated acutely by IL-12 that give rise to stable patterns of gene expression and are unique to the effector phenotype of TEM.
Discussion
Innate cytokines are critical signals that drive this differentiation process, with IL-12 and IFN-α/β playing centrals roles in the context of intracellular pathogen infections.5,19 Our previous studies defined unique roles for IL-12 and IFN-α/β in regulating effector and memory functions in both human CD4+ and CD8+ T cells.18,26 Although IL-12 was unique in its ability to regulate effector cell commitment, the downstream targets of IL-12 that mediate this activity have not been elucidated. In the current study, we used a hypothesis-driven approach to dissect IL-12–driven gene expression pathways governing human CD8+ T effector cell development. This analysis revealed both the acute transcriptional changes that occurred in response to IL-12 and the stable patterns of gene expression adopted by TEM cells in vivo.
The in vitro culture system used here allowed for the strict control of innate cytokine stimulation during priming as well as distinguishing dividing versus nondividing populations. As cells divided in response to TCR activation, IL-12 instructed a program of effector development in the daughter cells as assessed by increased lytic activity and cytokine secretion. The differential expression of effector genes within this population directly paralleled their effector function and underscored the unique effects exerted by IL-12, but not IFN-α. Although many genes were induced by IL-12 in the undivided population, the magnitude of induction of those genes was greater as the cells divided, suggesting greater sensitivity to IL-12.18 The differences in gene expression signatures regulated by IL-12 versus IFN-α were striking. Regardless of whether the cells were dividing or not, > 10% of the genes altered by IL-12 were commonly regulated by IFN-α. Importantly, the main effector cytokine genes IFNG, TNF, IL8, and LTA were selectively induced by IL-12, but not by IFN-α. This result is in contrast to observations in mice where both IFN-α and IL-12 share some common effects by promoting effector cell development.15,16 Thus, these data suggest that, unlike mice, IL-12 is unique in its ability to program effector cell development in human CD8+ T cells and further supports the conclusion that IL-12 and IFN-α are not redundant signals.
Acute effector cells die en masse after the resolution of an infection, yet small pools of antigen-experienced TEM cells escape this attrition and retain their effector phenotype. These endogenous TEM displayed lytic activity and secreted inflammatory cytokines and paralleled cells that were activated with IL-12 in vitro. Although the TEM that we identified ex vivo potentially contained various additional populations of cells that differed in their effector function, we used these cells to determine whether any subsets of genes that were acutely regulated by IL-12 in vitro were stably expressed in TEM in vivo. First, compared with the CCR7hi subpopulation, the gene signatures in TEM isolated ex vivo revealed a strong bias for specific ontology terms describing lymphocyte activation, immune response, and other biological processes consistent with their effector phenotype. Previous studies have examined the functional abilities and gene expression profiles of several subsets of CD8+ TEM cells, and our analysis is in agreement with those defined as effector memory cells.27-30 As expected based on the purification scheme, the TEM population expressed higher levels of CXCR3 along with selective expression of KLRG1 and IL18R1. Further, even though these cells were not overtly activated, the steady-state levels of IFNG, TNF, and CCL5 were elevated, further indicating effector potential. Although we were able to detect higher intracellular protein levels of perforin and granzyme B in CCR7loCXCR3hi CD8+ T cells, only granzymes A and K displayed statistically significant differential expression (Figure 6C). This result is in agreement with previous studies that have shown that, although resting memory CD8+ T cells have open chromatin sites at Prf1 and Gzmb, relatively low levels of active transcription occur at these genes without antigen stimulation.31 Overall, the TEM isolated from peripheral blood express gene signatures reflective of their unique effector function that we demonstrated to be acutely regulated in vitro by IL-12.
In response to TCR activation, both the dividing and nondividing cells responded to IL-12, as evidenced by the differential regulation of both induced and repressed genes. We focused on the IL-12–induced genes within the dividing population to identify specific subsets of genes expressed at higher levels in the CCR7loCXCR3hi subset isolated ex vivo. We identified genes with the intrinsic potential to regulate fate commitment. Within this set of genes, we identified 5 molecular regulators (signaling intermediates and transcription factors) that were both induced by IL-12 and more highly expressed in the TEM population. Not surprisingly given its importance in regulating effector CTLs in mice,32,33 Eomes was included in this group, and this is the first demonstration that Eomes is an IL-12–induced gene in human CD8+ T cells. In contrast, the related T-box family member T-bet was not differentially regulated by IL-12 or IFN-α, nor was it differentially expressed within the CXCR3hi population in vivo. This observation supports previous studies suggesting a reciprocal relationship between Eomes and T-bet in regulating effector functions in CD8+ versus CD4+ T cells.32,34 However, in mice, IL-12 induces T-bet expression in CD8+ T cells,24,35 and the graded expression of T-bet drives terminal effector differentiation as cells respond to infection.36 By contrast, we found no differential regulation of T-bet by IL-12, nor were there any significant difference in its expression in human CD8+ T cells in vivo. Further, in human, both Eomes and T-bet are regulated by IL-12 in CD8+ and CD4+ T cells, respectively.18,26,37 As such, it is unclear how the IL-12 signaling pathway regulates only one of the 2 transcription factors in each cell type and whether species differences can account for this regulation.
In addition to Eomes, this analysis revealed 4 additional unique IL-12–induced intracellular regulators that were expressed in TEM in vivo. First, we identified both a dual-specificity phosphatase (DUSP5) and a MAP kinase (MAP3K8), both of which have been implicated in directly regulating effector T-cell activity.38-40 Given their known enzymatic activities, it is possible that enhanced expression of DUSP5 and MAP3K8 may create a molecular on/off switch to transmit signals during recall responses to antigen. In addition, ERN1, an endoplasmic reticulum protein that acts as an endoplasmic reticulum stress signaling molecule,41 was also induced by IL-12. Stable expression of ERN1 within the TEM might allow effectors to maintain viability during synthesis of large quantities of effector proteins, such as cytokines. Finally, the cyclin-dependent kinase inhibitor family member CDKN2D was expressed in TEM, and its activity may be involved in the differential ability of TEM versus TCM to divide in response to antigen stimulation in the absence of exogenous IL-2, as demonstrated in Figure 2A. Collectively, the induction of these 5 genes by IL-12 and their stable expression within TEM may represent a unique pathway that defines their phenotype and regulates their function.
Perhaps the most intriguing result of this analysis was the identification of 3 genes that were highly expressed within the TEM yet were not regulated by either IL-12 or IFN-α: ZEB2, PRDM1, and BCL6. All 3 of these factors have been demonstrated as transcriptional repressors. ZEB2 is often found to be mutated in Mowat-Wilson syndrome42 and regulates epithelial-to-mesenchymal transition during embryogenesis.43 Until now, expression of ZEB2 has not been described in T cells, although a recent study demonstrated a critical role for this factor during murine hematopoiesis.44 In contrast, BCL6 and PRDM1 (Blimp-1) have been shown to exert distinct activities during memory cell development that seem to balance the ratios of TEM to TCM.45 In murine CD8+ T cells, Blimp-1 and Bcl6 are reciprocally expressed in TEM and TCM,46,47 and elevated Blimp-1 levels drive terminal effector cell differentiation associated with an exhausted phenotype during persistent viral infections.48 In human CD8+ T cells, we found that both BCL6 and PRDM1 were elevated within the TEM compared with TCM, which seems to contradict the notion that the 2 factors stand in opposing negative regulation of each other. However, given the strong and selective regulation of effector function by IL-12, it was surprising to find that neither BCL6 nor PRDM1 was regulated by IL-12. Perhaps other signals, such at TCR activation or IL-2, promote induction of these genes at early time points, whereas IL-12 retains expression of these factors at later time points that were not assessed in this study. Alternatively, ZEB2, BCL6, and PRDM1 could be downstream targets of other acute IL-12–induced genes, such as EOMES, which could promote terminal differentiation of effectors during later points of activation or during secondary responses to antigen. Indeed, Wirth et al reported increased expression of both Eomes and Prdm1 in murine CD8+ T cells undergoing repeated rounds of antigen stimulation, suggesting a contribution by TCR stimulation to the stable induction of these genes.49
In conclusion, IL-12 is a primary innate cytokine that directly regulates effector cell development in human CD8+ T cells. Although some gene signatures are shared between IL-12 and IFN-α, IL-12 uniquely regulates a subset of genes that are either direct effector molecules, such as IFN-γ, or are regulators of their expression. Future studies will focus on the coordinated action of this subset of regulatory proteins and determine specific downstream targets during infection and vaccination.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank University of Texas Southwestern Flow Cytometry Facility for cell-sorting assistance; the University of Texas Southwestern Microarray Core facility for performing the microarray hybridization and scanning; Megan Kong and Namir Chowdhury for help with data analysis; and Dr Michelle Gill, Sarah Gonzales, and Jonathan Huber for helpful discussions and critically reading the manuscript.
This work was supported by National Institutes of Health grants (AI005284, F.Z.C.; AI068622, H.J.R.; AI45764, J.F.; and AI56222, J.D.F.).
National Institutes of Health
Authorship
Contribution: F.Z.C. designed and performed research, analyzed data, and wrote the paper; H.J.R. designed and performed research; L.S.D. analyzed data; J.F. designed research; and J.D.F. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: J. David Farrar, Department of Immunology, University of Texas Southwestern Medical Center, 5323 Harry Hines Blvd, Dallas, TX 75390-9093; e-mail: david.farrar@utsouthwestern.edu.