Abstract
The aim of this study was to evaluate the nonchemotactic function of CCL18 on human dendritic cells (DCs). In different protocols of DC differentiation, CCL18 was highly produced, suggesting that it may constitute a mandatory mediator of the differentiation process. Differentiation of monocytes from healthy subjects in the presence of granulocyte-macrophage colony-stimulating factor and CCL18 led to the development of DCs with a semimature phenotype, with intermediate levels of costimulatory and MHC class II molecules, increased CCR7 expression, which induced, in coculture with allogenic naive T cells, an increase in IL-10 production. The generated T cells were able to suppress the proliferation of effector CD4+CD25− cells, through a cytokine-dependent mechanism, and exhibited characteristics of type 1 T regulatory cells. The generation of tolerogenic DCs by CCL18 was dependent on the production of indoleamine 2,3-dioxigenase through an interleukin-10-mediated mechanism. Surprisingly, when DCs originated from allergic patients, the tolerogenic effect of CCL18 was lost in relation with a decreased binding of CCL18 to its putative receptor. This study is the first to define a chemokine able to generate tolerogenic DCs. However, this function was absent in allergic donors and may participate to the decreased tolerance observed in allergic diseases.
Introduction
Chemokines are a large family of related molecules classified on the basis of structural properties, according to the number and position of conserved cysteine residues, with 2 major (CXC and CC) and 2 minor (C and CX3C) chemokine subfamilies. They act through 7-transmembrane domain G protein-coupled receptors on their target cells.1,2 These small and secreted proteins are mainly involved in leukocyte chemoattraction and cytokine-like activities, including regulation of angiogenesis, fibrosis, proliferation, and apoptosis,1 but also have other immune functions, including T-cell activation3 and differentiation.4 Some chemokines have been shown to directly regulate the ability of dendritic cells (DCs) to prime a Th1 cell response by modifying their maturation and/or cytokine production.5-7 DCs are key players in activation of the adaptive immune system by their capacity of antigen presentation to and priming of T cells. DCs serve as sentinels, recognizing antigens, and on maturation migrate to the draining lymph nodes to initiate the effector primary immune response. Moreover, an increasing body of evidence suggests that, besides their immunogenic role in inflammatory conditions, DCs may also play a role in induction of tolerance, in particular in steady-state conditions, predominantly by inducing regulatory T cells (Tregs).8
CCL18 is a chemokine expressed at high levels in lung and lymph nodes, mainly produced by antigen-presenting cells, such as monocytes, macrophages, and immature DCs.9,10 CCL18 was first identified as a chemotactic factor for naive CD4+ T cells11 and immature DCs,10 suggesting a main role in primary immune responses; however, it can also attract skin-homing memory T cells12 and Th2 cells and basophils,13 suggesting a role also in secondary immune responses. CCL18 is mainly induced by the Th2 type cytokines IL-4 and IL-13 and inhibited by IFN-γ.9 Accordingly, an overexpression of this chemokine has been identified in allergic diseases, such as asthma and atopic dermatitis.12,13 In contrast, CCL18 is able to inhibit CCR3-dependent chemoattraction of eosinophils by eotaxin14 and is induced by the suppressive cytokine IL-10, and we recently showed that it could generate adaptive regulatory T cells,15 suggesting that CCL18 may also play an antagonist role in allergic diseases. CCL18 is also dysregulated in a number of other diseases without preferential Th2 polarization, such as leukemia and Gaucher disease.16,17 It is of interest that the CCL18 gene is thought to be absent in rodents18 and is still an orphan chemokine without known receptor, precluding experimental studies to assess its functionality in vivo.
Because DCs appear as major targets and producers of CCL18, the purpose of this study was to assess the direct immune effect of CCL18 on human DC differentiation, maturation, and polarization of T-cell immune responses according to the atopic status of the donors. This study shows, for the first time, to our knowledge, that a chemokine can differentiate DCs in tolerogenic cells able to prime regulatory T cells. This effect of CCL18 was mediated through the induction of IL-10 and indoleamine 2,3-dioxigenase (IDO) by DCs obtained from healthy subjects and suggests that the constitutive expression of CCL18 in the lung may play a tolerogenic role in steady-state conditions. However, in allergic patients, this property was lost, in association with a decreased binding of CCL18 to its putative receptor.
Methods
Subjects
Venous blood was obtained from 33 healthy nonallergic (NA) subjects, with no history of allergic diseases, exhibiting total immunoglobulin E (IgE) levels < 100 kU/L and absence of allergen-specific IgE antibodies. Venous blood was also collected from 15 allergic (A) patients. These patients exhibited a history of allergic rhinitis and/or intermittent asthma, positive skin prick tests to common aeroallergens, positive allergen-specific IgE antibodies (> 3 kU/L), and elevated total IgE levels. None had received oral or inhaled corticosteroids within 2 months before sample collection. Patients were under β2-agonists as needed. The study was approved by the Comité consultatif de protection des personnes dans la recherche biomédicale de Lille (CP 04/45). All donors signed an informed consent form in accordance with the Declaration of Helsinki.
Generation of DCs
Peripheral blood mononuclear cells (PBMCs) were isolated via density gradient centrifugation using Ficoll-Hypaque (GE Healthcare). CD14+ monocytes were isolated by magnetic column purification on the basis of positive selection with anti-CD14 microbeads (Miltenyi Biotec) with a purity of 96% and used to generate DCs by culturing 1 × 106 CD14+ cells in RPMI 1640 medium with 2 mmol/mL glutamine, 100 μg/mL ticarpen, and 10% FCS (complete RPMI) supplemented with various cytokine cocktails for 6 days according to protocols previously described in the literature.19-23 In 5 of these protocols, granulocyte-macrophage colony-stimulating factor (GM-CSF) at 25 ng/mL was associated with one of the following: cytokine, IL-4 (10 ng/mL), IL-13, TGF-β1, IL-15, IFN-α, and IL-3, all at 5 ng/mL. In the last one, IFN-α was associated with IL-3, both at 5 ng/mL. In some experiments, neutralizing anti-CCL18 antibody (R&D Systems) was added to the culture at day 0, 2, and 4 at a concentration of 10 μg/mL. Blocking activity of the CCL18 antibody was evaluated by its ability to inhibit CCL18-induced naive T-cell chemotaxis, which was ∼ 45% (data not shown). Three protocols were thereafter routinely used, one as negative control with GM-CSF alone at 25 ng/mL (GMDCs), the classic protocol of DC generation with GM-CSF and IL-4 (GM/IL-4DCs), and one consisting of the addition of 10−7M CCL18 to GM-CSF (GM/CCL18DCs; R&D Systems). Preliminary dose-response experiments showed that the optimal CCL18 concentration was 10−7M, although an effect was also observed at 10−8M (data not shown). In other experiments, 10 μg/mL neutralizing anti–IL-10 (R&D Systems) or 10 μg/mL isotype antibody was added to the cultures.
DCs were then matured for 48 hours with 10 ng/mL IL-1β and 20 ng/mL TNF-α (R&D Systems). Supernatants were recovered at different time points of the cultures and stored at −20°C for further evaluation. Cells were recovered 48 hours after maturation and assessed by flow cytometry for cell surface marker expression and used for T-cell coculture or proliferation experiments.
Isolation of CD4+CD45RA+ naive T cells and DC/T-cell cocultures
Allogenic CD4+CD45RA+ naive T cells were obtained from PBMCs exclusively of healthy donors by negative selection using naive CD4+ T Cell Isolation Kit II (Miltenyi Biotec). Mature DCs were cocultured with naive T cells at a 1:10 ratio at 106 cells/mL in complete RPMI for 5 days. Supernatants were recovered and frozen at −20°C until use.
Flow cytometry sorting of T-cell subsets
CD4+ T cells were purified from PBMCs by magnetic separation (Miltenyi Biotec). Purified CD4+ T cells were stained with anti-CD4 FITC (BD Biosciences PharMingen) and anti-CD25–phycoerythrin (PE; BD Biosciences PharMingen), for 30 minutes on ice, washed in PBS, 2% FCS, and sorted by flow cytometry (FACSAria, BD Biosciences). CD4+CD25−, CD4+CD25high T-cell purity was between 96% and 98%.
Assessment of the cytokine profile
Concentrations of CCL18, IL-10, IL-4, IFN-γ, IL-23, IL-6, and TGF-β1 (R&D Systems) and IDO (Uscn Life Science) in DCs or DC/T-cell coculture supernatants were measured by ELISA, according to the manufacturer's recommendations, and expressed in picograms per milliliter. The level of sensitivity was 1.1 pg/mL for IL-4, 12.5 pg/mL for IL-10, IL-23, IL-6, CCL18, and IFN-γ, 15.6 pg/mL for TGF-β1, and 0.51 IU/mL for IDO. Results were expressed as mean ± SEM.
Phenotype analysis
DC phenotype was evaluated by flow cytometry using a standardized protocol as previously described.24 Cells were kept on ice during all the procedures. For the extracellular markers, cells were stained with anti–HLA-DR–FITC, anti-CD80–PE, anti-CD86–allophycocyanin, anti-CCR7–PE, CD1a-FITC, anti-CD14–FITC, anti-CD16–FITC, anti-CD11c–allophycocyanin, anti-CD206–PE, anti-CD40–FITC, anti-ICOS–L-PE, anti-PD–L1-PE, and anti-PDL2–allophycocyanin (BD Biosciences PharMingen). For the intracellular markers, cells were labeled using anti–human Foxp3-FITC staining set (eBioscience) and anti–IL-10 antibody (BD Biosciences PharMingen) according to the manufacturer's recommendations. For CCL18 receptor expression, cells were labeled using biotinylated CCL18 (Sigma-Aldrich) followed with streptavidin-PE (R&D systems). The specificity of the biotinylated CCL18 was shown by inhibition with nonlabeled CCL18 (data not shown).
As a control, we used a small molecular weight protein derived from Plasmodium falciparum, as previously described.15
Suppression assays
A fixed number of purified flow cytometry sorted CD4+CD25− T cells (5 × 104/well) were stimulated in triplicate wells with soluble anti-CD3 monoclonal antibody (10 ng/mL) and anti-CD28 monoclonal antibody (10 ng/mL; BD Biosciences PharMingen) in 96-well round-bottom plates (Corning Costar), in the absence or presence of an increasing number of T lymphocytes primed with GMDCs, GM/CCL18DCs, or GM/IL-4DCs at suppressor/responder ratios of 1:4, 1:2, and 1:1, respectively. To get rid of the remaining DCs in the cocultures, T cells were purified by magnetic bead selection using the anti-CD3 Miltenyi kit, with a purity of 95%-98%. Irradiated (5000 cGy) autologous PBMCs were used as antigen-presenting cells (5 × 104/well).). Sorted CD4+CD25+ and CD4+CD25− T cells were used as positive and negative controls of T-cell suppression, respectively. Proliferation was measured after [methyl-3H]-thymidine (1 μCi/well; GE Healthcare) incubation for the last 18 hours. Cultured cells were harvested on glass fiber filter (Printed Filtermat A) using the harvester (Tomtec, Wallac) and sealed in a sample bag after drying and the addition of scintillation liquid (Beckman Coulter). Incorporated radioactive thymidine was detected by scintillation counting using a 1450 Trilux β-counter (Wallac) and estimated in counts per minute. Results were expressed as mean ± SEM (counts per minute). In some experiments, 10 μg/mL neutralizing anti–IL-10 (R&D Systems), 10 μg/mL anti-TGFβ1 (R&D Systems), or 10 μg/mL isotype antibody was added to the cultures. Blocking activity of IL-10 antibody was evaluated by the manufacturer by neutralization of IL-10–induced proliferation of the MC/9-2 mouse mast cell line, and for TGF-β1 antibody by inhibition of IL-4–dependent proliferation of the HT-2 mouse T-cell line.
Statistical analysis
Statistical analysis for production of cytokines was first performed within the subgroups using the analysis of variance test and, when significant, followed by the post-Dunnett Multiple Comparison test. The paired t test was performed for phenotype analysis and suppression assays. P values < .05 were regarded as statistically significant. Statistical analysis was performed using GraphPad Prism Version 4.0 software.
Results
CCL18 production increases during DC differentiation and is higher in DCs from allergic than healthy subjects
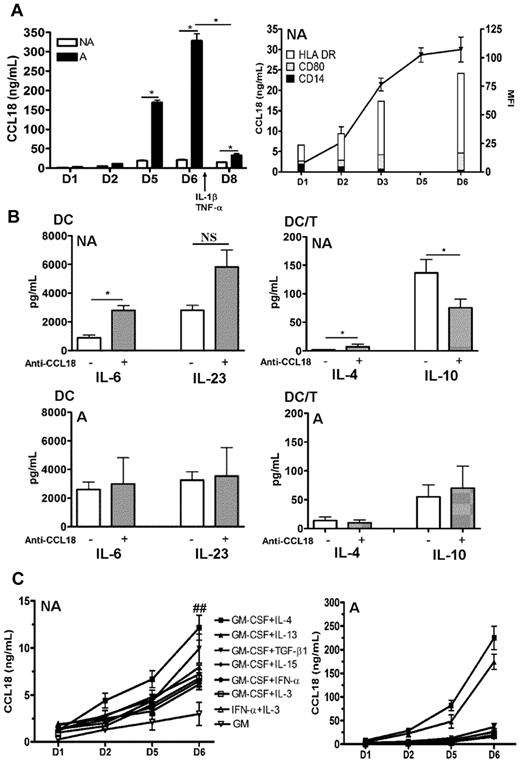
CCL18 has been shown to be produced by immature DCs at high levels in standard protocols of differentiation associating GM-CSF and Th2 cytokines.10 We first examined the kinetics of production of CCL18 in GM plus IL-4 differentiated DCs in nonallergic versus allergic subjects. As shown in Figure 1A, DCs from allergic donors produced more CCL18 than healthy subjects in both their immature and mature stages. The increase in CCL18 production also occurred when the cells were washed every 2 days, showing that it was not related to time accumulation (data not shown). Intrinsic CCL18 production correlated with the appearance of the DC differentiation markers HLA-DR and CD80 and the loss of the monocyte marker CD14 (Figure 1A). To evaluate the effect of CCL18 on DC maturation, and because immature DCs produce CCL18, which may have an autocrine effect, we used a neutralizing antibody against CCL18, during differentiation of DCs obtained from healthy subjects by the conventional IL-4 plus GM-CSF protocol. Despite high doses and repetitive administration of the antibody, the production of CCL18 by GM/IL-4DCs was only neutralized by 45% as assessed by ELISA (data not shown). The partial neutralization of CCL18 by a blocking antibody did not modify the DC surface phenotype (data not shown), increased the DC production of proinflammatory cytokines IL-6, but not of IL-23 and IL-10, and led in DC/T-cell cocultures to a decrease in IL-10 and an increase in IL-4 productions in nonallergic donors, whereas no modifications were observed in allergic donors (Figure 1B). We therefore decided to evaluate other protocols of differentiation described in the literature to determine a protocol that would not induce the production of CCL18. Surprisingly, all protocols tested generated the production of CCL18 at high levels, even those not using CCL18-inducing Th2 cytokines (Figure 1C). When monocytes were differentiated with GM-CSF alone, a very small production of CCL18 was observed (Figure 1C). Furthermore, when the atopic status was taken into account, DCs from allergic patients secreted more CCL18 than nonallergic subjects in all DC differentiation protocols but especially in those using Th2 cytokines (10-fold increase for IL-13 and IL-4, respectively, and 5-fold for the others; Figure 1C). The constant secretion of CCL18 in all protocols let us suppose that this chemokine may be an obligatory mediator involved in DC differentiation. To test this hypothesis, we evaluated a protocol combining CCL18 and GM-CSF (GM/CCL18DCs) and compared it with GM-CSF alone as negative control (GMDCs) and the standard GM-CSF plus IL-4 as positive control (GM/IL-4DCs). It is of note that monocytes treated without growth factor or with CCL18 alone died very rapidly after a few days of culture.
Kinetics of CCL18 production by differentiated DCs and effect of CCL18 neutralization. Monocytes were purified from nonallergic (NA) or allergic (A) subjects and differentiated for 6 days with different cytokines. (A) GM-CSF plus IL-4–differentiated DCs were analyzed for CCL18 production by ELISA at day 1, 2, 5, and 6 of differentiation and at day 8 after maturation. Evolution of CCL18 production (black line) and flow cytometry DC phenotype (histograms) expressed as mean fluorescence intensity (MFI) for the indicated markers during differentiation. (B) GM-CSF plus IL-4 differentiated DCs from NA or A donors were treated or not with a neutralizing anti-CCL18 antibody, and cytokine production was quantified by ELISA 48 hours after DC maturation and 5 days after coculture with allogenic naive T cells (DC/T). (C) DCs differentiated for 6 days with the indicated combinations of cytokines were evaluated for CCL18 production by ELISA at different time points of differentiation. Results are expressed as mean ± SEM (ng/mL) for CCL18 and mean ± SEM (pg/mL) for the other cytokines. n = 3-6 per condition for panel A, n = 8 NA and 5 A for panel B, and n = 10 or 11 for panel C. *P < .05, NA versus A. ##P < .01, NA versus A. NS indicates not significant.
Kinetics of CCL18 production by differentiated DCs and effect of CCL18 neutralization. Monocytes were purified from nonallergic (NA) or allergic (A) subjects and differentiated for 6 days with different cytokines. (A) GM-CSF plus IL-4–differentiated DCs were analyzed for CCL18 production by ELISA at day 1, 2, 5, and 6 of differentiation and at day 8 after maturation. Evolution of CCL18 production (black line) and flow cytometry DC phenotype (histograms) expressed as mean fluorescence intensity (MFI) for the indicated markers during differentiation. (B) GM-CSF plus IL-4 differentiated DCs from NA or A donors were treated or not with a neutralizing anti-CCL18 antibody, and cytokine production was quantified by ELISA 48 hours after DC maturation and 5 days after coculture with allogenic naive T cells (DC/T). (C) DCs differentiated for 6 days with the indicated combinations of cytokines were evaluated for CCL18 production by ELISA at different time points of differentiation. Results are expressed as mean ± SEM (ng/mL) for CCL18 and mean ± SEM (pg/mL) for the other cytokines. n = 3-6 per condition for panel A, n = 8 NA and 5 A for panel B, and n = 10 or 11 for panel C. *P < .05, NA versus A. ##P < .01, NA versus A. NS indicates not significant.
GM/CCL18DCs from healthy subjects have characteristics of semimature DCs and produce IL-10
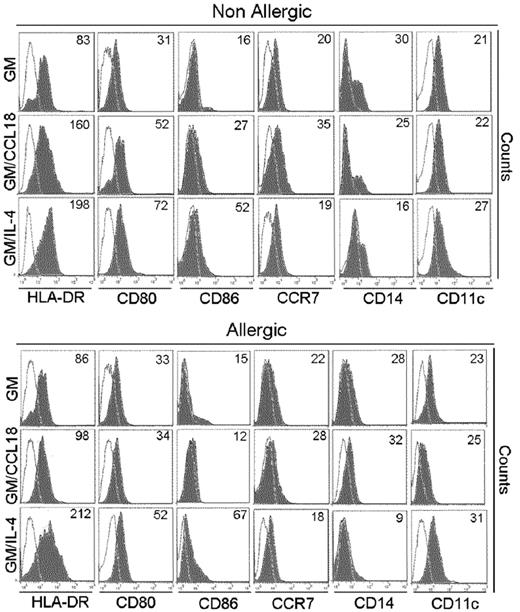
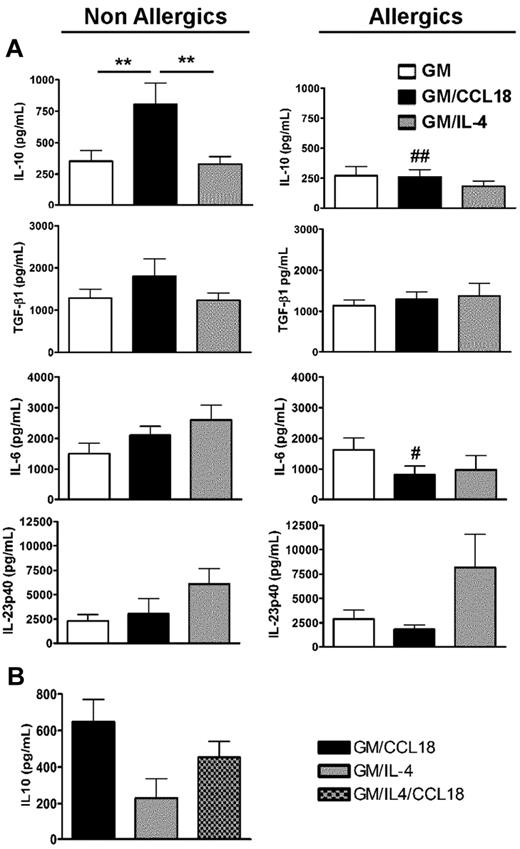
GM/CCL18DCs were generated from monocytes obtained from healthy subjects. The microscopic morphology of the generated DCs was assessed. After maturation, GM/IL-4DCs exhibited typical DC morphology, with large cell bodies and sharp cytoplasmic projections, did not adhere to plastic and promoted few cluster formations. GM/CCL18DCs were bigger with spread cytoplasmic projections and more adherent than GM/IL-4DCs, and aggregated in clusters. GM-CSFDCs were also big and granular but with fewer cytoplasmic projections than GM/IL-4DCs (data not shown). The cell surface phenotype was then evaluated by flow cytometry. Examples of representative histograms are shown in Figure 2. GM/IL-4DCs exhibited a characteristic phenotype of mature DCs with up-regulated expression of the costimulatory molecules HLA-DR, CD80, CD86, ICOS-L, PD-L1, and down-regulated expression of the monocyte/macrophage markers CD14/CD16 and of the mannose receptor CD206 compared with GMDCs (Table 1). In contrast, GM/CCL18DCs expressed CD14, CD16, and CD206 levels similar to GMDCs but exhibited intermediate levels of the DC maturation markers HLA-DR, CD80, and CD86, in between GMDCs and GM/IL-4DCs. Interestingly, GM/CCL18DCs also showed elevated expression of the lymph node homing receptor CCR7 compared with both GM/IL-4DCs and GMDCs (Table 1). Surprisingly, when GM/CCL18DCs were generated from allergic subjects, they exhibited characteristics similar to the negative control GMDCs (Table 1; Figure 2). To further assess the differences between the generated DCs, cytokines were assayed in the mature DC supernatants. IL-10 and TGF-β were assessed as anti-inflammatory cytokines and IL-12, IL-6, and IL-23 as proinflammatory cytokines. GM/CCL18DCs produced enhanced amounts of IL-10, compared with GM/IL-4DCs and GMDCs (Figure 3A). The other regulatory cytokine TGF-β was also slightly increased, but the difference did not reach statistical significance. All the other cytokines were not significantly modified (Figure 3A) or were absent, such as for IL-12 (data not shown). Similarly to the observations for the cell surface phenotype, GM/CCL18DCs obtained from allergic donors did not show differences with GMDCs (Figure 3A) and exhibited less IL-10 and IL-6 production than GM/CCL18DCs obtained from nonallergic subjects. The effect of addition of exogenous CCL18 to GM/IL-4DCs from nonallergic subjects was evaluated on IL-10 production. As shown in Figure 3B, IL-10 production was 2-fold higher than for GM/IL-4DCs but one-third lower than for GM/CCL18DCs. Altogether, these data suggest that in healthy subjects CCL18 induces IL-10–producing DCs, described to be tolerogenic, whereas in allergic patients these DCs seemed unable to respond to CCL18.
Phenotypic characteristics of mature differentiated DCs. HLA-DR, CD86, CD80, CD14, and CCR7 expression on mature GMDCs, GM/CCL18DCs, and GM/IL-4DCs from a nonallergic and allergic subject. One representative flow cytometry experiment of 8 is shown. Values in the quadrant indicate the mean fluorescence intensity after subtraction of the isotype control (unfilled black line).
Phenotypic characteristics of mature differentiated DCs. HLA-DR, CD86, CD80, CD14, and CCR7 expression on mature GMDCs, GM/CCL18DCs, and GM/IL-4DCs from a nonallergic and allergic subject. One representative flow cytometry experiment of 8 is shown. Values in the quadrant indicate the mean fluorescence intensity after subtraction of the isotype control (unfilled black line).
Phenotypic profile of DCs
| . | Nonallergics . | Allergics . | ||||
|---|---|---|---|---|---|---|
| GMDCs . | GM/CCL18DCs . | GM/IL-4DCs . | GMDCs . | GM/CCL18DCs . | GM/IL-4DCs . | |
| HLA-DR | 72.7 ± 33 | 121.2 ± 39.8* | 177 ± 42.6§ | 82.3 ± 28 | 77.5 ± 26.6#,** | 189.1 ± 57.3¶ |
| CD80 | 28.7 ± 15 | 68.3 ± 33.8† | 73.8 ± 41.4‖ | 37.5 ± 14.2 | 27.5 ± 11.3#,‡ | 69.8 ± 51.8 |
| CD86 | 18.7 ± 10 | 33.3 ± 13.8† | 48.1 ± 27.1‖ | 14.3 ± 8.9 | 16.8 ± 7.5#,‡ | 33.3 ± 20.4‖ |
| CD11c | 26.2 ± 10.2 | 21.6 ± 9.4 | 23.4 ± 4.9 | 25.2 ± 4.7 | 20.2 ± 4.2 | 27.0 ± 5.1 |
| CD206 | 24.1 ± 4.2 | 15.1 ± 3.3 | 9.7 ± 3.0¶ | 20.6 ± 4.6 | 20.7 ± 7.8 | 15.1 ± 3.0 |
| CD1a | 9.2 ± 5.3 | 6.2 ± 4.9†‡ | 21.2 ± 9.2‖ | 5.8 ± 6 | 3.7 ± 3.1** | 20.0 ± 8.2¶ |
| CD14 | 47.5 ± 22.3 | 34.8 ± 18.3 | 19.7 ± 9.9‖ | 48.5 ± 32 | 42.7 ± 13.3** | 14.0 ± 6.2‖ |
| CD16 | 27.2 ± 5.8 | 26.6 ± 4.8‡ | 9.7 ± 2.9¶ | 14.5 ± 2 | 18.1 ± 4.6‡ | 5.4 ± 3.0¶ |
| CCR7 | 21.6 ± 4.4 | 30.4 ± 7.6†‡ | 20.2 ± 6.2 | 18.5 ± 8.0 | 31.7 ± 10.7 | 20.5 ± 8.7 |
| CD40 | 3.5 ± 0.2 | 3.8 ± 0.6 | 3.7 ± 1.0 | 4.4 ± 1.8 | 4.5 ± 0.8 | 4.9 ± 1.7 |
| ICOS-L | 0.5 ± 0.4 | 1.6 ± 0.5 | 6.4 ± 1.2¶ | 0.5 ± 0.2 | 1.3 ± 0.7 | 4.0 ± 1.3‖ |
| PD-L1 | 1.3 ± 1.2 | 2.6 ± 1 | 5.8 ± 2.0‖ | 1.1 ± 0.7 | 1.4 ± 0.6 | 4.7 ± 1.4‖ |
| PD-L2 | 6.2 ± 1.8 | 7.7 ± 1.8 | 8.4 ± 3.2 | 4.4 ± 1.4 | 5.2 ± 1.8 | 7.8 ± 4.6 |
| . | Nonallergics . | Allergics . | ||||
|---|---|---|---|---|---|---|
| GMDCs . | GM/CCL18DCs . | GM/IL-4DCs . | GMDCs . | GM/CCL18DCs . | GM/IL-4DCs . | |
| HLA-DR | 72.7 ± 33 | 121.2 ± 39.8* | 177 ± 42.6§ | 82.3 ± 28 | 77.5 ± 26.6#,** | 189.1 ± 57.3¶ |
| CD80 | 28.7 ± 15 | 68.3 ± 33.8† | 73.8 ± 41.4‖ | 37.5 ± 14.2 | 27.5 ± 11.3#,‡ | 69.8 ± 51.8 |
| CD86 | 18.7 ± 10 | 33.3 ± 13.8† | 48.1 ± 27.1‖ | 14.3 ± 8.9 | 16.8 ± 7.5#,‡ | 33.3 ± 20.4‖ |
| CD11c | 26.2 ± 10.2 | 21.6 ± 9.4 | 23.4 ± 4.9 | 25.2 ± 4.7 | 20.2 ± 4.2 | 27.0 ± 5.1 |
| CD206 | 24.1 ± 4.2 | 15.1 ± 3.3 | 9.7 ± 3.0¶ | 20.6 ± 4.6 | 20.7 ± 7.8 | 15.1 ± 3.0 |
| CD1a | 9.2 ± 5.3 | 6.2 ± 4.9†‡ | 21.2 ± 9.2‖ | 5.8 ± 6 | 3.7 ± 3.1** | 20.0 ± 8.2¶ |
| CD14 | 47.5 ± 22.3 | 34.8 ± 18.3 | 19.7 ± 9.9‖ | 48.5 ± 32 | 42.7 ± 13.3** | 14.0 ± 6.2‖ |
| CD16 | 27.2 ± 5.8 | 26.6 ± 4.8‡ | 9.7 ± 2.9¶ | 14.5 ± 2 | 18.1 ± 4.6‡ | 5.4 ± 3.0¶ |
| CCR7 | 21.6 ± 4.4 | 30.4 ± 7.6†‡ | 20.2 ± 6.2 | 18.5 ± 8.0 | 31.7 ± 10.7 | 20.5 ± 8.7 |
| CD40 | 3.5 ± 0.2 | 3.8 ± 0.6 | 3.7 ± 1.0 | 4.4 ± 1.8 | 4.5 ± 0.8 | 4.9 ± 1.7 |
| ICOS-L | 0.5 ± 0.4 | 1.6 ± 0.5 | 6.4 ± 1.2¶ | 0.5 ± 0.2 | 1.3 ± 0.7 | 4.0 ± 1.3‖ |
| PD-L1 | 1.3 ± 1.2 | 2.6 ± 1 | 5.8 ± 2.0‖ | 1.1 ± 0.7 | 1.4 ± 0.6 | 4.7 ± 1.4‖ |
| PD-L2 | 6.2 ± 1.8 | 7.7 ± 1.8 | 8.4 ± 3.2 | 4.4 ± 1.4 | 5.2 ± 1.8 | 7.8 ± 4.6 |
DCs differentiated by different protocols were evaluated for their cell-surface markers by flow cytometry. Data are presented as mean fluorescence intensity ± SEM.
P < .01 (GM/CCL18DCs vs GMDCs).
P < .05 (GM/CCL18DCs vs GMDCs).
P < .05 (GM/CCL18DCs vs GM/IL-4DCs).
P < .001 (GM/IL-4DCs vs GMDCs).
P < .05 (GM/IL-4DCs vs GMDCs).
P < .01 (GM/IL-4DCs vs GMDCs).
P < .05 (GM/CCL18DCs from allergics vs nonallergics).
P < .01 (GM/CCL18DCs vs GM/IL-4DCs).
Cytokine production by mature differentiated DCs. (A) Production of IL-10, TGF-β1, IL-6, and IL-23 in mature DC culture supernatants was quantified by ELISA. Data are pg/mL ± SEM for n = 9 nonallergics (NA) and n = 8 allergics (A). **P < .01. P < .05 (A vs NA); and ##P < .01 (A vs NA). (B) Production of IL-10 in mature DC culture supernatants was quantified by ELISA and presented as above. n = 3 NA subjects.
Cytokine production by mature differentiated DCs. (A) Production of IL-10, TGF-β1, IL-6, and IL-23 in mature DC culture supernatants was quantified by ELISA. Data are pg/mL ± SEM for n = 9 nonallergics (NA) and n = 8 allergics (A). **P < .01. P < .05 (A vs NA); and ##P < .01 (A vs NA). (B) Production of IL-10 in mature DC culture supernatants was quantified by ELISA and presented as above. n = 3 NA subjects.
CD4+T cells primed by GM/CCL18DCs from healthy subjects produce more IL-10 but do not express more Foxp3 than GM/IL-4DCs
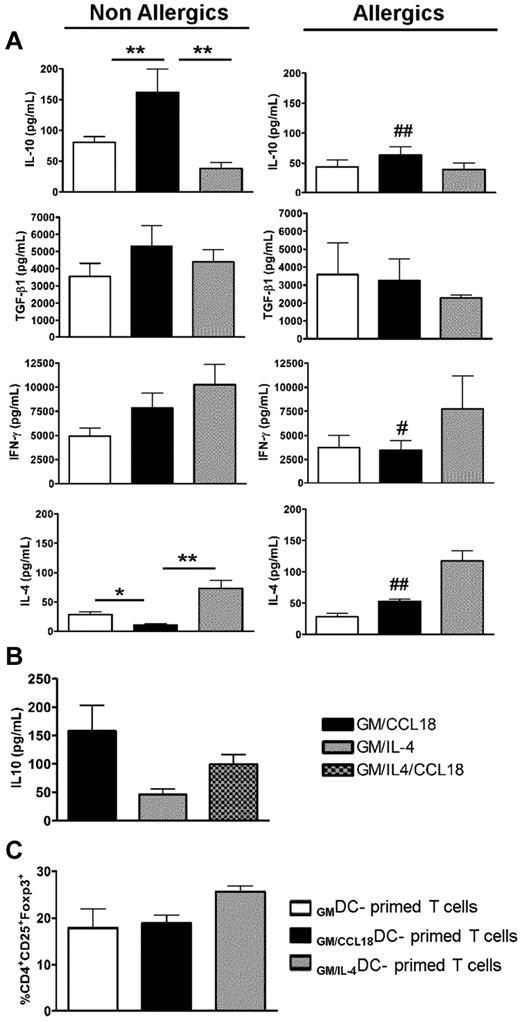
To further assess whether GM/CCL18DCs from healthy subjects were able to induce T cells with a regulatory phenotype, the generated DCs were cocultured with allogenic naive T cells from nonallergic donors for 5 days and evaluated for their pattern of cytokine secretion. IL-10 production was significantly increased in supernatants of GM/CCL18DC/T-cell cocultures, whereas IL-4 synthesis was reduced compared with GM/IL-4DCs (Figure 4A). The secretion of TGF-β1 was slightly, but not significantly, increased, whereas IFN-γ showed intermediate levels compared with GMDCs and GM/IL-4DCs. The increase in IL-10 production was not related to an increased T-cell proliferation as shown by the enumeration of the T cells at the end of the cocultures, which showed identical numbers of T cells in the GM and GM plus CCL18 protocols and a slightly increased number in the GM plus IL-4 protocol (data not shown). Intracellular staining showed that IL-10 was produced by 20% of the T cells and by 2% of the DCs (data not shown). The same experimental protocol, but with GM/CCL18DCs obtained from allergic donors, did not lead to significant changes in cytokine production compared with GMDCs. However, they exhibited less IL-10 and IFN-γ and more IL-4 production than GM/CCL18DCs obtained from nonallergic donors (Figure 4A). As for DCs, T-cell cocultures with GM/IL-4/CCL18DCs from nonallergic subjects generated more IL-10 production than with GM/IL-4DCs and less than with GM/CCL18DCs (Figure 4B). To further evaluate the phenotype of the generated T cells, the percentage of CD4+CD25+Foxp3+ cells, a subset of regulatory T cells, was evaluated in the different DC differentiation conditions. No differences were observed in the percentage of CD4+CD25+Foxp3+ cells between GMDC (17.9% ± 4%), GM/CCL18DC (18.9% ± 2%), and GM/IL-4DC (25.6% ± 2%) primed T cells (Figure 4C).
Cytokine profile in DC/T-cell cocultures and percentages of CD4+CD25+Foxp3+ cells after priming by differentiated DCs. (A) Production of IL-10, TGF-β1, IFN-γ, and IL-4 in DC/T-cell coculture supernatants was quantified by ELISA. Data are pg/mL ± SEM for n = 8 nonallergics (NA) and n = 7 allergics (A). P < .05; **P < .01. P < .05 (A vs NA). ##P < .01(A vs NA). (B) Production of IL-10 in DC/T-cell coculture supernatants was quantified by ELISA and presented as in Figure 6A. n = 3 NA subjects. (C) The percentage of CD4+CD25+Foxp3+ cells among T cells primed by differentiated DCs was evaluated by flow cytometry (n = 3 NA).
Cytokine profile in DC/T-cell cocultures and percentages of CD4+CD25+Foxp3+ cells after priming by differentiated DCs. (A) Production of IL-10, TGF-β1, IFN-γ, and IL-4 in DC/T-cell coculture supernatants was quantified by ELISA. Data are pg/mL ± SEM for n = 8 nonallergics (NA) and n = 7 allergics (A). P < .05; **P < .01. P < .05 (A vs NA). ##P < .01(A vs NA). (B) Production of IL-10 in DC/T-cell coculture supernatants was quantified by ELISA and presented as in Figure 6A. n = 3 NA subjects. (C) The percentage of CD4+CD25+Foxp3+ cells among T cells primed by differentiated DCs was evaluated by flow cytometry (n = 3 NA).
The data in the preceding paragraph suggest that DCs from healthy subjects differentiated in the presence of CCL18 favor the generation of T cells overexpressing the anti-inflammatory cytokine IL-10, but not Foxp3.
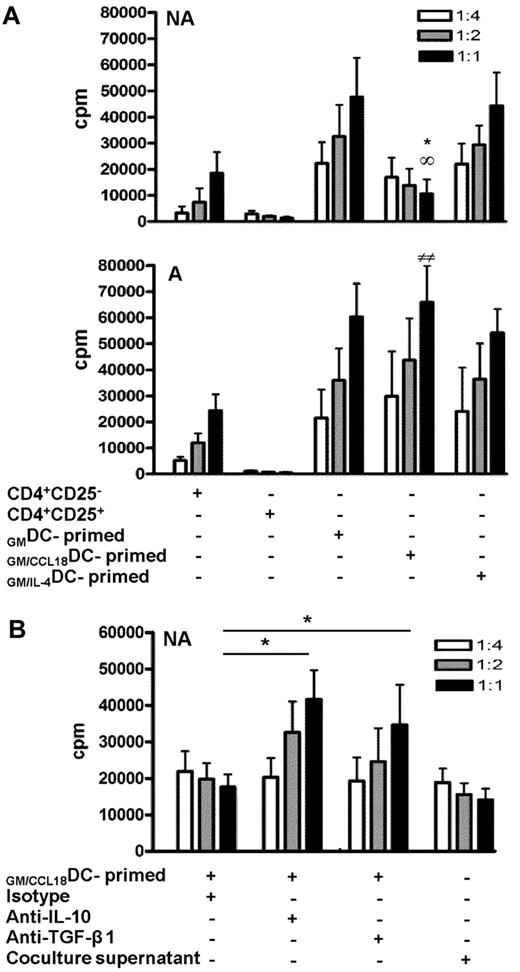
CD4+ T cells primed by GM/CCL18DCs from healthy subjects inhibit CD4+CD25− effector T-cell proliferation by cytokine-dependent mechanisms
To definitely determine whether the CD4+ T cells induced by GM/CCL18DCs obtained from nonallergic subjects were regulatory T cells, we evaluated whether T cells primed by GM/CCL18DCs had suppressive abilities on autologous flow cytometry sorted CD4+CD25− T cells. For this purpose, CD4+CD25− T-cell proliferation of a fixed number of cells was measured by radioactive thymidine incorporation in the presence of increasing ratios of T cells primed by different types of DCs. The addition of CD4+CD25− effector cells (negative control) resulted in increased T-cell proliferation, whereas the addition of CD4+CD25+ regulatory T cells (positive control) resulted in a ratio-dependent suppression of proliferation (75% inhibition at 1:1 ratio compared with the negative control; Figure 5A). A dose-dependent inhibition of CD4+CD25− proliferation was observed with CD4+ T cells primed with GM/CCL18DCs (72% ± 2% at a 1:1 ratio compared with both GMDCs and GM/IL-4DCs), whereas CD4+ T cells primed by GM/IL-4DCs and GMDCs increased effector T-cell proliferation. In contrast to healthy subjects, GM/CCL18DCs generated from allergic patients did not suppress but enhanced effector T-cell proliferation (Figure 5A). Regulatory T cells have been shown to act through 2 main mechanisms: production of suppressive cytokines such as IL-10 and TGF-β and through cell contact inhibition. To determine the mechanism involved in the suppressive effect of T cells primed by GM/CCL18DCs generated from healthy subjects, inhibition of IL-10 and TGF-β1 and experiments using cell supernatants were performed. Blocking anti–IL-10 and anti–TGF-β1 antibodies were added to the proliferation assays to evaluate their involvement in the suppressive ability of T cells primed by GM/CCL18DCs. Blockade of IL-10 and of TGF-β1 significantly restored the proliferation of T cells primed by GM/CCL18DCs compared with the isotype treatment (Figure 5B). To determine whether part of the suppressive effect was cell-contact dependent, supernatants of T cells primed by GM/CCL18DCs were tested in suppression assay toward effector T cells. The inhibition of CD4+CD25− effector cell proliferation with the supernatants was equivalent to the inhibition in the presence of T cells primed by GM/CCL18DCs, suggesting that the inhibition of CD4+CD25− effector cell proliferation was mainly cytokine-dependent (Figure 5B).
Inhibitory effect of GM/CCL18DC-primed T cells on proliferation of CD4+CD25− effector cells. (A) T cells primed by GMDCs, GM/CCL18DCs, and GM/IL-4DCs from nonallergic (NA) or allergic (A) donors were added at suppressor/responder ratios of 1:4, 1:2, and 1:1 to autologous CD4+CD25− effector cells stimulated with soluble anti-CD3 and CD28 antibodies. CD4+CD25− and CD4+CD25+ T cells were used as negative and positive controls, respectively. Irradiated autologous PBMCs were used as antigen-presenting cells. Proliferation was measured by incorporation of 3H-thymidine at 48 hours. Data are counts per minute ± SEM. *P < .05 (GM/CCL18DCs vs GMDCs). ∞P < .05 (GM/CCL18DCs vs GM/IL-4DCs). ##P < .01 (GM/CCL18DCs from A vs NA). (B) Control isotype, anti–IL-10, or anti–TGF-β1 neutralizing antibodies were added to the DC/T-cell coculture system. T cells primed by GM/CCL18DCs or supernatants from DC/T-cell cocultures at different dilutions were added to effector cells as described as in Figure 5A. Data are counts per minute ± SEM. *P < .05 versus isotype-treated T cells primed by GM/CCL18DCs. n = 3 experiments with triplicate wells for each condition.
Inhibitory effect of GM/CCL18DC-primed T cells on proliferation of CD4+CD25− effector cells. (A) T cells primed by GMDCs, GM/CCL18DCs, and GM/IL-4DCs from nonallergic (NA) or allergic (A) donors were added at suppressor/responder ratios of 1:4, 1:2, and 1:1 to autologous CD4+CD25− effector cells stimulated with soluble anti-CD3 and CD28 antibodies. CD4+CD25− and CD4+CD25+ T cells were used as negative and positive controls, respectively. Irradiated autologous PBMCs were used as antigen-presenting cells. Proliferation was measured by incorporation of 3H-thymidine at 48 hours. Data are counts per minute ± SEM. *P < .05 (GM/CCL18DCs vs GMDCs). ∞P < .05 (GM/CCL18DCs vs GM/IL-4DCs). ##P < .01 (GM/CCL18DCs from A vs NA). (B) Control isotype, anti–IL-10, or anti–TGF-β1 neutralizing antibodies were added to the DC/T-cell coculture system. T cells primed by GM/CCL18DCs or supernatants from DC/T-cell cocultures at different dilutions were added to effector cells as described as in Figure 5A. Data are counts per minute ± SEM. *P < .05 versus isotype-treated T cells primed by GM/CCL18DCs. n = 3 experiments with triplicate wells for each condition.
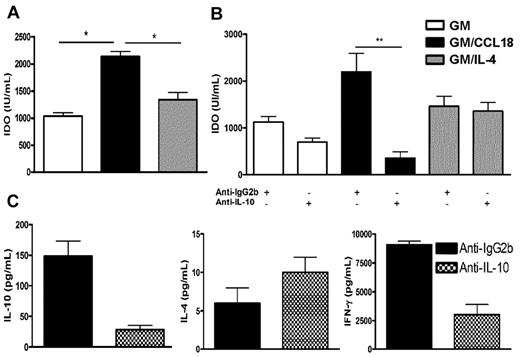
CCL18 generates tolerogenic DCs in healthy donors through IL-10-induced IDO production
Next, the mechanism whereby CCL18 was inducing tolerogenic DCs in healthy subjects was evaluated. Dysregulation of the tryptophan metabolism in DCs has been shown to generate regulatory T cells, in particular through the production of the tryptophan-catabolizing enzyme IDO.25 Therefore, the production of IDO was assessed in the supernatants of mature differentiated DCs from healthy donors. Increased levels of IDO were detected in supernatants of GM/CCL18DCs compared with the other protocols of DC differentiation (Figure 6A). As IL-10 has been described as an inducer of IDO,25,26 the effect of IL-10 inhibition during differentiation of DCs was examined using a neutralizing IL-10 antibody. Compared with isotype-treated DCs, anti–IL-10–treated GM/CCL18DCs exhibited a statistically significant decrease of IDO production, whereas GMDC and GM/IL-4DC IDO levels were not affected (Figure 6B). To evaluate whether anti–IL-10–treated GM/CCL18DCs were impaired in their ability to generate Tregs, they were cocultured for 5 days with naive T cells and their cytokine secretion determined. As shown in Figure 6C, there was a strong reduction in the production of IL-10 and IFN-γ, whereas IL-4 production remained elevated. Altogether, these results suggest that CCL18 induces tolerogenic DCs through IL-10–induced IDO, able to promote T cells producing the IL-10 suppressive cytokine.
IDO production by mature differentiated DCs from nonallergic subjects and effect of IL-10 neutralization on GM/CCL18DC priming effect. (A) IDO production in supernatants from mature differentiated DCs. (B) Effect of IL-10 neutralization during DC differentiation on IDO production by DCs compared with isotype-treated DCs. (C) Effect of IL-10 neutralization during GM/CCL18DC differentiation on the cytokine production by DC/T-cell cocultures compared with isotype-treated DCs. Data are mean IU/mL for IDO and pg/mL for cytokines ± SEM of n = 6 experiments for panel A, n = 3 to 6 experiments for panel B, and n = 2 experiments for panel C. *P < .05. **P < .01.
IDO production by mature differentiated DCs from nonallergic subjects and effect of IL-10 neutralization on GM/CCL18DC priming effect. (A) IDO production in supernatants from mature differentiated DCs. (B) Effect of IL-10 neutralization during DC differentiation on IDO production by DCs compared with isotype-treated DCs. (C) Effect of IL-10 neutralization during GM/CCL18DC differentiation on the cytokine production by DC/T-cell cocultures compared with isotype-treated DCs. Data are mean IU/mL for IDO and pg/mL for cytokines ± SEM of n = 6 experiments for panel A, n = 3 to 6 experiments for panel B, and n = 2 experiments for panel C. *P < .05. **P < .01.
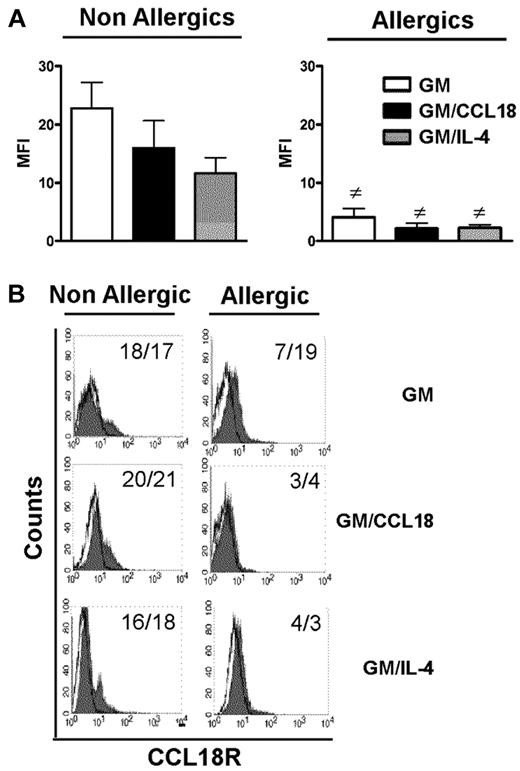
CCL18 does not bind to DCs from allergic patients
For all examined parameters, the results observed with GM/CCL18DCs obtained from allergic subjects were similar to those obtained with GMDCs, suggesting that CCL18 could not act on its putative receptor in these patients. Although CCL18 receptor is still unknown, its presence can be visualized using its labeled ligand. Therefore, its expression was examined by flow cytometry using biotinylated CCL18. The results showed that all differentiated DCs from allergic subjects bound less CCL18 than healthy subjects as shown by decreased mean fluorescence intensity and percentage of expression after subtraction of the biotinylated control protein (Figure 7). Taken together, these data suggest that, in allergic subjects, decreased CCL18R binding on DCs may explain the absence of tolerogenic effect of CCL18.
Cell surface expression of CCL18 receptor(s) on mature differentiated DCs from nonallergic and allergic subjects. (A) Baseline expression of CCL18R evaluated on the surface of mature GMDCs, GM/CCL18DCs, and GM/IL-4DCs from nonallergic (NA) and allergic (A) subjects expressed as mean fluorescence intensity (MFI) ± SEM for n = 6 subjects. #P < .05 (A vs NA). (B) Representative histograms of CCL18R staining on mature differentiated DCs from NA and A subjects. Values in the quadrant indicate the MFI/percentage of positive cells after subtraction of the control protein (unfilled black line).
Cell surface expression of CCL18 receptor(s) on mature differentiated DCs from nonallergic and allergic subjects. (A) Baseline expression of CCL18R evaluated on the surface of mature GMDCs, GM/CCL18DCs, and GM/IL-4DCs from nonallergic (NA) and allergic (A) subjects expressed as mean fluorescence intensity (MFI) ± SEM for n = 6 subjects. #P < .05 (A vs NA). (B) Representative histograms of CCL18R staining on mature differentiated DCs from NA and A subjects. Values in the quadrant indicate the MFI/percentage of positive cells after subtraction of the control protein (unfilled black line).
Discussion
In this study, we evaluated the immune nonchemotactic function of CCL18 on DCs. CCL18 endogenous production was much higher in DCs obtained from allergic than nonallergic subjects, in particular for the protocols using IL-4 and IL-13 as differentiating cytokines. This might be linked to a priming effect already present in vivo, related to the Th2 cytokines present in allergic patients, which are known to induce CCL18.9 Alternatively, this may also be related to an intrinsic capacity of DCs from allergic patients to produce more CCL18 than nonallergic donors. This latter hypothesis is supported by the elevated level of CCL18 that persisted in the maturation phase at day 8 in supernatants from allergic subjects, even after DC extensive washing and removal of Th2 cytokines. It is also consistent with the ability of DCs obtained from allergic subjects to behave differently from those obtained from nonallergic subjects. For example, we and others have previously shown that DCs from allergic patients are able to favor a Th2 profile when cocultured with naive T lymphocytes versus a Th1 profile for nonallergic subjects.27-29 In our initial experiments, the inhibition of half of the endogenous CCL18 production during the differentiation process did lead to an increase in the ratio of proinflammatory versus anti-inflammatory cytokines in DCs and to a decrease in IL-10 production in DC/T-cell cocultures. However, this endogenous production of CCL18 by DCs from healthy subjects was not enough to induce a regulatory phenotype in T cells as shown by the absence of inhibition of effector T-cell proliferation by GM/IL-4DCs. We also performed dose-response experiments, which showed that the minimal exogenous CCL18 concentration required to obtain a tolerogenic effect on DCs was 10−8M (ie, 80 ng/mL), therefore 5-fold more than the quantity produced by DCs from healthy subjects. Such high environmental concentrations may be achieved in the lung, where CCL18 is constitutively expressed. It has been previously shown that alveolar macrophages produce high levels of CCL18,9,13 5 million alveolar macrophages being enough to attain at baseline the 10−8M concentration. In the present study, exogenous CCL18 was found to drive DCs with characteristics of semimature DCs only in nonallergic subjects. Indeed, their GM/CCL18DCs still expressed macrophage lineage markers, such as CD14, CD16, and the macrophage mannose receptor CD206, but also exhibited a partial maturation with intermediate levels of costimulatory and major histocompatibility complex class II molecules, and a decreased production of the proinflammatory cytokine IL-23p40. Semimature DCs have been previously shown to develop in response to different environmental stimuli, such as IL-6,30 TNF-α,31,32 vitamin D3 and dexamethasone.33,34 The generation of semimature DCs by CCL18 was not related to an overproduction of IL-6 as shown in DC supernatants; and although TNF-α was used in DC maturation phase in all protocols, only the CCL18 protocol led to a semimature phenotype. It is of interest that it has been previously shown that semimature DCs induced by vitamin D3 strongly promote CCL18 secretion.10 Different surface phenotypes have been identified among semimature DCs, including increased expression of inhibitory costimulatory molecules, such as PD-L1 or ICOS-L.34-36 In the present study, CCL18 primed DCs did not show increased expression of these inhibitory molecules, suggesting that they were not involved in the observed tolerogenic effect. Interestingly, CCL18 primed DCs expressed high levels of CCR7, a chemokine receptor necessary to enter secondary lymphoid tissues. In vivo, it has been reported that CCR7 expression by semimature DCs is critical to tolerance induction in steady-state conditions, by allowing these cells to immigrate into lymph nodes where they interact with specific T cells.37 It has been recently shown that antigen transport by CCR7-dependent migrating lung-derived DCs to the draining bronchial lymph nodes was a necessary requirement for successful induction of tolerance toward innocuous inhaled antigens and for protection against the development of allergic airway diseases.38 As CCL18 is constitutively expressed in the lung, these data and our results suggest that in healthy subjects, in steady-state conditions, CCL18 may constitute a novel priming mechanism of semimature lung DCs able to migrate to lymph nodes and to participate to the maintenance of tolerance against inhaled antigens, including allergens. Semimature DCs are known to produce increased quantities of IL-10, a cytokine able to alter DC maturation process. Exposure of immature DCs to IL-10 results in a reduced expression of costimulatory and MHC class II molecules and production of proinflammatory cytokines,39 and leads to the development of adaptive regulatory T cells, including Tr1 cells,40,41 whereas fully mature DCs are resistant to IL-10 effects.42 In our study, the induction of IL-10 by DCs differentiated in the presence of CCL18 led to Tregs that had the features of Tr1 cells because they did not overexpress Foxp3, exhibited low levels of IL-4, and intermediate levels of IFN-γ, and acted through a soluble cytokine-dependent mechanism, as originally described for Tr1 cells.40,43-45 A high level of IL-10 was detected in DC/T-cell cocultures, which was higher than the level produced by DCs alone, with 10-fold more IL-10–producing lymphocytes than DCs, showing that the production of IL-10 in the cocultures was also derived from T cells. Until recently, the mechanisms involved in IL-10–induced Tr1 induction remained elusive; however, a recent study showed that induction of Tr1 cells by IL-10–primed DCs was IL-10–dependent and required the ILT4/HLA-G signaling pathway.46 We now provide here a new mechanism of Tr1 differentiation, involving activation of DCs through the CCL18 pathway. IL-10 appeared as the main mediator responsible for the generation of Tregs in healthy subjects. Indeed, its inhibition during the differentiation of DCs in the presence of CCL18 changed the T-cell coculture cytokine profile from an antiinflammatory to a proinflammatory profile, through the inhibition of IDO. We have previously shown that CCL18 was also able to convert effector memory CD4+CD25− T cells into adaptive regulatory T cells, through a TGF-β-dependent mechanism.15 Although GM/CCL18DC-primed regulatory T cells did also exert their suppressive function partly through TGF-β, the initial mechanism of DC priming did not seem related to TGF-β as shown by the absence of difference in TGF-β production among the different differentiation protocols. Altogether, CCL18 appears as an anti-inflammatory chemokine in nonallergic subjects, able to favor a regulatory profile. Regulatory DCs are promising tools for clinical application in transplantation, autoimmunity, or allergy. A variety of biologic and pharmacologic agents have been shown to induce tolerogenic DCs47 ; therefore, CCL18 may be added to the arsenal of therapeutic tools devoted to induce tolerance. However, in allergic subjects, no change was observed in surface markers, cytokine profiles, and function of DCs, which was consistent with the decreased CCL18R staining on DC cell surface. Although the precise nature of CCL18R is still unknown, decreased binding of CCL18 to its putative receptor may be related to a desensitization phenomenon linked to the elevated endogenous levels of CCL18 found in DC supernatants from allergic subjects compared with control subjects. However, although we treated DCs from healthy subjects with twice the endogenous concentration of CCL18 found in DCs from allergic patients, CCL18R was still expressed on DCs from healthy subjects, suggesting that another mechanism might take place. Accordingly, no differences were observed between the different differentiation protocols in G protein-coupled receptor kinase 2 and β-arrestin expression, 2 molecules controlling chemokine desensitization (data not shown). The exact mechanisms regulating CCL18R expression in allergic subjects need further investigation. However, these data suggest that the absence of feedback regulation by CCL18R in allergic subjects may participate to the lack of resolution of allergic inflammatory reactions.
In conclusion, this study shows, for the first time, that CCL18 can generate functional tolerogenic DCs able to prime regulatory T cells in healthy subjects, suggesting that CCL18 may play a potent role in inducing and maintaining tolerance to inhaled antigens through its constitutive lung expression and priming of semimature DCs. In allergic subjects, this effect was lost and may account for sustained allergic inflammation in these patients.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Stanilas Tomavo for his kind gift of control protein.
This work was supported by ANR Physio, Santelys, and Region Nord Pas de Calais.
Authorship
Contribution: I.A. and S.A.Y. conceived, designed, coordinated, and performed research, analyzed data, and wrote the paper; Y.C., H.V., Y.F., and C.P. performed research and analyzed data; O.M. and N.D. performed radioactive experiments; A.-B.T. and B.W. recruited the patients and analyzed data; and A.T. conceived, designed, coordinated, and analyzed data and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Anne Tsicopoulos, Centre for Infection and Immunity of Lille, Pulmonary Immunity, Institut Pasteur de Lille, 1 rue du Prof Calmette, BP 245, 59019 Lille, France; e-mail: anne.tsicopoulos@pasteur-lille.fr.
References
Author notes
I.A. and S.A.Y. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal