To the editor:
We read with great interest the article by Charles et al1 describing anergy of VH1-69+ CD21low B cells from patients with hepatitis C virus (HCV)–associated mixed cryoglobulinemia (MC). Anergy of these cells was made evident by decreased calcium mobilization and low production of IgM. These authors reported that the VH1-69+ B cells with a CD21high phenotype were not anergic, but data from our laboratory challenge this conclusion. It is worth noting that in Charles et al's study, calcium mobilization in different subsets of B cells was very variable, and that the analyses could be biased by contaminating VH1-69− CD21high B cells because G6+ cells were not gated in those experiments. Furthermore, IgM production does not appear to be a reliable marker of anergy in CD21low B cells, because the CD21low B cells of patients with common variable immunodeficiency or with HIV infection have defects of proliferative responses although they produce significant amounts of IgM.2,3 Unfortunately, proliferation studies were not reported in Charles et al's article.
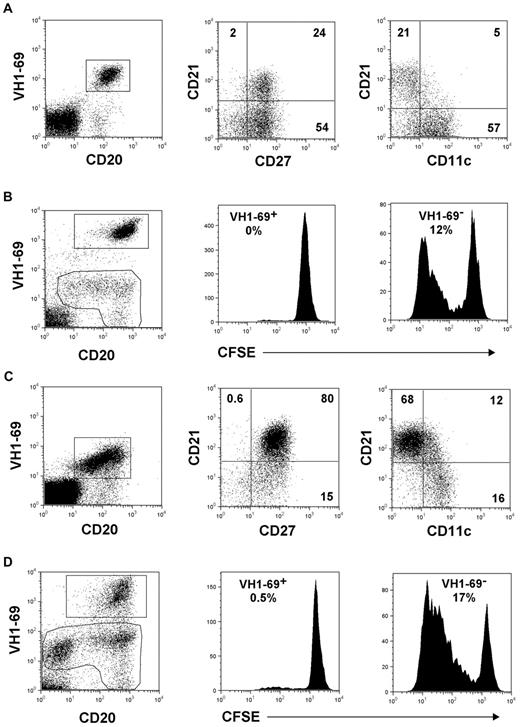
We investigated the functional properties of VH1-69+ B cells from several patients with HCV-associated MC. As with Charles's study, we exploited the G6 antibody4 (provided by R. Jefferis, University of Birmingham, Birmingham, United Kingdom) to identify these cells. Phenotypic characteristics suggest that the VH1-69+ CD21high (IgM+CD27+) marginal zone (MZ)–like B cells are the precursors of their CD21low (CD11c+) counterparts, considering that some of them express low-level CD11c (Figure 1A,C). The proliferative responses of VH1-69+ B cells were analyzed by the CFSE dilution flow cytometric method5 ; the strategy of analysis is illustrated in Figure 1B and D. We found that both the CD21low (Figure 1B) and the CD21high (Figure 1D) VH1-69+ B cells failed to proliferate in response to the stimulation of Toll-like receptor (TLR) 9 with CpG; similar findings were obtained with the TLR7 ligand R848 (not shown). Furthermore, VH1-69+ B cells failed to differentiate to CD20low/neg plasmablasts (Figure 1B,D). The data shown in Figure 1D are representative of the findings in 3 HCV+ MC patients with highly enriched (> 75% of total B cells) CD21high VH1-69+ B cells.
Phenotype and proliferative responses of CD21low and CD21high VH1-69+ B cells. (A-B) FACS plots from a representative (n = 8) MC patient with predominance of CD21low VH1-69+ B cells. (A) Expression of surface markers by electronically gated VH1-69+ B cells stained with the G6 antibody.4 (B) Gating strategy for the analysis of proliferative responses of VH1-69+ and VH1-69− B cells to CpG (2.5 μg/mL, 5-day culture). Cells were permeabilized before staining. The regions of analysis encompass CD20+ B cells and CD20low/neg plasmablasts, which were also characterized as CD38+ cytoplasmic-IgMhigh cells (not shown). Plasmablasts are present only in the VH1-69− gating region. Percent values in the histograms denote the number of cells entering division (precursor cohort5 ). (C-D) FACS plots from a representative (n = 3) MC patient with highly enriched CD21high VH1-69+ B cells. Analyses of surface marker expression (C) and of proliferative responses to CpG (D) were done as in panels A and B, respectively.
Phenotype and proliferative responses of CD21low and CD21high VH1-69+ B cells. (A-B) FACS plots from a representative (n = 8) MC patient with predominance of CD21low VH1-69+ B cells. (A) Expression of surface markers by electronically gated VH1-69+ B cells stained with the G6 antibody.4 (B) Gating strategy for the analysis of proliferative responses of VH1-69+ and VH1-69− B cells to CpG (2.5 μg/mL, 5-day culture). Cells were permeabilized before staining. The regions of analysis encompass CD20+ B cells and CD20low/neg plasmablasts, which were also characterized as CD38+ cytoplasmic-IgMhigh cells (not shown). Plasmablasts are present only in the VH1-69− gating region. Percent values in the histograms denote the number of cells entering division (precursor cohort5 ). (C-D) FACS plots from a representative (n = 3) MC patient with highly enriched CD21high VH1-69+ B cells. Analyses of surface marker expression (C) and of proliferative responses to CpG (D) were done as in panels A and B, respectively.
Clonal B cells of HCV+ MC patients provide a unique model for investigating the pathophysiology of human MZ B cells. Murine MZ B cells expand massively on stimulation by blood-borne microbial antigens6 and, similarly, the antigenic pressure of HCV appears to result in the robust clonal expansion of VH1-69+ MZ B cells in MC.7 We provide evidence that these cells undergo premature proliferative anergy, which takes place when the VH1-69+ B cells still retain a CD21high MZ-like phenotype and have not yet shifted to a fully “exhausted”3 CD21low phenotype. Interestingly, microarray gene expression profiling studies8 showed that murine MZ B cells stimulated in vivo by Streptococcus pneumoniae rapidly up-regulate Stra13, a negative regulator of B-cell proliferation. Thus, early proliferative anergy may serve to constrain the excessive expansion of MZ B cells activated by microbial antigens, and to attenuate the risk of autoimmune disease and of malignant transformation.
Authorship
Acknowledgments: This work was supported by the Intramural Research Program of the Sapienza University of Rome.
Contribution: M.V., M. Cagliuso, V.C., and M.F. designed the experiments and analyzed the data; M.V., M. Cagliuso, and V.C. performed flow cytometric analyses and cell cultures; M. Carbonari supervised flow cytometric analyses; M. Casato recruited and monitored patients; and M.F. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Massimo Fiorilli, Department of Clinical Immunology, Sapienza University of Rome, Viale dell'Università 37, 00185 Rome, Italy; e-mail: massimo.fiorilli@uniroma1.it.
References
Author notes
M.V., M. Cagliuso, and V.C. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal