Abstract
Defective Fas signaling leads to resistance to various anticancer therapies. Presence of potential inhibitors of Fas which could block Fas signaling can explain cancer cells resistance to apoptosis. We identified promyelocytic leukemia protein (PML) as a Fas-interacting protein using mass spectrometry analysis. The function of PML is blocked by its dominant-negative form PML–retinoic acid receptor α (PMLRARα). We found PMLRARα interaction with Fas in acute promyelocytic leukemia (APL)–derived cells and APL primary cells, and PML-Fas complexes in normal tissues. Binding of PMLRARα to Fas was mapped to the B-box domain of PML moiety and death domain of Fas. PMLRARα blockage of Fas apoptosis was demonstrated in U937/PR9 cells, human APL cells and transgenic mouse APL cells, in which PMLRARα recruited c-FLIPL/S and excluded procaspase 8 from Fas death signaling complex. PMLRARα expression in mice protected the mice against a lethal dose of agonistic anti-Fas antibody (P < .001) and the protected tissues contained Fas-PMLRARα-cFLIP complexes. Taken together, PMLRARα binds to Fas and blocks Fas-mediated apoptosis in APL by forming an apoptotic inhibitory complex with c-FLIP. The presence of PML-Fas complexes across different tissues implicates that PML functions in apoptosis regulation and tumor suppression are mediated by direct interaction with Fas.
Introduction
Maintenance of cell life-death homeostasis by widely expressed cell surface death receptors carries potential risks of inadvertent apoptosis, thus death receptors must be tightly regulated. Fas (APO-1, CD95) is a potent death receptor that eliminates autoreactive lymphocytes during lymphocyte development, but is less known for its proliferative functions such as its ability to stimulate regeneration of liver tissue.1 Levels of Fas expression in cancers vary, and Fas activation by ligand or agonistic antibodies is often blocked. A minority of cancer cells acquires disabling mutations of Fas or Fas signaling mediators, and various cancers rather express inhibitors of Fas signaling such as c-FLIP and other recently recognized Fas-associated inhibitors, for example, hepatocyte growth factor receptor and human herpesvirus 8 protein K1.2-4
Defective Fas signaling is an important cause of cancer resistance to therapy. Many genotoxic therapies including radiation depend on intact Fas signaling to eradicate cancer cells.5 For example, Fas defective cells are significantly hindered in undergoing apoptosis after treatment with conventional doses of chemotherapy and radiation.6,7 Restoring Fas apoptosis or sensitizing cancer cells to Fas-mediated apoptosis would improve the efficacy of many cancer therapies. However, stimulation of Fas in cancer cells has also triggered apoptosis of noncancerous cells.8 To elucidate a role for specific regulators of Fas signaling in cancer cells, we sought to identify potential modulators of Fas expressed in cancers and target them to selectively sensitize cancer cells to Fas-mediated apoptosis as a component of chemotherapy. This idea is appealing based on the assumption that cancer cells are abnormal in numerous aspects and, although poised to undergo apoptosis, survive through blockage of the apoptotic pathways.
In this study, we screened cells for potential regulators of the Fas death receptor. Using mass spectrometric analysis of Fas-associated proteins, we identified peptides derived from promyelocytic leukemia (PML) protein. PML is a tumor suppressor whose expression is ubiquitous, but it is significantly decreased in 60% of hematologic and epithelial cancers mostly because of enhanced degradation.9 The dominant negative form of PML is the oncogenic promyelocytic leukemia–retinoic acid receptor α (PMLRARα), formed by the translocation of chromosomes 15 and 17 t(15;17).10-12 PMLRARα has known proproliferative and antiapoptotic activities.13 Thus, we investigated whether the dominant negative PMLRARα regulates Fas-mediated apoptosis. We demonstrated that PMLRARα binds to Fas in APL cell lines and primary cells, and blocks Fas-mediated apoptosis through recruitment of c-FLIP in several cell models and in mice, suggesting that PMLRARα interrupts Fas-mediated apoptosis by binding directly to the Fas receptor complex.
Methods
Cell culture and transfection
Cell culture conditions and transfection methods are described in supplemental Methods (available on the Blood Web site: see the Supplemental Materials link at the top of the online article) and previous reports.14-18
Purification and identification of Fas-associated proteins
BJAB cells were screened for potential binding modulators of Fas as described in supplemental Methods.
Immunoprecipitation and Western blot analysis
Cellular fractionation
Propidium iodide staining
Propidium iodide (PI) staining was performed using PI/RNase staining buffer (BD Biosciences Pharmingen) as described in supplemental Methods.
Flow cytometry analysis of apoptosis
Apoptosis analysis by flow cytometry was carried out using the FITC– annexin V apoptosis detection kit I (BD Biosciences) as described in supplemental Methods.
Flow cytometry analysis of surface Fas expression level
U937/PR9 cells were treated with or without 200μM ZnSO4 for 24 hours. The cells (1 × 106) were then washed twice with 0.1M PBS + 1% FBS and incubated with FITC-conjugated monoclonal anti-Fas antibody (APO-1-1; Santa Cruz Biotechnology) for 20 minutes at 4°C. After 3 washes with cold PBS + 10% FBS, cells were resuspended in 150 μL of PBS + 2% FBS and analyzed using a FACS Calibur flow cytometer and BD CellQuest for data acquisition (both from BD Biosciences). Data were analyzed and plotted using FlowJo Version 7.5.5 software.
Experiments using shRNA
Lentiviral particles containing PML and a nontargeting shRNA sequence (Sigma Aldrich) were transduced into NB4 or U937 cells using the supplier's instructions. Transduced cells were selected in RPMI-1640 medium containing puromycin (2 μg/mL) for 2 weeks and suppression of protein expression was confirmed by Western blotting.
Survival of PMLRARα-expressing mice challenged with agonistic anti-Fas antibody
Six- to 8-week-old C57BL/6 female mice were purchased from the National Cancer Institute (Bethesda, MD). All mice were housed and treated in accordance with the guidelines of The University of Texas M. D. Anderson Cancer Center's Institutional Animal Care and Use Committee.
To assess the role of PMLRARα expression in vivo, we expressed PMLRARα in mouse liver by transfection of 100μg of PMLRARα plasmid or empty-vector plasmid (12 mice per group) by tail vein inoculation using a hydrodynamic transfection method.22 Twenty-four hours after transfection, mice were challenged with 2 μg/g mouse weight of agonistic Fas antibody Jo2 intraperitoneally and monitored for survival.23
Immunohistochemical staining and TUNEL assay of mouse liver tissues
Immunohistochemical staining and TUNEL assay were performed with the indicated antibodies as previously described.17
Statistical analysis
Experimental data are reported as means ± SD of 3 independent samples as indicated. Differences between 2 groups were assessed using the 2-tailed Student t test and a P value < .05 was considered to be statistically significant.
Results
Identification of PML as a Fas-associated protein by liquid chromatography tandem mass spectrometry analysis
BJAB cells, which show intermediate resistance to Fas-mediated apoptosis, were analyzed for potential modulators of Fas. The purification scheme described in “Purification and identification of Fas-associated proteins” revealed a distinct Fas-associated protein band of 75 kDa which was present in the Fas B-10 antibody lane, but was absent in control anti-TRAIL antibody lane. This band of interest was excised, digested with trypsin, and analyzed by liquid chromatography tandem mass spectrometry. Three peptides identified with acceptable confidence, TDGFDEFKVR, APASEEEFQFLR, and LRQEEPQSLQAAVR, are specifically present in both PML and PMLRARα, 2 dimerizing proteins with proapoptotic and antiapoptotic properties, respectively.
PMLRARα interacts with Fas in APL NB4 cells and APL primary cells
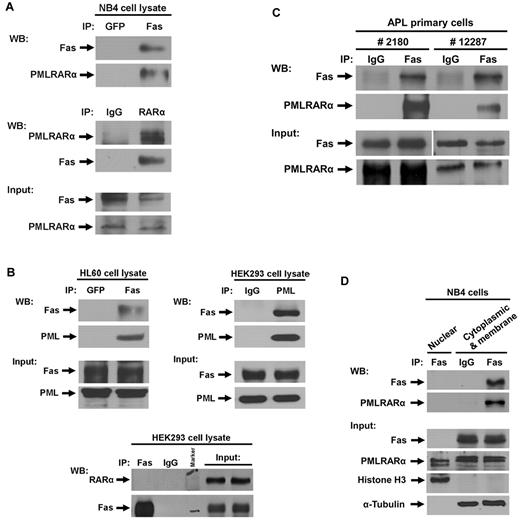
We examined the interaction of PMLRARα with Fas in human APL-derived NB4 cells, which spontaneously express endogenous PMLRARα. Anti-Fas antibody, but not the control anti-GFP antibody, coprecipitated PMLRARα (Figure 1A). In a reciprocal coimmunoprecipitation, anti-RARα antibody precipitated PMLRARα-Fas complex (Figure 1A). Notably, NB4 cells expressed RARα as well as PMLRARα, but immunoprecipitation of NB4 cell extracts with anti-Fas antibody did not coprecipitate RARα, suggesting that only the PML component of PMLRARα fusion protein is responsible for association with Fas. We also analyzed the association of PML with Fas in cell lysates from the acute myeloleukemic cell line HL60 and HEK293 cells by immunoprecipitation and Western blot (IP/WB) analysis. PML coprecipitated with anti-Fas antibody B-10 and Fas coprecipitated with anti-PML antibody PG-M3 in both tested cell lines; but RARα did not coprecipitate with anti-Fas antibody (Figure 1B).
PMLRARα Interacts with Fas in acute promyelocytic leukemia (APL) cell line and APL primary cells. (A) Cell extracts of APL NB4 cells were immunoprecipitated using antibodies against green fluorescent protein (GFP) and Fas, or normal IgG and RARα followed by Western blot analysis with antibodies against Fas and RARα for detection of Fas and PMLRARα, respectively. The expression levels of Fas and PMLRARα in whole-cell extracts are also shown by Western blot. (B) Cell extracts of HL60 cells and HEK293 cells were immunoprecipitated using antibodies against GFP and Fas, IgG and PML, or Fas and IgG. The precipitates were subjected to immunoblot analysis using anti-Fas, anti-PML, and anti-RARα antibodies. The expression levels of Fas, PML and RARα in whole-cell extracts are also shown. (C) APL primary cells isolated from 2 patients were lysed and cell extracts were immunoprecipitated using antibodies against normal IgG and Fas. The precipitates were subjected to Western blot analysis using an anti-Fas antibody for detection of Fas, and anti-RARα antibody for detection of PMLRARα. The expression levels of Fas and PMLRARα in whole-cell extracts are shown on the bottom panels. (D) To assess location of Fas-PMLRARα complexes, APL NB4 cells were subjected to subcellular fractionation and the presence of proteins and complexes in the fractions was assessed by IP/WB analysis. Histone H3 and α-tubulin were used as nuclear and cytosol/membrane fraction control proteins, respectively.
PMLRARα Interacts with Fas in acute promyelocytic leukemia (APL) cell line and APL primary cells. (A) Cell extracts of APL NB4 cells were immunoprecipitated using antibodies against green fluorescent protein (GFP) and Fas, or normal IgG and RARα followed by Western blot analysis with antibodies against Fas and RARα for detection of Fas and PMLRARα, respectively. The expression levels of Fas and PMLRARα in whole-cell extracts are also shown by Western blot. (B) Cell extracts of HL60 cells and HEK293 cells were immunoprecipitated using antibodies against GFP and Fas, IgG and PML, or Fas and IgG. The precipitates were subjected to immunoblot analysis using anti-Fas, anti-PML, and anti-RARα antibodies. The expression levels of Fas, PML and RARα in whole-cell extracts are also shown. (C) APL primary cells isolated from 2 patients were lysed and cell extracts were immunoprecipitated using antibodies against normal IgG and Fas. The precipitates were subjected to Western blot analysis using an anti-Fas antibody for detection of Fas, and anti-RARα antibody for detection of PMLRARα. The expression levels of Fas and PMLRARα in whole-cell extracts are shown on the bottom panels. (D) To assess location of Fas-PMLRARα complexes, APL NB4 cells were subjected to subcellular fractionation and the presence of proteins and complexes in the fractions was assessed by IP/WB analysis. Histone H3 and α-tubulin were used as nuclear and cytosol/membrane fraction control proteins, respectively.
To confirm the relevance of PMLRARα-Fas association in human cancer cells, we analyzed extracts from APL primary cells isolated from 2 patients with newly diagnosed APL. As expected, PMLRARα coimmunoprecipitated with anti-Fas antibody but not with control IgG antibody (Figure 1C).
To determine the location of PMLRARα–Fas complexes in cells, we separated subcellular compartments of NB4 cells using the nuclear/cytosol fractionation kit. Fas was found only in the cytosolic/membrane fraction, whereas PMLRARα was present in both cytosolic/membrane and nuclear fractions (Figure 1D). In agreement with these results, the PMLRARα-Fas complexes were found only in the cytosolic/membrane fraction (Figure 1D). High-resolution separation showed exclusive presence of α-tubulin and histone H3 in the cytosolic/membrane and nuclear fractions, respectively (Figure 1D).
PMLRARα suppresses Fas-mediated apoptosis in U937/PR9 cells
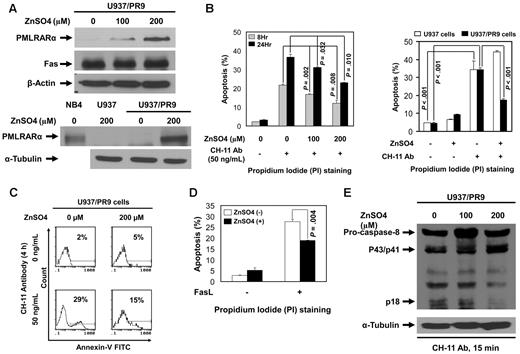
We studied engineered U937 cells, which express PMLRARα under a zinc-inducible promoter (U937/PR9 cells) to determine the effects of PMLRARα on Fas-mediated apoptosis. U937/PR9 cells incubated with ZnSO4 for 24 hours expressed PMLRARα in a dose-dependent manner without changes in Fas levels. Control ZnSO4-treated U937 cells did not express PMLRARα (Figure 2A). To test the effect of PMLRARα on Fas-mediated apoptosis, U937 and U937/PR9 cells incubated with or without ZnSO4 were then incubated with 50 ng/mL agonistic anti-Fas antibody CH-11 to induce Fas-mediated apoptosis. The levels of apoptosis were evaluated by flow cytometry of PI-stained cells. PMLRARα-expressing U937/PR9 cells showed significantly lower levels of apoptosis than did U937/PR9 cells not expressing PMLRARα at 8 and 24 hours after induction of apoptosis (P = .008 and .010, respectively; Figure 2B). CH-11 antibody induced a comparable level of apoptosis in control U937 cells and ZnSO4-treated U937 cells (Figure 2B).
Expression of PMLRARα blocks Fas-mediated apoptosis. (A) ZnSO4 (0, 100 and 200μM) was used to induce PMLRARα expression in U937/PR9 cells for 24 hours. Whole-cell lysates were subjected to Western blot analyses using an anti-RARα (for detection of PMLRARα), anti-Fas and anti–β-actin antibodies. NB4 cells and U937 cells treated with ZnSO4 were used as a positive and a negative control for PMLRARα expression, respectively. (B) U937/PR9 cells were treated with 100 and 200μM ZnSO4 for 24 hours followed by incubation with 50 ng/mL CH-11 antibody for 8 and 24 hours (left panel). U937 and U937/PR9 cells were treated with ZnSO4 for 24 hours followed by incubation with 50 ng/mL CH-11 antibody for 24 hours (right panel). The cells were stained with propidium iodide (PI) and analyzed by flow cytometry for the rates of apoptosis. The values shown are the means ± SD of 3 independent experiments. (C) U937/PR9 cells were treated with ZnSO4 for 24 hours, washed and incubated with or without 50 ng/mL CH-11 antibody for 4 hours. The cells were stained with annexin V–FITC and analyzed by flow cytometry for the rates of apoptosis. Similar results were obtained in 2 independent experiments. (D) U937/PR9 cells were treated with or without ZnSO4 for 24 hours, then washed and incubated with or without FasL for 24 hours. The cells were stained with PI and analyzed for the apoptosis rates by flow cytometry. The data represent the means ± SD of 3 independent experiments. (E) U937/PR9 cells were treated with 0, 100, and 200μM ZnSO4 for 24 hours. Cells were then washed and incubated with CH-11 antibody (50 ng/mL) for 15 minutes. Whole cell lysates were subjected to Western blot analyses using anti–caspase-8 and anti–α-tubulin antibodies for detection of cleaved/active caspase-8 fragments and α-tubulin, respectively.
Expression of PMLRARα blocks Fas-mediated apoptosis. (A) ZnSO4 (0, 100 and 200μM) was used to induce PMLRARα expression in U937/PR9 cells for 24 hours. Whole-cell lysates were subjected to Western blot analyses using an anti-RARα (for detection of PMLRARα), anti-Fas and anti–β-actin antibodies. NB4 cells and U937 cells treated with ZnSO4 were used as a positive and a negative control for PMLRARα expression, respectively. (B) U937/PR9 cells were treated with 100 and 200μM ZnSO4 for 24 hours followed by incubation with 50 ng/mL CH-11 antibody for 8 and 24 hours (left panel). U937 and U937/PR9 cells were treated with ZnSO4 for 24 hours followed by incubation with 50 ng/mL CH-11 antibody for 24 hours (right panel). The cells were stained with propidium iodide (PI) and analyzed by flow cytometry for the rates of apoptosis. The values shown are the means ± SD of 3 independent experiments. (C) U937/PR9 cells were treated with ZnSO4 for 24 hours, washed and incubated with or without 50 ng/mL CH-11 antibody for 4 hours. The cells were stained with annexin V–FITC and analyzed by flow cytometry for the rates of apoptosis. Similar results were obtained in 2 independent experiments. (D) U937/PR9 cells were treated with or without ZnSO4 for 24 hours, then washed and incubated with or without FasL for 24 hours. The cells were stained with PI and analyzed for the apoptosis rates by flow cytometry. The data represent the means ± SD of 3 independent experiments. (E) U937/PR9 cells were treated with 0, 100, and 200μM ZnSO4 for 24 hours. Cells were then washed and incubated with CH-11 antibody (50 ng/mL) for 15 minutes. Whole cell lysates were subjected to Western blot analyses using anti–caspase-8 and anti–α-tubulin antibodies for detection of cleaved/active caspase-8 fragments and α-tubulin, respectively.
To confirm this novel finding, we also analyzed apoptosis by annexin V staining in cells treated with CH-11 for 4 hours. In the agreement with the previous experiment, we found lower proportions of annexin V-positive PMLRARα-expressing cells than nonexpressing cells (15% compared with 29%), confirming that PMLRARα suppresses Fas-mediated apoptosis (Figure 2C). Because agonistic antibodies do not always reproduce the effects of ligands, we incubated U937/PR9 cells with and without ZnSO4 followed by induction of apoptosis by FasL. Again, the PMLRARα-expressing cells showed lower apoptosis rates than did the PMLRARα-nonexpressing cells (P = .004; Figure 2D).
Alterations of Fas apoptosis rates should correspond with the levels of activation of the initiator procaspase 8. We examined the levels of cleaved procaspase 8 (p18) in extracts of U937/PR9 cells and found that PMLRARα expression is associated with lower levels of procaspase 8 cleavage/activation (Figure 2E).
Down-regulation of PMLRARα sensitizes transgenic mouse APL cells and human APL NB4 cells to Fas-mediated apoptosis
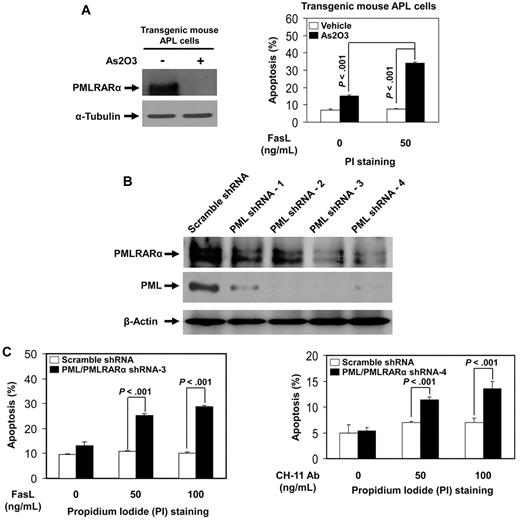
To confirm the relevance of the inhibitory effects of PMLRARα on Fas apoptosis found in U937/PR9 cells, we analyzed Fas-mediated apoptosis in transgenic mouse APL cells and human APL NB4 cells. Transgenic mouse APL cells were treated with arsenic trioxide (As2O3), an effective drug for human APL, which induced degradation of PMLRARα (Figure 3A). As2O3-treated mouse APL cells showed a significantly higher level of FasL-induced apoptosis compared with controls (Figure 3A). Human APL NB4 cells with knocked-down expression of PML and PMLRARα (clone 3 and clone 4 generated by 2 different specific shRNA sequences) showed higher levels of apoptosis in response to Fas stimulation compared with cells expressing control scrambled shRNAs (Figure 3B-C). The net enhancing effect of combined PMLRARα and PML knockdown on apoptosis is consistent with the dominant negative effect of antiapoptotic PMLRARα over the suggested proapoptotic effects of PML.
Down-regulation of PMLRARα enhances Fas-mediated apoptosis in transgenic mouse APL cells and human APL NB4 cells. (A) Transgenic mouse APL cells were treated with vehicle or 1μM arsenic trioxide (As2O3) for 24 hours. Whole cell lysates were analyzed for protein expression with anti-PML and anti–α-tubulin antibodies (left). Cells were then incubated with or without 50 ng/mL FasL for additional 24 hours, and stained with PI to analyze apoptosis by flow cytometry. The data represented are the means ± SD of 3 independent experiments (right). (B) Human APL NB4 cells were transduced with scrambled shRNA or 4 different PML/PMLRARα-targeting shRNAs as described in “Experiments using shRNA.” After selection with puromycin, cells were analyzed for protein expression with anti-RARα, anti-PML, and anti–β-actin antibodies to confirm down-regulation of targets. (C) NB4 clone 3 and clone 4 cells with knockdown of PML and PMLRARα expression were incubated with indicated concentrations of FasL or CH-11 antibody for 48 hours. The cells were then stained with PI and analyzed for apoptosis by flow cytometry. The data represent the means ± SD of 3 independent experiments.
Down-regulation of PMLRARα enhances Fas-mediated apoptosis in transgenic mouse APL cells and human APL NB4 cells. (A) Transgenic mouse APL cells were treated with vehicle or 1μM arsenic trioxide (As2O3) for 24 hours. Whole cell lysates were analyzed for protein expression with anti-PML and anti–α-tubulin antibodies (left). Cells were then incubated with or without 50 ng/mL FasL for additional 24 hours, and stained with PI to analyze apoptosis by flow cytometry. The data represented are the means ± SD of 3 independent experiments (right). (B) Human APL NB4 cells were transduced with scrambled shRNA or 4 different PML/PMLRARα-targeting shRNAs as described in “Experiments using shRNA.” After selection with puromycin, cells were analyzed for protein expression with anti-RARα, anti-PML, and anti–β-actin antibodies to confirm down-regulation of targets. (C) NB4 clone 3 and clone 4 cells with knockdown of PML and PMLRARα expression were incubated with indicated concentrations of FasL or CH-11 antibody for 48 hours. The cells were then stained with PI and analyzed for apoptosis by flow cytometry. The data represent the means ± SD of 3 independent experiments.
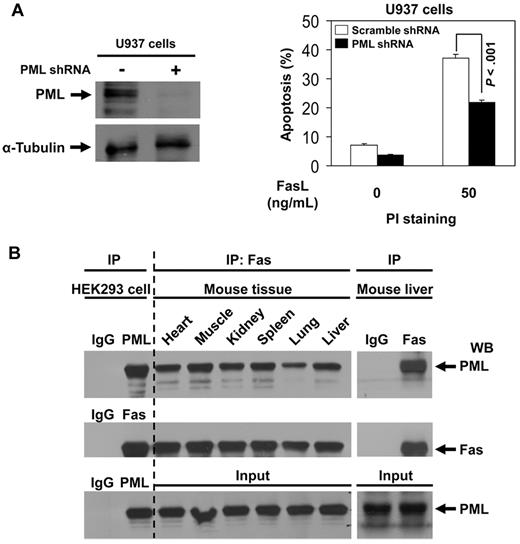
To demonstrate whether PML is indeed involved in Fas-mediated apoptosis, we knocked down PML expression by PML shRNA in U937 cells, which express endogenous PML but not PMLRARα (Figure 4A). Knockdown of PML significantly decreased the level of FasL-induced apoptosis compared with control cells (Figure 4A), confirming the suspected proapoptotic effects of PML. We used coimmunoprecipitation and Western blot analysis and confirmed the presence of PML-Fas complexes in all tested mouse tissues (Figure 4B).9 These results suggest that PML could modulate Fas apoptosis in many normal tissues.
PML enhances Fas-mediated apoptosis and PML-Fas association is common in noncancerous tissues. (A) U937 cells were transduced with scrambled shRNA or PML-specific shRNAs as described in “Experiments using shRNA.” After selection with puromycin, cells were analyzed for protein expression with anti-PML and anti–α-tubulin antibodies to confirm down-regulation of PML. The cells were incubated with 0 or 50 ng/mL of FasL for 24 hours and then stained with propidium iodide and analyzed for apoptosis by flow cytometry. The data represent the means ± SD of 3 independent experiments. (B) Cell extracts of various mouse tissues were immunoprecipitated using antibody against Fas or IgG followed by Western blot analysis with antibodies against PML and Fas. The precipitates from HEK293 cell extracts by normal IgG antibody were used as negative control; the precipitates from HEK 293 cell extracts by anti-PML or anti-Fas antibody were used as positive control, respectively.
PML enhances Fas-mediated apoptosis and PML-Fas association is common in noncancerous tissues. (A) U937 cells were transduced with scrambled shRNA or PML-specific shRNAs as described in “Experiments using shRNA.” After selection with puromycin, cells were analyzed for protein expression with anti-PML and anti–α-tubulin antibodies to confirm down-regulation of PML. The cells were incubated with 0 or 50 ng/mL of FasL for 24 hours and then stained with propidium iodide and analyzed for apoptosis by flow cytometry. The data represent the means ± SD of 3 independent experiments. (B) Cell extracts of various mouse tissues were immunoprecipitated using antibody against Fas or IgG followed by Western blot analysis with antibodies against PML and Fas. The precipitates from HEK293 cell extracts by normal IgG antibody were used as negative control; the precipitates from HEK 293 cell extracts by anti-PML or anti-Fas antibody were used as positive control, respectively.
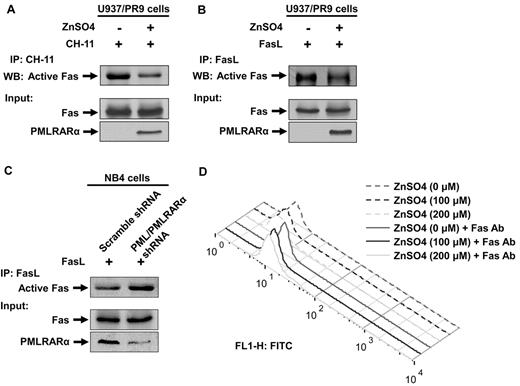
PMLRARα blocks CH-11 antibody and Fas ligand binding to Fas
The newly discovered interaction of Fas with PMLRARα in the cytosolic/membrane fraction and the negative effects of PMLRARα on Fas-mediated apoptosis prompted us to examine a possible mechanism of interference of PMLRARα with the early stages of Fas activation.
We first analyzed the ability of Fas to bind agonistic antibody in the U937/PR9 cells treated with ZnSO4 for 24 hours to induce expression of PMLRARα or buffer alone treated cells. Cells were subsequently incubated with 200 ng/mL agonistic anti-Fas antibody CH-11. Unbound antibody was removed by washing. Cells were lysed and precipitated with goat anti–mouse IgM antibody to coprecipitate CH-11–bound Fas (Figure 5A top panel). More Fas was pulled down with CH-11 from the non-PMLRARα–expressing cells than PMLRARα-expressing cells. The total levels of cellular Fas were not affected by expression of PMLRARα (Figure 5A bottom panel).
PMLRARα blocks CH-11 antibody and Fas ligand binding to Fas. (A) U937/PR9 cells were treated with or without ZnSO4 for 24 hours to induce expression of PMLRARα and subsequently incubated with agonistic anti-Fas antibody CH-11. Unbound antibody was removed by washing. Cells were lysed and precipitated with goat anti–mouse IgM antibody to coprecipitate CH-11–bound Fas (Active Fas, top panel). Total cell extracts were also Western blotted with anti-Fas and anti-RARα antibodies for detection of Fas and PMLRARα. (B) Expression of PMLRARα was induced as in panel A and cells were incubated with FasL. Excess of FasL was washed off and cells lysed. FasL complexes were precipitated to detect activation accessible Fas. (C) Scrambled shRNA expressing cells and NB4 clone 4 cells were incubated with FasL as in panel B. Cell extracts were used to immunoprecipitate FasL to detect FasL-bound/activation accessible Fas (top panel). Whole cellular levels of Fas and PMLRARα were analyzed by Western blot. (D) U937/PR9 cells were treated with 0, 100, and 200μM ZnSO4 for 24 hours and stained with anti-Fas antibody, to evaluate cell surface Fas levels by flow cytometry as described in “Flow cytometry analysis of surface Fas expression level.”
PMLRARα blocks CH-11 antibody and Fas ligand binding to Fas. (A) U937/PR9 cells were treated with or without ZnSO4 for 24 hours to induce expression of PMLRARα and subsequently incubated with agonistic anti-Fas antibody CH-11. Unbound antibody was removed by washing. Cells were lysed and precipitated with goat anti–mouse IgM antibody to coprecipitate CH-11–bound Fas (Active Fas, top panel). Total cell extracts were also Western blotted with anti-Fas and anti-RARα antibodies for detection of Fas and PMLRARα. (B) Expression of PMLRARα was induced as in panel A and cells were incubated with FasL. Excess of FasL was washed off and cells lysed. FasL complexes were precipitated to detect activation accessible Fas. (C) Scrambled shRNA expressing cells and NB4 clone 4 cells were incubated with FasL as in panel B. Cell extracts were used to immunoprecipitate FasL to detect FasL-bound/activation accessible Fas (top panel). Whole cellular levels of Fas and PMLRARα were analyzed by Western blot. (D) U937/PR9 cells were treated with 0, 100, and 200μM ZnSO4 for 24 hours and stained with anti-Fas antibody, to evaluate cell surface Fas levels by flow cytometry as described in “Flow cytometry analysis of surface Fas expression level.”
To confirm whether lower Fas binding to agonistic antibody holds true for FasL, we repeated the experiment (Figure 5A) by incubating cells with FasL. In agreement with the results obtained with the agonistic antibody, less Fas was able to bind FasL in the PMLRARα-expressing cells than in PMLRARα-negative cells (Figure 5B).
FasL binding may have been affected by exposure to ZnSO4, thus, we tested binding of FasL to Fas in the previously described NB4 clone 4 with suppressed expression of PML and PMLRARα. In agreement with the results obtained with U937/PR9 cells, more Fas receptor associated with FasL in PML and PMLRARα- knockdown cells than in control cells (Figure 5C).
Although our Western blot analysis consistently showed that expression of PMLRARα has no effect on the total cellular levels of Fas (Figure 5A-C), a possible explanation of the lower binding to Fas would be the interference of PMLRARα with plasma membrane localization of Fas. Therefore, we analyzed cell surface expression of Fas before and after induction of PMLRARα expression with ZnSO4. As shown in Figure 5D, expression of PMLRARα did not affect levels of Fas receptor at the cell surface.
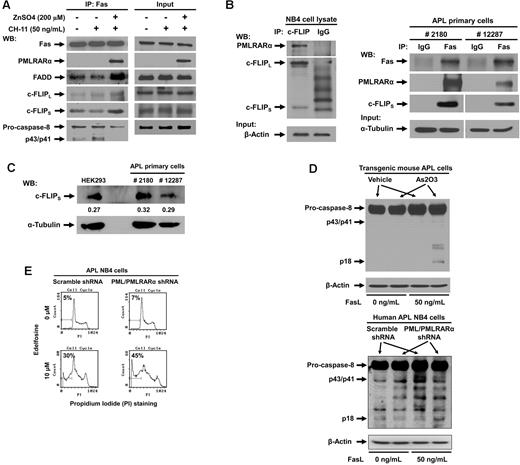
PMLRARα forms complex with Fas and recruits c-FLIP
We next proceeded to analyze the effect of PMLRARα on formation of death-inducing signaling complex (DISC) in U937/PR9 cells induced to express PMLRARα by incubation with ZnSO4 and triggered to undergo apoptosis with 50 ng/mL CH-11. Cell lysates were immunoprecipitated with anti-Fas antibody and analyzed by Western blot for the components of DISC (Figure 6A). As expected, PMLRARα coprecipitated with Fas. Furthermore, expression of PMLRARα was associated with higher levels of FADD and both forms of c-FLIP at DISC but with lower levels of full-length and cleaved forms of caspase-8 compared with PMLRARα nonexpressing cells (Figure 6A).
PMLRARα recruits c-FLIP to Fas and inhibits procaspase-8 binding and cleavage. (A) U937/PR9 cells treated with or without 200μM ZnSO4 were activated with 50 ng/mL of CH-11 antibody and immunoprecipitated with anti-Fas antibody. The precipitants were analyzed for the presence of DISC subunits by Western blot (left panel). The panel on the right shows input protein levels in whole cell extracts. (B) APL NB4 cell extracts were immunoprecipitated using antibody against c-FLIP and control IgG antibody, and precipitates were Western blotted using anti-RARα and anti–c-FLIP antibodies (left panel). Equal input protein levels were confirmed by immunoblot analysis of β-actin in the whole cell lysates. APL primary cells from 2 patients were lysed and immunoprecipitated with anti-Fas or control IgG antibody (right panel). (This is the same experiment as shown in Figure 1C with extended analysis of c-FLIP). Precipitates were analyzed for the presence of PMLRARα and c-FLIP by Western blot. The quality of input material is shown in bottom panels. (C) Extracts from APL primary cells and HEK293 cells were subjected to Western blot analysis using anti–c-FLIP and anti–α-tubulin antibodies to compare relative protein expression levels. Quantification of bands with reference to tubulin levels was performed using Adobe Photoshop CS3 software. (D) Vehicle and arsenic trioxide-treated transgenic mouse APL cells (top) or scrambled shRNA cells and NB4 clone 4 cells (bottom) were treated with 0 or 50 ng/mL of FasL for 15 minutes. Whole cell lysates were then subjected to Western blot analysis using anti–caspase-8 and anti–β-actin antibodies. (E) NB4 clone 4 and scrambled shRNA NB4 cells were incubated with or without 10μM edelfosine, stained by propidium iodide and analyzed for the degree of apoptosis by flow cytometry.
PMLRARα recruits c-FLIP to Fas and inhibits procaspase-8 binding and cleavage. (A) U937/PR9 cells treated with or without 200μM ZnSO4 were activated with 50 ng/mL of CH-11 antibody and immunoprecipitated with anti-Fas antibody. The precipitants were analyzed for the presence of DISC subunits by Western blot (left panel). The panel on the right shows input protein levels in whole cell extracts. (B) APL NB4 cell extracts were immunoprecipitated using antibody against c-FLIP and control IgG antibody, and precipitates were Western blotted using anti-RARα and anti–c-FLIP antibodies (left panel). Equal input protein levels were confirmed by immunoblot analysis of β-actin in the whole cell lysates. APL primary cells from 2 patients were lysed and immunoprecipitated with anti-Fas or control IgG antibody (right panel). (This is the same experiment as shown in Figure 1C with extended analysis of c-FLIP). Precipitates were analyzed for the presence of PMLRARα and c-FLIP by Western blot. The quality of input material is shown in bottom panels. (C) Extracts from APL primary cells and HEK293 cells were subjected to Western blot analysis using anti–c-FLIP and anti–α-tubulin antibodies to compare relative protein expression levels. Quantification of bands with reference to tubulin levels was performed using Adobe Photoshop CS3 software. (D) Vehicle and arsenic trioxide-treated transgenic mouse APL cells (top) or scrambled shRNA cells and NB4 clone 4 cells (bottom) were treated with 0 or 50 ng/mL of FasL for 15 minutes. Whole cell lysates were then subjected to Western blot analysis using anti–caspase-8 and anti–β-actin antibodies. (E) NB4 clone 4 and scrambled shRNA NB4 cells were incubated with or without 10μM edelfosine, stained by propidium iodide and analyzed for the degree of apoptosis by flow cytometry.
The strikingly high levels of FADD and caspase-8 inhibitor c-FLIP recruited to Fas receptor in PMLRARα-expressing cells deserved further exploration. To examine the possibility of PMLRARα binding to c-FLIP in the DISC complex, we subjected NB4 cell lysates to immunoprecipitation with anti-cFLIP antibody or isotype-matched control antibody. PMLRARα indeed coprecipitated with c-FLIP (Figure 6B). To prove clinical relevance of PMLRARα association with c-FLIP, we analyzed presence of c-FLIP in Fas complexes from primary APL cells. As expected, both PMLRARα and c-FLIP coprecipitated with anti-Fas antibody, but not with control IgG antibody (Figure 6B). These results are an extension of the experiment shown in Figure 1C. Thus, in addition to interfering with binding of agonistic antibody and FasL to Fas, PMLRARα also recruits caspase-8 inhibitor c-FLIP to the Fas receptor, which inhibits processing of the initiator caspase-8 and blocks Fas-mediated apoptosis even further.
To explore a possibility that increased levels of c-FLIP at Fas receptor are because of increased expression of c-FLIP in PMLRARα expressing cells, we compared the relative expression levels of c-FLIP in the APL primary cells and HEK293 cells (Figure 6C). Importantly the c-FLIP/α-tubulin ratios were comparable between tested cells confirming that increase of c-FLIP at Fas complex is not because of increased c-FLIP expression in APL cells but rather because of recruitment of c-FLIP by PMLRARα.
If PMLRARα inhibits Fas apoptosis, targeting of PMLRARα may restore early Fas signaling including procaspase-8 activation. To confirm this expectation, we incubated As2O3-treated transgenic mouse APL cells and human APL NB4 clone 4 cells with 50 ng/mL FasL. Cells extracts were analyzed for cleavage/activation of initiator caspase-8. In both cell types, targeted destruction of PMLRARα led to increased cleavage of caspase-8 to the active p18 form in response to FasL compared with vehicle-treated or scrambled shRNA-expressing control cells (Figure 6D).
Overall, our data suggested that PMLRARα blocks initiation of Fas-mediated apoptosis at membrane proximal steps. To confirm the early interruption of Fas-mediated apoptosis by PMLRARα, we used a ligand-independent method to activate Fas apoptotic signaling. NB4 clone 4 and control cells were incubated with 10μM edelfosine, which activates Fas by transporting the receptor into lipid rafts independently of FasL.24 Although edelfosine-induced activation of Fas bypasses the requirement for Fas activation by ligand, it still depends on activation of caspase-8. NB4 clone 4 cells with suppressed expression of PML and PMLRARα showed enhanced rates of apoptosis compared with scrambled shRNA control clone (45% vs 30%), showing that PMLRARα affects activation of caspase-8 regardless of the mechanism of Fas activation (ligand dependent or independent) (Figure 6E).
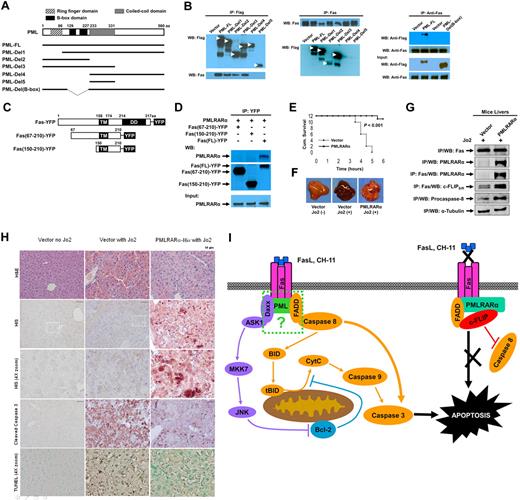
The B-box domain of PMLRARα is required for Fas binding
Our previous experiments indicated that PML but not RARα binds to Fas and suggested that the PML fragment of PMLRARα might mediate binding to Fas. To further map the interacting domain, we expressed Flag-tagged PML deletion mutants (Figure 7A) in HEK293 cells. Anti-Flag antibody immunoprecipitated adequate levels of tagged full-length PML (PML-FL) and PML-deletion mutants. Deletion mutants Del1-3, but not mutants Del4 and Del5 coprecipitated Fas (Figure 7A-B). To corroborate these mapping results, we reversed the order of antibodies used in the IP/WB analysis (Figure 7B). The use of antibodies in reversed order confirmed association of Fas with all PML deletion mutants except for mutants Del4 and Del5 (Figure 7B) lacking PML B-box domain (amino acids 120-230). To confirm involvement of B-box region in interaction with Fas, we constructed a PML B-box deletion mutant PML-Del (B-box) which indeed failed to bind to Fas, confirming that the B-box domain of PML is essential for PML- and PMLRARα-Fas complex formation.
PMLRARα-Fas binding domains and protection of mouse against Fas-induced death. (A) Schematic representation of PML deletion mutants. (B) HEK293 cells were transfected with pcDNA3-Flag vector, pcDNA3-Flag-PML-FL, or pcDNA3-Flag-deletion mutants illustrated in panel A. Cells lysates were immunoprecipitated using anti-Flag antibody (left) or anti-Fas antibody (middle). HEK293 cells were transfected with pcDNA3-Flag vector, pcDNA3-Flag-PML-FL or pcDNA3-Flag-PML-Del (B-box) mutant, lysed and immunoprecipitated with anti-Fas antibody (right). The precipitates were Western blotted with anti-Flag and anti-Fas antibody. (C) Schematic representation of Fas deletion mutants used to map binding to PMLRARα. Deletion of pre-ligand binding assembly domain (aa. 1-67) prevents trimerization of Fas deletion mutants with endogenous Fas. TM indicates transmembrane domain; and DD, death domain. (D) HEK293 cells were cotransfected with pcDNA3-PMLRARα, and plasmids expressing indicated YFP-tagged Fas mutants. Whole-cell lysates were immunoprecipitated using an anti-GFP antibody and the precipitates were Western blotted using anti-RARα and anti-GFP antibodies. The equal expression levels of PMLRARα in whole-cell extracts were confirmed by immunoblot analysis. (E) C57BL/6 mice were transfected with 100 μg of PMLRARα or empty vector plasmid using the hydrodynamic methodology.22 Twenty-four hours later, the mice were inoculated intraperitoneally with a lethal dose of anti–mouse Fas antibody (Jo2) and monitored for survival 6 hours after challenge when the surviving mice were killed to harvest liver tissues for comparative analysis. (F) Representative images of the livers harvested at the time of death are shown. (G) Liver extracts of PMLRARα and vector-transfected mice were analyzed for PMLRARα-Fas complexes by immunoprecipitation and Western blotting using anti-RGSHis, anti-Fas, anti–c-FLIP, and anti–caspase-8 antibodies. (H) To confirm that PMLRARα blocks Fas-mediated apoptosis, the livers from panel E were subjected to immunohistochemical and TUNEL staining. Representative H&E-stained slides (top row), anti-RGS His antibody staining showing PMLRARα-RGSHis expression (2nd and 3rd rows), anti–cleaved caspase-3 antibody and TUNEL staining (4th and bottom row, respectively) are shown. (I) Model of PMLRARα-mediated inhibition of Fas apoptosis. PMLRARα in complex with Fas recruits FADD and c-FLIP and excludes procaspase-8 to block Fas-mediated apoptosis. At the same time, Fas complexed with PMLRARα is unable to bind FasL and CH-11. ASK1 indicates apoptosis signal-regulating kinase; FADD, Fas-associated death domain protein; JNK, c-Jun NH2-terminal kinase; tBID, truncated Bid; CytC, cytochrome C; MKK7: mitogen-activated protein kinase kinase 7; and c-FLIP, cellular-FLICE inhibitory protein.
PMLRARα-Fas binding domains and protection of mouse against Fas-induced death. (A) Schematic representation of PML deletion mutants. (B) HEK293 cells were transfected with pcDNA3-Flag vector, pcDNA3-Flag-PML-FL, or pcDNA3-Flag-deletion mutants illustrated in panel A. Cells lysates were immunoprecipitated using anti-Flag antibody (left) or anti-Fas antibody (middle). HEK293 cells were transfected with pcDNA3-Flag vector, pcDNA3-Flag-PML-FL or pcDNA3-Flag-PML-Del (B-box) mutant, lysed and immunoprecipitated with anti-Fas antibody (right). The precipitates were Western blotted with anti-Flag and anti-Fas antibody. (C) Schematic representation of Fas deletion mutants used to map binding to PMLRARα. Deletion of pre-ligand binding assembly domain (aa. 1-67) prevents trimerization of Fas deletion mutants with endogenous Fas. TM indicates transmembrane domain; and DD, death domain. (D) HEK293 cells were cotransfected with pcDNA3-PMLRARα, and plasmids expressing indicated YFP-tagged Fas mutants. Whole-cell lysates were immunoprecipitated using an anti-GFP antibody and the precipitates were Western blotted using anti-RARα and anti-GFP antibodies. The equal expression levels of PMLRARα in whole-cell extracts were confirmed by immunoblot analysis. (E) C57BL/6 mice were transfected with 100 μg of PMLRARα or empty vector plasmid using the hydrodynamic methodology.22 Twenty-four hours later, the mice were inoculated intraperitoneally with a lethal dose of anti–mouse Fas antibody (Jo2) and monitored for survival 6 hours after challenge when the surviving mice were killed to harvest liver tissues for comparative analysis. (F) Representative images of the livers harvested at the time of death are shown. (G) Liver extracts of PMLRARα and vector-transfected mice were analyzed for PMLRARα-Fas complexes by immunoprecipitation and Western blotting using anti-RGSHis, anti-Fas, anti–c-FLIP, and anti–caspase-8 antibodies. (H) To confirm that PMLRARα blocks Fas-mediated apoptosis, the livers from panel E were subjected to immunohistochemical and TUNEL staining. Representative H&E-stained slides (top row), anti-RGS His antibody staining showing PMLRARα-RGSHis expression (2nd and 3rd rows), anti–cleaved caspase-3 antibody and TUNEL staining (4th and bottom row, respectively) are shown. (I) Model of PMLRARα-mediated inhibition of Fas apoptosis. PMLRARα in complex with Fas recruits FADD and c-FLIP and excludes procaspase-8 to block Fas-mediated apoptosis. At the same time, Fas complexed with PMLRARα is unable to bind FasL and CH-11. ASK1 indicates apoptosis signal-regulating kinase; FADD, Fas-associated death domain protein; JNK, c-Jun NH2-terminal kinase; tBID, truncated Bid; CytC, cytochrome C; MKK7: mitogen-activated protein kinase kinase 7; and c-FLIP, cellular-FLICE inhibitory protein.
The death domain of Fas is necessary for Fas-PMLRARα complex formation
Because cytoplasmic PMLRARα can bind to Fas and block Fas-mediated apoptosis, and the cytoplasmic death domain (DD) is essential for Fas signal transduction, we investigated whether the Fas death domain is involved in binding to PMLRARα. Fas PLAD (extracellular pre-ligand assembly domain; aa. 18-67) deleted constructs were tested to prevent oligomerization of tested Fas mutants with the endogenous Fas, which could lead to false-positive results because of association of PMLRARα with the endogenous Fas subunits. IP/WB analysis of HEK 293 cells transiently expressing PMLRARα and YFP-tagged Fas death domain deletion mutants showed that only the full-length Fas was able to coprecipitate PMLRARα (Figure 7C-D), suggesting that the death domain of Fas is required for formation of Fas-PMLRARα complex.
PMLRARα protects mice against challenge with a lethal dose of agonistic anti–mouse Fas antibody Jo2
To confirm that PMLRARα blocks Fas-mediated apoptosis in an in vivo system, we expressed RGSHis-tagged PMLRARα in the liver tissue of mice, which is extremely susceptible to Fas-mediated apoptosis.25 The liver tissue showed widespread expression of reporter GFP plasmid using the hydrodynamic transfection method.17 Transfected mice were challenged with a lethal dose of agonistic anti–mouse Fas antibody Jo2 and monitored for survival up to 6 hours after the challenge. The mice transfected with the PMLRARα-expressing plasmid had a significantly higher survival rate than did control mice transfected with vector alone (Figure 7E; 11 of 12 vs 0 of 12; P < .001).
On gross examination, the livers of the antibody-killed mice showed signs of hemorrhaging on > 50% of the liver surface (Figure 7F).26 In contrast, livers from the surviving (PMLRARα-transfected) mice had fewer signs of hemorrhaging.
Because results from tissue culture experiments indicated that PMLRARα-Fas interaction was associated with protection against Fas-mediated apoptosis, we probed for PMLRARα-Fas complexes in liver tissues. Representative PMLRARα-expressing and vector control liver extracts contained comparable levels of Fas and Fas precipitated PMLRARα from PMLRARα-positive extracts (Figure 7G) confirming the presence of PMLRARα-Fas complexes. Higher levels of c-FLIPS in Fas complex and intact procaspase-8 in whole tissue extract were observed in PMLRARα-transfected livers than in vector-transfected livers confirming a protective effect against Fas-mediated apoptosis expected from our tissue culture data.
Immunohistochemical staining of liver sections with hematoxylin and eosin showed hemorrhaging and the presence of numerous shrunken, presumably apoptotic nuclei in vector-transfected liver tissues challenged with agonistic Jo2 antibody, whereas PMLRARα-transfected livers challenged with agonistic Jo2 antibody were comparable with nonchallenged vector controls (Figure 7H). Anti-RGS His antibody staining showed successful expression of PMLRARα-RGSHis in polygonal shaped cells that contained centrally located nuclei (characteristic of hepatocytes), as expected using this transfection method, which targets hepatocytes with high efficiency (Figure 7H).
To analyze the extent of apoptosis in liver tissues challenged with agonistic anti-Fas antibody Jo2, we stained representative thin sections with anti–cleaved caspase-3 antibody and TUNEL which showed regional distribution of positive staining (Figure 7H). PMLRARα-transfected mice had fewer cleaved caspase-3–positive cells and fewer TUNEL-positive cells than did the vector control mice. Thus, as clearly indicated by several methods, PMLRARα expression interferes with Fas-mediated apoptosis in vitro and in vivo.
Discussion
PML and PMLRARα have been long recognized as modulators of Fas apoptotic signaling, but evidence of a detailed and convincing mechanism for this regulation has not been offered.13 Here we show for the first time that PML and oncogenic protein PMLRARα associate with Fas in human APL cell line, APL primary cells, mouse APL cells and in vivo. Further analysis showed that binding of the cancer-related protein PMLRARα to Fas prevents Fas-mediated apoptosis in all tested cell models and in mice. Association of PMLRARα with Fas enhances the recruitment of FADD and the caspase-8 inhibitor c-FLIP to the Fas receptor complex, which effectively blocks initiation of Fas-apoptotic signaling - cleavage of the initiator caspase-8 in response to ligand-dependent and ligand-independent Fas activation. In addition to inhibition of caspase-8 cleavage, PMLRARα interferes with binding of agonistic anti-Fas antibody CH-11 and FasL to Fas receptor.
The PMLRARα fusion protein harbors the N-terminus of PML containing the RING finger domain followed by 2 B-boxes and an α-helical coiled-coil motif, referred to as the RBCC domain, which mediates protein-protein interactions and is responsible for multimerization and heterodimerization of PML.27-30 PMLRARα also retains the C-terminal region of RARα including the retinoic acid-binding domain and DNA-binding domain.27,31 PMLRARα acts in a dominant negative manner and disrupts the normal functions of both PML and RARα, including PML-related enhancement of apoptosis and RARα-related cell differentiation.13,32 PMLRARα is known to block the p53-dependent as well as p53-independent apoptotic pathways.13 The details of PMLRARα-related suppression of p53-mediated apoptosis are unclear, but the effect has been attributed to disruption of nuclear transcription, primarily by transcriptional repression.33 Several more recent reports suggest that PMLRARα also affects p53-independent apoptosis.34 In agreement with those reports, we did not observe PMLRARα effect on total cellular Fas levels in several cells and in mice and on the levels of Fas at the cell surface (Figures 5, 6A, and 7G), demonstrating PMLRARα-mediated protection against Fas apoptosis is independent of p53. Our finding of direct interaction of PMLRARα with Fas receptor seems to be a plausible explanation of how PMLRARα regulates Fas-mediated apoptosis.
The conventional role of PMLRARα is in the nucleus, where it disrupts nuclear bodies and, in at least one arm of its effects, blocks apoptosis through transcriptional regulation. In this study, we demonstrated a role of cytoplasmic PMLRARα in direct apoptosis control. We separated cellular compartments and showed that PMLRARα-Fas complexes were exclusively present in the cytosolic/membrane fractions (Figure 1D). There is growing support for the notion that PMLRARα and PML have meaningful roles in the cytoplasm, for example: (1) some PML isoforms (cPML) function exclusively within the cytoplasm; (2) during virus infections, PML is transported to the cytoplasm where it binds to viral proteins and suppresses infection; (3) PML binds to cytoplasmic targets such as Elf4e to block translation; (4) PML regulates apoptosis at endoplasmic reticulum by modulating calcium release; (5) PMLRARα binds to TGFβR and blocks PML-mediated transport of activated TGFβR into early endosomes; and (6) PML is present in complexes with the cytoplasmic resident protein Fas in all mouse tissues analyzed (Figure 4B).35-37 Thus, it seems likely that PMLRARα- and PML-mediated regulation of apoptosis can occur through direct interaction with Fas at the cytoplasmic leaflet of the plasma membrane. This direct regulation represents another layer of apoptosis control with advantages over nuclear-based control, as the early signaling steps can be effectively extinguished or rerouted.38
The domains of Fas and PMLRARα required for complex formation have been preliminary assigned to the B-boxes of PML moiety and the death domain of Fas, both known to mediate interactions with other proteins. Interestingly, the B-box region of PML contains a sumoylation site K160, which is a pivotal regulatory site of PML turnover.39 We observed that PMLRARα blocks Fas signaling by 2 potential mechanisms: PMLRARα prevents recognition of cell surface Fas by FasL and agonistic anti-Fas antibody (Figure 5); and PMLRARα enhances formation of a Fas complex enriched with c-FLIP (Figures 6A-B and 7G). This mechanism differs from PMLRARα-mediated down regulation of tumor necrosis factor α receptor levels that prevented apoptosis in other leukemic cells40 as we show that PMLRARα does not affect total cellular and cell surface levels of Fas (Figure 5). Accumulation of c-FLIP at the Fas receptor has proven to be potent mechanism for blocking apoptosis.41 Blocking of procaspase-8 activation or ligand binding should suffice to block apoptosis. Thus, the interesting question is why PMLRARα blocks apoptosis of a single receptor at 2 sites. PMLRARα presence in Fas complex may produce a mix of Fas receptors blocked alternatively by recruitment of cFLIP or blocked at agonist binding step. Of interest is the possibility that PMLRARα exerts inside-out signaling on Fas by the active release of FasL as a form of intercellular signaling. Intracellular modulation of Fas could alter cells binding to ligand and associated proteins and mediate: (1) regulation of circulating FasL, (2) apoptosis escape mechanisms, (3) cell migration into FasL constitutively expressing tissues, and (4) the high levels of FasL often observed in malignancies with poor prognosis. Thus, intracellular oncogene modulation of FasL binding merits further investigation.
Taken together, in this study we found that PMLRARα can bind directly to Fas and suppress Fas-mediated apoptosis. In the nucleus, PMLRARα alters the normal protein interactions of PML, disrupts formation of nuclear bodies and transcription complexes.37 In the cytoplasm, PMLRARα binds to the Fas complex and greatly facilitates binding of cFLIP and FADD to produce a stable inactive complex that excludes procaspase 8 binding and prevents its activation (Figure 7I). In addition, PMLRARα prevents binding of Fas ligand and agonistic antibody CH-11 to Fas suggesting a role of inside-out signaling, which could be of further importance. PML serves as a scaffold protein in the nuclear bodies and this function may extend to the cytoplasm where PML may promote Fas-FADD-procaspase 8 complexes or destabilize Fas-FADD-cFLIP complexes – the opposing effects to PMLRARα. The apoptosis inhibitory death receptor-FADD-cFLIP complexes are present in some cancers42 and selective down-regulation of cFLIP is sufficient to induce apoptosis. Our model predicts that PML positively enhances Fas receptor signaling in tissues and would be especially important during conditions of cell stress when cytoplasmic levels of the PML rise and PML tumor suppressor properties are most prominent.
Fas apoptosis has an established indispensible role in response to numerous chemotherapies and irradiation, which was readily demonstrated by a lack of response to these treatments in cancer cells with defective Fas signaling.43 The newly discovered direct regulation of Fas apoptosis by PML/ PMLRARα suggests an important mechanism implicated in cancer chemotherapy resistance, because > 60% of all cancers show depressed levels or mutations of tumor suppressor PML.9,44 The role of PML in apoptosis has been underestimated because PML enhances apoptosis primarily under stress conditions such as during exposure to toxins, inflammation, chemotherapy, or radiation. We have identified an attractive potential target for regulation of apoptosis at the PMLRARα-Fas and PML-Fas interface. In fact, the effectiveness of cancer therapies such as interferon α and proteasome inhibitors may be based on enhanced responses to Fas-mediated apoptosis through enhanced expression and blocked degradation of positive Fas regulator PML, respectively.45 The use of arsenic trioxide has been a key component of therapies producing durable remissions for the majority of individuals with APL by targeting PMLRARα for degradation.45-47 Arsenic trioxide kills cells in a Fas-dependent manner and this mechanism may require removal of PMLRARα from the inhibitory Fas complex for apoptosis to proceed (Figure 3A).48,49 By neutralizing the inhibitory effect of Fas-binding proteins such as PMLRARα and/or promoting positive Fas modulators such as PML, we can improve responses to many chemotherapeutic treatments that merits further investigation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Pardee Foundation and the Richard Spencer Lewis Memorial Foundation and patients' families who have also supported this research. They also thank Dr Jagannadha Sastry and Dr Hector Martinez-Valdez for their encouragement and critical review of the manuscript.
This work was supported by the National Institutes of Health (CA1206173, CA153170, CA158692, and DK091490 to F.S.), the Leukemia & Lymphoma Society (R6132-06 and R6187-09 to F.S.), the American Cancer Society (118447-MRSG-10-052-01-LIB to ZB), The University of Texas M. D. Anderson Cancer Center Multidisciplinary Research Program and Institutional Research Grant (to F.S.), and Johns Hopkins Cancer Center Core Grant (5P30 CA06973-46 to J.E.K.).
National Institutes of Health
Authorship
Contribution: R.-H.T. designed the research studies, performed most of the experiments, analyzed the data, and wrote the manuscript; Z.B. prepared the animal protocol, designed the B-box mutant, and wrote the manuscript; J.F.W. analyzed surface Fas expression level; A.-H.R. performed tissue staining; U.D. and X.A. contributed to the writing of the manuscript; D.H.H. sequenced Fas-associated peptides; J.E.K. provided tissue samples and contributed to writing; and H.-K.L., J.J.M., and F.S. contributed to research design and writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Felipe Samaniego, MD, Department of Lymphoma and Myeloma, The University of Texas M. D. Anderson Cancer Center, 7455 Fannin St, Houston, TX 77054; e-mail: fsamaniego@mdanderson.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal